Abstract
Chromophobe renal cell carcinoma (chRCC) is the third most common subtype of RCC, after clear cell (ccRCC) and papillary RCC. Its lower incidence and frequent exclusion from clinical trials might be why chRCC characteristics have not been extensively studied. The aim of our study was to examine tumor characteristics and long-term prognosis of chRCC compared to ccRCC. We collected 4,210 evaluable patients subjected to surgery for chRCC (n = 176) or ccRCC (n = 4,034) at five centers in Germany (University Hospitals of Hannover, Homburg/Saar, Mainz, Ulm, and Marburg) between 1990 and 2010. Patients with chRCC were significantly younger (mean, 60.1 vs. 62.1 years) and tended to be more frequently female (43.8 vs. 36.5 %). Although Fuhrman grade and median tumor diameter were not significantly different, significantly fewer patients with chRCC than with ccRCC presented with high tumor stage or metastasis at diagnosis (18.5 vs. 43.8 %). Moreover, significantly more chRCC patients were treated with partial nephrectomy (41.5 vs. 26.2 %). Accordingly, 5-year cancer-specific survival (CSS) rates were 83.2 % for chRCC against 75.8 % for ccRCC patients (p = 0.014, log rank). However, in multivariate analysis, chromophobe subtype was not confirmed as a significant positive prognostic factor for RCC (HR 0.88, 95 % CI 0.63–1.24; p = 0.48 Cox regression). This is one of the largest studies to date showing that chRCC is associated with a significantly lower risk of locally invasive tumor growth and metastatic disease than ccRCC. We conclude that the clinical behavior of chRCC is less aggressive than that of ccRCC, independent of Fuhrman grade or tumor size.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clear cell renal cell carcinoma (ccRCC) is the most common histopathological subtype of malignant kidney tumor and accounts for approximately 75–80 % of all cases. As most clinical studies and guidelines focus on patients with this subtype, those with less common tumor entities such as papillary, chromophobe, or collecting duct tumors have often been excluded [1].
Chromophobe RCC (chRCC) comprises 5–10 % of the total cases of RCC. Mean age of presentation is in the fifth decade, and it is more commonly observed in women (52 %) than in men (48 %) [2, 4, 5].
The prognostic significance of different histological subtypes has been investigated in a series of interinstitutional and collaborative national and international studies with patient populations as large as 11,618 individuals [2–4]. Despite these large case series, study results are not consistent. Some authors identified histological subtype of RCC as prognostic factor [4, 5], while others saw no survival advantage for any specific histological subtype [3, 6, 7]. In particular, differences between histological subtypes did not hold up in multivariate analyses, when tumor stage and Fuhrman grade were included as variables [3, 6, 8, 9], in spite of the established differences in gene abnormalities between the different morphological variants of RCC [10–12].
The aim of this large retrospective multicenter study was to compare incidence, tumor characteristics, and long-term prognosis of chRCC with that of ccRCC.
Methods
Patients and tumor characteristics
This retrospective study included 4,897 patients who underwent surgery because of a renal tumor between 1990 and 2010 at Hannover, Homburg, Mainz, Ulm (1995–2010), or Marburg (1990–2005) University Medical Centers. The tumor histological subtype was determined according to the 1997 UICC classification. In 176 (3.6 %) patients, a chRCC was diagnosed while in 4,034 (82.4 %) this was a ccRCC. Cases with ccRCC with sarcomatoid elements were excluded. Staging was performed according to the 2002 tumor, node, metastasis (TNM) classification. Information on patient and tumor characteristics, such as age, sex, stage, presence of regional lymph node or distant metastases, histological subtype, and Fuhrman grade, was obtained from institutional databases.
Follow-up
The duration of follow-up was calculated from date of surgery to date of death or last follow-up. Death was assessed as either cancer-related or cancer-unrelated. The primary end point of this study was cancer-specific survival (CSS). Follow-up assessment ended in August 2013.
