Abstract
In the development and progression of hepatocellular carcinoma, tumor hypoxia plays an important role, as does activation of the Wnt pathway. The aim of this study was to characterize the expression and interrelationship between hypoxia and Wnt-pathway-associated proteins as prognostic factors for hepatocellular carcinoma. Expression of HIF-1α, CA-IX, E-cadherin, β-catenin, and Ki-67 was assessed by immunohistochemistry in 179 primary hepatocellular carcinoma cases. Univariate and multivariate analyses were performed to assess the relationship between the clinicopathological factors, protein expression, overall survival (OS), and recurrence-free survival (RFS). By univariate analysis, tumor stage, size, satellitosis, and vascular invasion were confirmed as prognostic factors for worse OS and RFS. High expression of HIF-1α, CA-IX, β-catenin, Ki-67, and E-cadherin was observed in 60, 15, 64, 8, and 64 % of tumors, respectively, and this was significantly associated with poor OS. CA-IX, HIF-1α, and E-cadherin were independent predictors of poor prognosis. We stratified 169 patients into four groups according to the expression level of hypoxia and Wnt pathway markers. The group with high expression of both hypoxia and Wnt-pathway-associated proteins showed worst OS. The poor survival of this group was also significant in patients with early stage disease and tumor size of less than 5 cm (p < 0.05). We identified a subgroup of hepatocellular carcinoma patients with high expression of both hypoxia and Wnt pathway proteins and found this predictive of poor survival. The therapeutic options for this group might need to be revisited.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is a lethal cancer associated with poor prognosis as the 5-year survival rate is less than 17 % [1]. HCC is the fifth most common cancer in men worldwide and is also currently the leading cause of cancer death in patients with cirrhosis. Hepatic resection and liver transplantation offers treatment to only 20 %, as most patients are diagnosed at late stage, with heavily impaired liver function or when tumor size no longer allows surgical management [2–5]. However, in the last two decades, various surveillance programs have been initiated (especially in Western countries) which has lead to early detection and diagnosis of HCC [6].
Recently, many studies have elucidated molecular mechanisms involved in the development of HCC, which provides a basis for molecular classification with prognostic or diagnostic relevance [7, 8]. These studies revealed that several signaling pathways regulating cell proliferation and survival are deregulated in HCC, including the Ras, PI3k/AKT/mTOR, and Wnt/β-catenin pathways [9]. In addition, mutation of TP53 and overexpression of cell cycle regulators such as cyclins and CDKs has been reported to be involved with enhanced cell proliferation. The Wnt signaling pathway might be the best characterized oncogenic pathway in HCC, its activation occurring most commonly though CTNNB1 mutation. E-cadherin and β-catenin are downstream targets of Wnt pathway, and the expression of which has been previously reported to predict poor prognosis in HCC.
Hypoxic environment in tumors enhances invasiveness and metastatic propensity. Hypoxia is associated with poor prognosis in HCC, promoting invasiveness, increased glycolysis, angiogenesis, and metastasis primarily through the Ras pathway, but it also decreases proliferation [10]. The response to hypoxic stress is mediated through hypoxia-inducible factor 1 (HIF-1), a heterodimeric transcription factor consisting of two subunits, HIF-1α and HIF-1β. HIF-1α overexpression is associated with poor prognosis in human cancers, including HCC [11–14]. Carbonic anhydrase IX (CA-IX) is a transmembrane protein with an extracellular catalytic site, the expression of which is activated by HIF-1α [15]. It plays a role in solid tumors like breast, head and neck, and non-small cell lung cancer [16–18]. Yu et al. reported that expression of CA-IX is induced by hypoxia in HCC cells and studied CA-IX protein expression in HCC patients [19]. An association of CA-IX expression with prognosis of HCC, however, has not been assessed.
Recent reports suggest that a hypoxic environment inactivates the Wnt pathway through HIF-1α in colon, lung, and renal cell carcinomas [20]. Therefore, we set out to study the expression of hypoxia-induced proteins HIF-1α and CA-IX and Wnt pathway target proteins β-catenin and E-cadherin in primary HCC. We focused on associations between patterns of expression and with clinicopathological features and patient survival.