Statistical methods
Chi-square or Fisher’s exact tests were conducted to assess differences in covariate distributions between histological subtypes. Continuous variables were reported as mean value and standard deviation (SD) or median value and interquartile ranges (IQR). Normal distribution of continuous parameters such as age and follow-up period was tested using the Kolmogorov-Smirnov test. Mann-Whitney and t tests were applied to compare continuous parameters between histological subtypes, in case of (non-)normal distribution. Kaplan-Meier estimates of survival time were calculated, and subgroups were compared by log rank test statistic. Multivariate Cox regression models were used to assess associations between survival and histological subtypes adjusted for different clinical and patient-specific covariates (i.e., age, sex, tumor grade, clinical symptoms, and metastatic status). SPSS 21.0 was used for statistical assessment. In all tests, a two-sided p < 0.05 was considered to indicate significance.
Results
Our patient population of 2,662 (63.2 %) men and 1,548 (36.8 %) women had a median/mean age of 63/62 (range, 17–93) years. The median/mean duration of follow-up was 46.0/59.8 months (IQR, 17.9–91.4) and did not differ significantly between patients with chRCC (median, 43 months) and those with ccRCC (median, 46 months; p = 0.08, Mann-Whitney U test). By the last day of data acquisition, 1,039 (24.7 %) had died from their tumor disease and 288 (6.8 %) from other causes. Detailed tumor and patients’ characteristics are summarized in Table 1.
Clinical parameters
Patients with chRCC were significantly younger than those with ccRCC (mean age, 60.1 vs. 62.1 years, p = 0.04, t test). Men comprised the majority in both groups, though their preponderance was less evident in the chRCC (56.3 %) than in the ccRCC group (63.5 %; p = 0.055, Fisher’s exact test). Partial nephrectomy was performed significantly more often for chRCC than for ccRCC (41.5 vs. 26.2 %; p < 0.001, chi-square test).
The rate of synchronous bilateral tumors was comparable (0 vs. 1.2 %, p = 0.19, chi-square test). The frequency of symptomatic RCC at diagnosis was not significantly different between the two subtypes (22.9 vs. 26.2 %, p = 0.44, chi-square test).
Tumor-specific parameters
Even though more chRCC patients were treated with a nephron sparing approach, the median/mean tumor diameter did not differ significantly between patients with chRCC (4.8/5.5 cm) and ccRCC (5.0/5.6 cm, p = 0.32, Mann-Whitney U test).
However, the chRCC group had a significantly higher number of organ-confined tumors (pT ≤ 2, N/M0; 81.5 vs. 56.2 %, p < 0.001; Fisher’s exact test). On the other hand, Fuhrman grade was similar between chRCC and ccRCC with 18.5 vs. 17.0 % G1, 62.4 vs. 66.3 % G2, and 19.1 vs. 16.7 % G3/4 (p = 0.50; chi-square test).
The frequency of lymph node metastases was not different between chRCC and ccRCC patients (4.8 vs. 8.6 %; p = 0.11, Fisher’s exact test), but synchronous distant metastases (pulmonary/visceral) at time of diagnosis were significantly less common in chRCC patients (5.3 vs. 15.0 %; p < 0.001, Fisher’s exact test).
Clinical course and oncological outcome
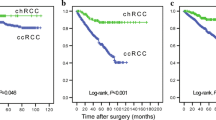
After a mean follow-up of about 5 years, the tumor-associated death rate was lower in the chRCC than in the ccRCC group (13.6 vs. 25.7 %; p < 0.001, Fisher’s exact test). The Kaplan-Meier 5-year CSS rate was 83.2 % for patients with chRCC and 75.8 % for those with ccRCC (p = 0.014, log rank, Fig. 1). In accordance, the Kaplan-Meier 5-year overall survival rate was 77.6 and 71.6 % for patients with chRCC and ccRCC, respectively (p = 0.04, log rank). However, in the group of patients with advanced disease at diagnosis (pT3–4 and/or N/M metastasis), chRCC was no longer associated with improved cancer-specific (46.2 vs. 52.9 %; p = 0.40) or overall (44.0 vs. 49.8 %; p = 0.51) 5-year survival.