Material and methods
Patients
We included in this study 179 HCC patients who had undergone curative hepatectomy between 1990 and 2009 at the National University Hospital in Singapore. Standard clinical data including survival were obtained from medical records. As pathological parameters, we included tumor stage, grade, vascular invasion, satellitosis, and background liver histology. Tumor grade was defined according to the Edmondson grading system [21]. Satellitosis was defined as a multifocal tumor, with a satellite nodule separated from the main tumor by a distance greater than the diameter of the satellite. Tumor stage was defined according to the sixth edition of the tumor-node-metastasis (TNM) classification of the International Union against Cancer [22]. Tumors were also classified according to the updated Barcelona Clinic Liver Cancer (BCLC) staging system, whenever relevant clinical information was available [23]. Clinicopathological features are summarized in Table 1.
Overall survival (OS) was defined as the interval between surgery and death or date of last observation. The death data were censored at the date of last follow-up for patients that were still alive. Patients were followed up until May 2010. Recurrence-free survival (RFS) was defined as the period from date of surgery until detection of recurrent tumor. RFS data were censored at time of last follow-up for patients without tumor recurrence. Ethics approval for this study was obtained from the National University Singapore-Institutional Review Board (NUS-IRB; 10-133).
Tissue microarrays and immunohistochemistry
Formalin-fixed, paraffin-embedded (FFPE) primary HCC tissue samples were obtained from the Department of Pathology, National University Hospital, Singapore. Tissue microarrays (TMAs) with triple punches of 1.5 mm in diameter (away from the center of the tumor) were constructed using an Advanced Tissue Arrayer (Chemicon International, CA) as described previously [24, 25]. Consecutive 4 μm sections were cut from each TMA and mounted on coated slides for immunohistochemical staining.
Immunohistochemistry (IHC) was performed using commercially available antibodies. The antigen retrieval and primary antibody details are summarized in Table 2. As detection system, we used peroxidase-labeled streptavidin-biotin (DAKO) with 3-3′-diaminobenzidine (DAKO, Glostrup, Germany) as chromogen according to the manufacturer’s instructions. Slides were then counterstained with hematoxylin. Appropriate positive and negative controls were run concurrently for each antibody.
Evaluation of protein expression
Immunoreactivity for each marker was assessed semi-quantitatively by evaluating the fraction of stained tumor as a percentage of the total number of tumor cells. Immunohistochemical staining was assessed by two independent pathologists (SS and BT). HIF-1α protein staining was classified as 0 for no staining, 1 for nuclear staining of less than 1 % of cells, 2 for nuclear staining of 1–10 % of cells and/or cytoplasmic staining, 3 for nuclear staining of 10–50 % of cells and/or cytoplasmic staining, and 4 for nuclear staining of more than 50 % of cells and/or strong cytoplasmic staining. Classes 0, 1, and 2 were considered as negative expression, while 3 and 4 were considered as positive expression [14]. CA-IX expression was classified as 0 for no staining, 1 for membranous staining of less than 5 % of the cells, 2 for membranous staining of 5–50 %, and 3 for membranous staining of 50 % or more of the cells [18]. Classes 0 and 1 were considered as negative, and 2 and 3 were considered as positive. Expression of β-catenin was classified as positive when more than 30 % of cancer cells showed membranous staining and otherwise as negative. When more than 10 % of cancer cells showed cytoplasmic or nuclei staining, this was classified as ectopic expression [26]. Loss of E-cadherin expression was called when E-cadherin immunoreactivity was reduced or lost in tumor cell membranes compared with adjacent nontumor epithelial cells. The Ki-67 labeling index was determined as the fraction of tumor cells with nuclear staining as a percentage of all tumor cells counted, regardless of staining intensity [27].
Statistical analysis
Statistical analysis was performed using SPSS version 15.0 for Windows (SPSS, Chicago, IL). The chi-squared test and Fisher’s exact test were used to examine associations between protein expression and clinicopathological features. Cumulative OS and RFS were calculated by the Kaplan-Meier method and analyzed by the log-rank test. Factors identified as statistically significant using Kaplan-Meier analyses were included in subsequent multivariate analyses using Cox proportional hazard regression model. The correlation significance was analyzed by Spearman and Pearson correlation analysis. A p value ≤0.05 was considered statistically significant.
Results
Clinicopathological features
Our cohort counted 142 male and 37 female patients with a mean age of 57.5 years. Of these patients, 59 (33 %) were alcoholic, 112 (62 %) had positive serology for hepatitis B virus (HBV), and 10 (6 %) were positive for hepatitis C virus (HCV) infection. Of 117 patients for which BCLC staging data were available, 2 were of stage 0 and underwent segment resection, 88 were stage A and underwent segment or lobe resection or hepatectomy with liver transplant, and 27 were stage B and underwent hepatectomy with liver transplant. Relapse of HCC occurred in 49 % of patients. Mean OS was 98.60 ± 9.12 months and mean RFS 71.64 ± 8.12 months.
Protein expression patterns
Data on expression of the proteins studied by immunohistochemistry are summarized in Table 2. Representative images of immunohistochemical staining are presented in Fig. 1. Expression of HIF-1α was positively correlated with that of β-catenin (r = 0.221; p = 0.005) and Ki-67 (r = 0.176; p = 0.025) and negatively correlated with that of E-cadherin (r = −0.186; p = 0.022). A significant positive correlation was observed between expression of CA-IX and of Ki-67 (r = 0.172; p = 0.029). Expression of E-cadherin was negatively correlated with that of β-catenin (r = −0.241; p = 0.002). HIF-1α expression was significantly more frequent in male patients (69 %, p = 0.026). β-Catenin expression was observed more frequently in HBV-positive cases (62 %; p = 0.035).
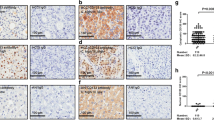
Representative images of protein marker expression by immunohistochemistry in the tissue microarray sections: a hematoxylin and eosin, b nuclear expression of β-catenin, c membranous and cytoplasmic expression of CA-IX, d nuclear and cytoplasmic expression of HIF-1α, e weak membranous expression of E-cadherin, and f nuclear expression of Ki-67 (original magnification, ×400)
Association of protein expression with OS and RFS
By univariate analysis, TNM stage, tumor size, satellitosis, and vascular invasion were confirmed as prognostic factors associated with worse OS and RFS (Table 3). High serum AFP was also associated with shorter RFS. High CA-IX expression was associated with worse OS (38 vs 102 months; p = 0.009) and RFS (24 vs 74 months; p = 0.014). Likewise, high expression of HIF-1α (112 vs 71 months; p = 0.042), β-catenin (85 vs 112 months; p = 0.042), E-cadherin (89 vs 120 months; p = 0.034), and Ki-67 (3 vs 101 months; p = 0.001) were associated with worse OS (supplementary Fig. 1). Multivariate Cox regression analysis indicated that CA-IX, HIF-1α, and E-cadherin are independent prognostic factors for OS (all p < 0.05), in addition to vascular invasion. For RFS, only vascular invasion and tumor size remained as independent prognostic factors.
Association of combined protein expression and survival
Using CA-IX and HIF-1α as markers of hypoxia and β-catenin and E cadherin as target proteins of the Wnt pathway, we developed a prognostic signature for HCC patients. To this end, we stratified the 169 patients for whom information on all four markers was available into four groups as follows: group 1 (Hypoxiahigh, only HIF-1α and CA-IX positive; n = 12), group 2 (Wnthigh, only β-catenin and E-cadherin positive; n = 48), group 3 (Hypoxiahigh and Wnthigh; n = 104), and group 4 (all markers negative; n = 5). Patients in group 3 had a mean overall survival of 61 months compared to 89 months in group 1, 126 months in group 2, and 104 months in group 4 (p = 0.033) (Table 4). The OS of the groups remained significantly different for early stage patients (p = 0.041) and for patients with small tumors (<5 cm) (p = 0.026) (Fig. 2). The differences in RFS (group 3, 47 months; group 1, 84 months; group 2, 88 months; group 4, 62 months) did not reach statistical significance (p = 0.181).
Discussion
The results of our study are in keeping with the notion that hypoxia and activation of the Wnt pathway are synergistic and might be used to determine tumor prognosis. We divided our HCC cohort into four groups: (1) hypoxiahigh, (2) Wnthigh, (3) hypoxiahigh as well as Wnthigh, and (4) no expression of any of the protein markers. The results show that group 3 (hypoxiahigh as well as Wnthigh) had significantly shorter OS than any of the other groups (Fig. 2). Even when stratified by tumor size and TNM stage, the prognostic value of our groups for OS remained robust.