Accordingly, by multivariate analysis including age, sex, tumor stage, presence of metastasis, and differentiation, chromophobe histology did not emerge as an independent factor prognostic for cancer-specific survival (HR 0.88, 95 % CI 0.63–1.24; p = 0.48, Cox regression, Table 2).
Discussion
To date, several studies have analyzed the role of histological subtype on prognosis in patients with RCC [3, 5, 9, 13, 14, 17–19]. In 1999, Ljungberg et al. [17] were the first to observe significant differences in cancer-specific survival between the three main RCC histopathological subtypes: patients with chRCC survived considerably longer than those with ccRCC. Moch et al. [13] confirmed these findings. In a large series of 2,528 patients from the Mayo Clinic, Cheville et al. [5] also reported that patients with ccRCC had a significantly poorer outcome, compared with patients with chRCC, even when the analysis was adjusted for TNM stage and nuclear grade. Accordingly, in the study published by Patard et al. [3], univariate analysis disclosed a trend towards better survival for chRCC with 5-year survival rates of 73.2 and 87.9 % for localized ccRCC and chRCC. However, multivariate analyses only identified TNM stage, grade, and clinical status but not histological subtype as independent prognostic markers. Gudbjartsson et al. [6], in a retrospective study from Iceland including 629 patients, did not find histological subtype prognostically significant, even after slide revision. Ficarra et al. [16] confirmed these findings.
In contrast, evaluating the National Cancer Institute Surveillance Epidemiology and End Results (SEER) database by multivariable analyses, Capitanio et al. [2] reported that histological subtype might independently influence CSS.
In our large multicenter study, chromophobe subtype was not independently associated with lower cancer-specific mortality. However, our calculated 5-year CSS rate of 83.2 % for chRCC patients is consistent with the excellent outcome generally reported in most earlier series, in which 5-year survival probability ranged from 80 to 100 % [3, 5, 6, 9, 14, 16]. Furthermore, in our cohort, the proportion of chRCC (3.4 %) was slightly lower than that in previously published surgical series (4 to 10 %) [5, 9, 13–15], but similar to that published by Patard et al. of 2.4 % [3].
Gudbjartsson et al. [6] concluded that improved survival of chRCC might be because they are diagnosed earlier and as a consequence present with lower tumor stage and grade. However, their case series contained only 15 patients with chRCC. In contrast, our larger cohort of 176 chRCC patients allows us to conclude that although chRCC and ccRCC do not differ significantly with respect to Fuhrman grade and median tumor diameter, chRCC is associated with a significantly lower rate of local tumor invasion and metastatic disease at diagnosis. Therefore, histological subtypes of RCC do not only differ in terms of morphology and genetics, but also in terms of clinical behavior. Specifically, ccRCC often presents at a more advanced stage and grade than chRCC [3, 5, 9, 13, 17]. In addition, the results of our study show that chRCC, in contrast to papillary RCC [4], are observed in younger patients and more often in female patients than in conventional or papillary RCC.
A striking finding in our case series, not earlier reported [3, 20–22], was that chRCC and ccRCC are not significantly different in terms of Fuhrman grade, while we would have expected chRCC to be more often of lower grade and associated with less aggressive tumor features. In contrast, chRCC frequently displays nuclear and nucleolar pleomorphism, which has led the “International Society of Urological Pathology (ISUP) Grading System for Renal Cell Carcinoma and Other Prognostic Parameters” to propose that, until further data have been accumulated, chRCC should not be graded at all [23].
The question arises why chromophobe histology goes along with less aggressive behavior than ccRCC. Identification of molecular mechanisms associated with and maybe responsible for different chRCC subtypes might help to differentiate between the majority of rather harmless chRCC, with a “pushing” rather than an “invading” growth pattern, and the few aggressive chRCC with a rather invasive growth pattern, early metastasis, and poor prognosis.