Liver cancer is characterized by abnormal vasculature and hypoxic stress, making it a potential target for antiangiogenic and antihypoxic drugs. Antihypoxic drugs inhibit HIF-1 activity by either decreasing the synthesis or increasing the rate of degradation of HIF-1α. HIF-1α might also be downregulated by inducing oncolytic Reovirus infection together with YC-1, an agent developed for circulatory disorders; combined treatment with these agents has been proposed as a novel strategy to treat solid tumors constitutively expressing HIF-1α [28]. In addition to antihypoxic drugs, drugs interfering with the Wnt pathway have therapeutic potential [29]. Dickkopf1 (Dkk1) binds to a low-density protein and prevents formation of the Fzd-Wnt-LRP6 complex [30]. XAV939 inhibits the enzyme tankyrase that normally destroys the scaffold protein axin, a crucial component of the β-catenin destruction complex [31]. Our data suggests that group 3 of HCC patients (hypoxiahigh and Wnthigh) might benefit from a combination of the abovementioned chemotherapeutic agents.
In colorectal carcinoma, β-catenin signaling can be diverted by hypoxia as HIF-1α competes with T cell factor 4 (TCF-4) for direct binding to β-catenin [32]. HIF-1α can modulate Wnt/β-catenin signaling in hypoxic embryonic stem cells by enhancing β-catenin activation and expression of the downstream effectors LEF-1 and TCF-1 [33]. Recently, Lehwald et al. showed that following ischemia, mice with β-catenin-deficient hepatocytes were significantly more susceptible to liver injury [34]. Together, these findings suggest that there is a significant cross-talk between the Wnt pathway and hypoxia. It has been proposed that hypoxia triggers a functional switch in β-catenin signaling, diverting it from its usual function of cell proliferation to a completely new role in tumorigenesis by enhancing HIF-1α-mediated transcriptional response and promoting cell survival and adaptation to hypoxia. β-Catenin contributes to hypoxia-induced metastatic potential [35]. Consistent with the current concept of the interrelationship between β-catenin and HIF-1α, we found that expression of these proteins positively correlated (r = 0.221, p = 0.005).
In vitro experiments have shown that carbonic anhydrase inhibitors, such as acetazolamide, might reduce invasiveness in mouse xenograft models of renal, colorectal, and breast carcinoma [36–39]. Yu et al. analyzed expression of CA-IX in 69 HCC patients and observed that CA-IX expression has prognostic value. They furthermore suggested that inhibition of CA-IX in combination with a hexokinase II inhibitor might be therapeutically useful in patients with HCC [19]. We found expression of CA-IX to be a significant prognostic factor both by univariate and multivariate analyses. HCC patients overexpressing CA-IX had shorter OS and RFS and CA-IX was an independent prognostic factor for poor survival. This finding might have therapeutic importance in the treatment of HCC, as hypoxia can lead to resistance to chemotherapy, and in such case, a CA-IX inhibitor might enhance the effect of the used drug.
HIF-1α plays a pivotal role in mediating oxygen-dependent transcriptional responses [40]. HIF-1α can be targeted and might have therapeutic potential in treating inflammatory diseases of the liver [41]. We observed that HCC patients with high HIF-1α had shorter OS (71 vs 112 months) than patients with low HIF-1α expression. In addition to its role in angiogenesis and glycolysis, HIF-1α is also a key mediator of cell growth and proliferation, potentially by inhibiting Myc and inducing cell cycle arrest [42, 43]. However, much controversy remains around its role in apoptosis and cell proliferation. We observed a positive correlation between Ki-67 labeling index and expression of HIF-1α (r = 0.176, p = 0.025), which supports a role for HIF-1α in the regulation of cell proliferation. This finding is at variance with previous reports, which however were based upon observations in vitro.
We also observed a significant negative correlation between expression of E-cadherin and HIF-1α (r = −0.186, p = 0.022). Jing et al. reported in esophageal carcinoma that hypoxia suppresses E-cadherin expression and facilitates migration of carcinoma cells via HIF-1α activation [44]. No other studies have previously reported this association.
We conclude that expression of CA-IX is a predictor of poor prognosis in primary HCC patients. We also show that the subgroup of patients with high expression of proteins associated with hypoxia as well as Wnt pathway activation has poor prognosis. These results should be validated in an independent cohort of HCC patients to determine clinical utility of these markers, which then might be used to support decisions on therapy.