Our study has several important limitations. The limitations are first and foremost those inherent to retrospective analysis, the lack of central pathology review and the multicenter setting. Moreover, our study population included only nonsarcomatoid RCC patients. Sarcomatoid features in any RCC subtype have been associated with aggressive growth and extremely poor clinical outcome, and their exclusion might have significantly influenced the results of our study. Furthermore, our results may be biased as all five participating urology departments are tertiary referral centers with a nonrepresentative patient population for lack of a sufficient number of patients with small tumors. However, all centers have significant experience in RCC management, which increases external validity of the data in comparison with those from a single-center, single-surgeon setting.
Conclusion
This is one of the largest studies to date, showing on chRCC and ccRCC patient groups that do not differ significantly in terms of Fuhrman grade and median tumor diameter, that chRCC is associated with a significantly lower risk of locally invasive tumor growth and metastatic disease. The less aggressive behavior of chRCC is not reflected in Fuhrman grade or tumor size only.
Improved understanding of the genetic and molecular events underlying different types of RCC will contribute to more differentiated classification systems in the emergence of new RCC subtypes based upon genetic and molecular characteristics. This will open new horizons in our understanding and will improve individualized treatment of RCC, beyond known histological subtypes.
References
Schrader AJ, Varga Z, Hegele A, Pfoertner S, Olbert P, Hofmann R (2006) Second-line strategies for metastatic renal cell carcinoma: classics and novel approaches. J Cancer Res Clin Oncol 132(3):137–149
Capitanio U, Cloutier V, Zini L, Isbarn H, Jeldres C, Shariat SF, Perrotte P, Antebi E, Patard JJ, Montorsi F et al (2009) A critical assessment of the prognostic value of clear cell, papillary and chromophobe histological subtypes in renal cell carcinoma: a population-based study. BJU Int 103(11):1496–1500
Patard JJ, Leray E, Rioux-Leclercq N, Cindolo L, Ficarra V, Zisman A, De La Taille A, Tostain J, Artibani W, Abbou CC et al (2005) Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol 23(12):2763–2771
Steffens S, Janssen M, Roos FC, Becker F, Schumacher S, Seidel C, Wegener G, Thuroff JW, Hofmann R, Stockle M et al (2012) Incidence and long-term prognosis of papillary compared to clear cell renal cell carcinoma—a multicentre study. Eur J Cancer 48(15):2347–2352
Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML (2003) Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol 27(5):612–624
Gudbjartsson T, Hardarson S, Petursdottir V, Thoroddsen A, Magnusson J, Einarsson GV (2005) Histological subtyping and nuclear grading of renal cell carcinoma and their implications for survival: a retrospective nation-wide study of 629 patients. Eur Urol 48(4):593–600
Kim H, Cho NH, Kim DS, Kwon YM, Kim EK, Rha SH, Park YW, Shim JW, Lee SS, Lee SN et al (2004) Renal cell carcinoma in South Korea: a multicenter study. Hum Pathol 35(12):1556–1563
Margulis V, Tamboli P, Matin SF, Swanson DA, Wood CG (2008) Analysis of clinicopathologic predictors of oncologic outcome provides insight into the natural history of surgically managed papillary renal cell carcinoma. Cancer 112(7):1480–1488
Amin MB, Amin MB, Tamboli P, Javidan J, Stricker H, de-Peralta Venturina M, Deshpande A, Menon M (2002) Prognostic impact of histologic subtyping of adult renal epithelial neoplasms: an experience of 405 cases. Am J Surg Pathol 26(3):281–291