References
Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds) (2013) SEER Cancer Statistics Review, 1975–2011, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission, posted to the SEER web site, April 2014
D’Amico G, Garcia-Tsao G, Pagliaro L (2006) Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 44:217e31
Tsochatzis EA, Bosch J, Burroughs AK (2014) Liver cirrhosis. Lancet 17:1749e61
Chen XP, Qiu FZ, Wu ZD, Zhang ZW, Huang ZY, Chen YF (2006) Long term outcome of resection of large hepatocellular carcinoma. Br J Surg 93(5):600–606
Vakili K, Pomposelli JJ, Cheah YL et al (2009) Living donor liver transplantation for hepatocellular carcinoma: increased recurrence but improved survival. Liver Transpl 15:1861–1866
Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M et al (2006) The natural history of compensated cirrhosis due to hepatitis C virus: a 17-year cohort study of 214 patients. Hepatology 43:1303e10
Lim HY, Sohn I, Deng S, Lee J, Jung SH, Mao M, Xu J, Wang K, Shi S, Joh JW, Choi YL, Park CK (2013) Prediction of disease-free survival in hepatocellular carcinoma by gene expression profiling. Ann Surg Oncol 20(12):3747–3753
Pang RW, Poon RT (2007) From molecular biology to targeted therapies for hepatocellular carcinoma: the future is now. Oncology 72:30–44
Villanueva A, Newell P, Chiang DY, Friedman SL, Llovet JM (2007) Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis 27:55–76
Gwak GY, Yoon JH, Kim KM, Lee HS, Chung JW, Gores GJ (2005) Hypoxia stimulates proliferation of human hepatoma cells through the induction of hexokinase II expression. J Hepatol 42:358–64
Gort EH, Groot AJ, van der Wall E, van Diest PJ, Vooijs MA (2008) Hypoxic regulation of metastasis via hypoxia-inducible factors. Curr Mol Med 8:60–67
Barsoum IB, Smallwood CA, Siemens DR, Graham CH (2014) A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res 74(3):665–6674
Lu X, Kang Y (2010) Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clin Cancer Res 16(24):5928–5935
Xie H, Song J, Liu K et al (2008) The expression of hypoxia-inducible factor-1α in hepatitis B virus-related hepatocellular carcinoma: correlation with patients’ prognosis and hepatitis B virus X protein. Dig Dis Sci 53:3225–3233
Pastorekova S, Ratcliffe PJ, Pastorek J (2008) Molecular mechanisms of carbonic anhydrase IX-mediated pH regulation under hypoxia. BJU Int 101:8–15
Pastorekova S, Zatovicova M, Pastorek J (2008) Cancer-associated carbonic anhydrases and their inhibition. Curr Pharm Des 14:685–98
Swinson DEB, Jones JL, Richardson D et al (2003) Carbonic anhydrase IX expression, a novel surrogate marker of tumor hypoxia, is associated with a poor prognosis in Non-small-cell lung cancer. J Clin Oncol 21:473–482
Beasley NJ, Wykoff CC, Watson PH et al (2001) Carbonic anhydrase IX, an endogenous hypoxia marker, expression in head and neck squamous cell carcinoma and its relationship to hypoxia, necrosis, and microvessel density. Cancer Res 61:5262–5267
Su-jong Y, Yoon J-h, Lee J-h et al (2011) Inhibition of hypoxia-inducible carbonic anhydrase-IX enhances hexokinase II inhibitor-induced hepatocellular carcinoma cell apoptosis. Acta Pharmacol Sin 32:912–920
Lim JH, Chun YS, Park JW (2008) Hypoxia-inducible factor-1α obstructs a Wnt signaling pathway by inhibiting the hARD1-mediated activation of β-catenin. Cancer Res 68:5177–5184
Edmondson HA, Steiner PE (1954) Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 7:462–503
Sobin LH, Wittekind C (eds) (2002) UICC (International Union against Cancer) TNM classification of malignant tumors, 6th ed. Wiley, New York, pp 1–264
Llovet JM, Bru C, Bruix J (1999) Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Disv 19:329e38
Das K, Mohd Omar MF, Ong CW et al (2008) TRARESA: tissue microarray-based hospital system for biomarker validation and discovery. Pathology 40:441–449
Srivastava S, Wong KF, Ong CW et al (2012) A morpho-molecular prognostic model for hepatocellular carcinoma. Br J Cancer 107:334–39
Guo C, Liu QG, Yang W, Zhang ZL, Yao YM (2008) Relation among p130Cas, E-cadherin and B-catenin expression, clinicopathologic significance and prognosis in human hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int 7:490–496
Saarnio J, Parkkila S, Parkkila AK et al (1998) Immunohistochemical study of colorectal tumors for expression of a novel transmembrane carbonic anhydrase, MN/CA IX, with potential value as a marker of cell proliferation. Am J of Pathol 153:279–285
Cho I-R, Koh S-S, Min H-J et al (2010) Down-regulation of HIF-1α by oncolytic reovirus infection independently of VHL and p53. Cancer Gene Ther 17:365–372
Zeng G, Apte U, Cieply B, Singh S, Monga SP (2007) siRNA-mediated beta-catenin knockdown in human hepatoma cells results in decreased growth and survival. Neoplasia 9:951–959
Li Y, Lu W, King TD et al (2010) Dkk1 stabilizes Wnt co-receptor LRP6: implication for Wnt ligand-induced LRP6 down-regulation. PLoS One 5:e11014
Huang SM, Mishina YM, Liu S et al (2009) Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461:614–620
Kaidi A, Williams AC, Paraskeva C (2007) Interaction between β-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat Cell Biol 9:210–217
Mazumdar J, O’Brien WT, Johnson RS, LaManna JC, Chavez JC, Klein PS, Simon MC (2010) O2 regulates stem cells through Wnt/β-catenin signalling. Nat Cell Biol 12:1007–1013
Lehwald N, Tao G-Z, Jang KY et al (2011) Wnt-β-catenin signaling protects against hepatic ischemia and reperfusion injury in mice. Gastroenterol 141:707–718
Liu L, Zhu XD, Wang WQ, Sorkin M, Knoefel WT, Sylvester KG (2010) Activation of β-catenin by hypoxia in hepatocellular carcinoma contributes to enhanced metastatic potential and poor prognosis. Clin Cancer Res 16:2740–2750
Teicher BA, Liu SD, Liu JT, Holden SA, Herman TS (1993) A carbonic anhydrase inhibitor as a potential modulator of cancer therapies. Anticancer Res 13:1549–1556
Parkkila S, Rajaniemi H, Parkkila AK et al (2000) Carbonic anhydrase inhibitor suppresses invasion of renal cancer cells in vitro. Proc Natl Acad Sci 97:2220–2224
Zatovicova M, Jelenska L, Hulikova A et al (2010) Carbonic anhydrase IX as an anticancer therapy target: preclinical evaluation of internalizing monoclonal antibody directed to catalytic domain. Curr Pharm Des 16(29):3255–3263
Lou Y, McDonald PC, Oloumi A et al (2011) Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res 71(9):3364–3376
Wang GL, Jiang B-H, Rue EA, Semenza GL (1995) Hypoxia-inducible factor 1 is a basic-helix–loop–helix–PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci 92:5510–5514
Wilson GK, Tennant DA, McKeating JA (2014) Hypoxia inducible factors in liver disease and hepatocellular carcinoma: current understanding and future directions. J of Hepatol 61(6):1397–1406
Dai C-X, Gao Q, Qiu S-J et al (2009) Hypoxia-inducible factor-1 alpha, in association with inflammation, angiogenesis and MYC, is a critical prognostic factor in patients with HCC after surgery. BMC Cancer 9:418
Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE (2004) HIF-1α induces cell cycle arrest by functionally counteracting Myc. EMBO J 23:1949–1956
Jing SW, Wang YD, Chen LQ et al (2013) Hypoxia suppresses E-cadherin and enhances matrix metalloproteinase-2 expression favoring esophageal carcinoma migration and invasion via hypoxia inducible factor-1 alpha activation. Dis Esophagus 26(1):75–83
Acknowledgments
The authors thank Madam Cheong Sok Lian for assisting in annotation of clinicopathological information and Madam Choo Shoonian for assistance in immunohistochemistry.
Conflict of interest
The authors have declared no conflict of interest
Funding
This study is supported by institutional grant R-173-000-129-646 to MST.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure S1
Kaplan-Meier survival plots of HCC patients according to expression of (A) CA-IX, (B) HIF-1α, (C) Ki-67, (D) β-catenin, and (E) E-cadherin expression (GIF 79 kb)
Rights and permissions
About this article
Cite this article
Srivastava, S., Thakkar, B., Yeoh, K.G. et al. Expression of proteins associated with hypoxia and Wnt pathway activation is of prognostic significance in hepatocellular carcinoma. Virchows Arch 466, 541–548 (2015). https://doi.org/10.1007/s00428-015-1745-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-015-1745-4