Linehan WM, Pinto PA, Srinivasan R, Merino M, Choyke P, Choyke L, Coleman J, Toro J, Glenn G, Vocke C et al (2007) Identification of the genes for kidney cancer: opportunity for disease-specific targeted therapeutics. Clin Cancer Res 13(2 Pt 2):671s–679s
Thoenes W, Storkel S, Rumpelt HJ, Moll R, Baum HP, Werner S (1988) Chromophobe cell renal carcinoma and its variants—a report on 32 cases. J Pathol 155(4):277–287
Nagashima Y (2000) Chromophobe renal cell carcinoma: clinical, pathological and molecular biological aspects. Pathol Int 50(11):872–878
Moch H, Gasser T, Amin MB, Torhorst J, Sauter G, Mihatsch MJ (2000) Prognostic utility of the recently recommended histologic classification and revised TNM staging system of renal cell carcinoma: a Swiss experience with 588 tumors. Cancer 89(3):604–614
Beck SD, Patel MI, Snyder ME, Kattan MW, Motzer RJ, Reuter VE, Russo P (2004) Effect of papillary and chromophobe cell type on disease-free survival after nephrectomy for renal cell carcinoma. Ann Surg Oncol 11(1):71–77
Crotty TB, Farrow GM, Lieber MM (1995) Chromophobe cell renal carcinoma: clinicopathological features of 50 cases. J Urol 154(3):964–967
Ficarra V, Martignoni G, Galfano A, Novara G, Gobbo S, Brunelli M, Pea M, Zattoni F, Artibani W (2006) Prognostic role of the histologic subtypes of renal cell carcinoma after slide revision. Eur Urol 50(4):786–793, discussion 793–784
Ljungberg B, Alamdari FI, Stenling R, Roos G (1999) Prognostic significance of the Heidelberg classification of renal cell carcinoma. Eur Urol 36(6):565–569
Levy DA, Slaton JW, Swanson DA, Dinney CP (1998) Stage specific guidelines for surveillance after radical nephrectomy for local renal cell carcinoma. J Urol 159(4):1163–1167
Amin MB, Corless CL, Renshaw AA, Tickoo SK, Kubus J, Schultz DS (1997) Papillary (chromophil) renal cell carcinoma: histomorphologic characteristics and evaluation of conventional pathologic prognostic parameters in 62 cases. Am J Surg Pathol 21(6):621–635
Delahunt B, Sika-Paotonu D, Bethwaite PB, William Jordan T, Magi-Galluzzi C, Zhou M, Samaratunga H, Srigley JR (2007) Fuhrman grading is not appropriate for chromophobe renal cell carcinoma. Am J Surg Pathol 31:957–960
Paner GP, Amin MB, Alvarado-Cabrero I, Young AN, Stricker HJ, Moch H, Lyles RH (2010) A novel tumor grading scheme for chromophobe renal cell carcinoma: prognostic utility and comparison with Fuhrman nuclear grade. Am J Surg Pathol 34:1233–1240
Przybycin CG, Cronin AM, Darvishian F, Gopalan A, Al-Ahmadie HA, Fine SW, Chen YB, Bernstein M, Russo P, Reuter VE, Tickoo SK (2011) Chromophobe renal cell carcinoma: a clinicopathologic study of 203 tumors in 200 patients with primary resection at a single institution. Am J Surg Pathol 35:962–970
Delahunt B, Cheville JC, Martignoni G, Humphrey PA, Magi-Galluzzi C, McKenney J, Egevad L, Algaba F, Moch H, Grignon DJ, Montironi R, Srigley JR (2013) The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol 37:1490–1504
Conflict of interest
None.
Source of funding
None.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Sandra Steffens, Frederik C. Roos, and Martin Janssen contributed equally to this work.
Rights and permissions
About this article
Cite this article
Steffens, S., Roos, F.C., Janssen, M. et al. Clinical behavior of chromophobe renal cell carcinoma is less aggressive than that of clear cell renal cell carcinoma, independent of Fuhrman grade or tumor size. Virchows Arch 465, 439–444 (2014). https://doi.org/10.1007/s00428-014-1648-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-014-1648-9