Abstract
Colorectal cancer (CRC) can be divided into non-mucinous and mucinous subtypes, of which the latter portends to have a worse clinical prognosis. A previous study suggested a putative link between SOX2 expression observed selectively in mucinous CRC and the induction of the gastric mucin MUC5AC. In this study, we re-evaluated the expression behavior of SOX2, MUC5AC, and CDX2 in both types of CRC. We performed immunohistochemical analysis on 90 cases of non-mucinous CRCs, 57 cases of mucinous CRCs, and 15 case-matched normal intestinal mucosa. In contrast to the previously suggested link between SOX2 and mucinous CRC, we observe aberrant expression of SOX2 at equal levels in both subtypes. Fluorescence in situ hybridization (FISH) analysis shows that expression is not attributed to genomic amplification. While SOX2 and CDX2 are normally expressed in a reciprocal manner, SOX2-positive tumor cells co-express CDX2. Furthermore, we show that MUC5AC is expressed independently of SOX2. In conclusion, we show that aberrant SOX2 expression is specifically linked neither to mucinous CRCs nor to the induction of MUC5AC, in contrast to previous suggestions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) affects over 400,000 people annually in Europe, resulting in approximately 220,000 deaths every year. CRC accounts for the most frequent malignancy within the European Union and is the second most common cause of cancer-related mortality. CRC has been classified into different subtypes according to criteria based on their histological appearance and mutational status [1]. Proper classification of CRC is becoming increasingly important in the clinic, as it will more often determine prognostic outcome and choice of therapeutic intervention.
Approximately 75–90 % of the CRC are non-mucinous, while the remaining 10–25 % are mucinous or signet ring cell carcinomas [2, 3]. Mucinous CRC is defined as a tumor with more than 50 % mucinous differentiation on histological examination, according to WHO criteria. These tumors are more common in the proximal colon and more often present with a flat appearance during colonoscopy, making their early identification more difficult. As a result, they present on average with a higher stage of tumor progression at first diagnosis. In addition to its delayed detection, mucinous differentiation by itself is associated with a modest increase in mortality compared with its non-mucinous counterpart, even when corrected for stage [3]. Elevated levels of mucin have also been associated with worse prognosis for various other tumor types [4–6]. Furthermore, in comparison to their non-mucinous counterparts, mucinous CRC more often show ectopic expression of MUC5AC, a mucin whose expression is normally restricted to the upper gastrointestinal tract [2, 7–9].
The etiology of this subset of cancers and the mechanism underlying the mucinous differentiation are still poorly understood. Previously, Park and co-workers reported an aberrant expression of the transcription factor SOX2 specifically in a subset of CRC with mucinous differentiation [9]. They also reported concordant expression of SOX2 with MUC5AC in colorectal cancers or cell lines thereof and the activation of a MUC5AC reporter construct by SOX2. In support of their results, others have shown that overexpression of SOX2 in COS-7 cells induced the mRNA expression of endogenous MUC5AC [10], indicating that the MUC5AC expression observed in mucinous CRC may indeed result from the ectopic SOX2 expression. The SOX2 gene encodes for a HMG-box containing transcription factor with a potent role in determining cell fate. In recent years, it has gained substantial attention as one of the factors required to obtain induced pluripotent stem (iPS) cells from adult somatic cells [11]. In the adult gastrointestinal tract, SOX2 expression is restricted to the gastric and esophageal epithelium, whereas it is completely excluded from the intestine [12]. Additionally, we and others have shown that during embryonic development, a strict balance between SOX2 and CDX2, which is a key transcription factor in determining intestinal identity, is essential for proper development of the intestinal tract [13, 14].
Recently, ectopic expression of SOX2 has been reported for a number of cancer types [15–19]. In these tumor types, aberrant SOX2 expression was associated with worse prognosis. As such, the ectopic SOX2 expression observed specifically in mucinous CRC may likewise affect their progression to malignancy. In this study, we re-evaluate the expression patterns of SOX2, CDX2, and MUC5AC in both mucinous and non-mucinous CRC. We show that SOX2 is not specifically expressed by mucinous tumors and is not correlated with MUC5AC expression.
Materials and methods
Paraffin-embedded CRC samples
Formalin-fixed, paraffin-embedded tissue blocks were obtained from the Department of Pathology, Erasmus Medical Centre, Rotterdam. We selected 45 mucinous colorectal cancers from a prospective population-based study conducted between May 2007 and September 2009 [20]. All these samples have been analyzed for the presence of a defect in mismatch repair by performing microsatellite instability (MSI) analysis and immunohistochemistry. An additional 12 mucinous samples were obtained from the pathology archive, resulting in a total of 57 mucinous colorectal cancers. MSI status was available for 53 tumors of which 11 (21 %) were scored as MSI-High. Mucinous CRC was defined as a tumor with more than 50 % mucinous differentiation on histological examination, according to the WHO 2010 criteria. For comparison, we also obtained 90 non-mucinous cancers and 15 control samples containing healthy intestinal tissue. For 82 non-mucinous cancers, the MSI status was available. MSI-High was observed in six (7 %) tumors, whereas an additional four (5 %) were scored as MSI-Low. Permission of the Medical Ethical Committee Erasmus MC was obtained: no. 193.948/2000/159. All H&E and immunohistochemically stained sections were evaluated by a pathologist (KB).
Immunohistochemistry
Sequential 5-μm paraffin sections were used for hematoxylin and eosin staining and immunohistochemistry. For immunohistochemistry, sections were deparaffinized and rehydrated, followed by antigen retrieval with microwave treatment in 10 mM Tris-HCl pH 8.0 and 1 mM EGTA. Sections were blocked with 5 % non-fat dry milk in phosphate-buffered saline (PBS) for 10 min and incubated with primary antibody diluted in 5 % non-fat dry milk in PBS overnight at 4 °C. The following antibodies were used: 1:500 SOX2 (immune systems, goat polyclonal), 1:200 MUC5AC (Abcam, mouse monoclonal 45M1), and 1:20 CDX2 (BioGenex, mouse monoclonal CDX2-88). Secondary antibodies against the correct IgG species were conjugated with peroxidase (Dako) using StreptABC complex/HRP (Dako) or HRP-DAB colorimetric detection. For all antibodies, appropriate negative and positive control tissues were included to confirm the specificity of our immunohistochemistry (IHC) protocol [14, 21, 22]. The intensity of staining was scored as 0, 1, 2, or 3 correlating to no, weak, moderate, or strong staining. Immunohistochemistry for SOX2 and CDX2 was scored as positive when at least 5 % of the tumor cells showed a clear nuclear staining, i.e., an intensity score of 2 or 3. MUC5AC was scored as positive when at least 5 % of the cells showed high intensity (2 or 3) cytoplasmic staining.
In situ hybridization assay for SOX2 amplification
Fluorescence in situ hybridization (FISH) was performed with a BAC probe (RP11-43F17) mapping to the SOX2 locus on chromosome 3q26.33 and a control probe mapping to the centromeric region of chromosome 12. Briefly, 5-μm paraffin sections were deparaffinized, pretreated in 0.01 M sodium citrate solution under high pressure in a pressure cooker, next with pepsin (4,000 U) at 37 °C, followed by washing and dehydration. Probes were labeled by nick-translation according to standard protocol, either with biotin-16-dUTP (SOX2) or digoxigenin-11-dUTP (chr. 12 centromere), and applied in a 10-μl hybridization mixture to the tissue slides. Probes and target were simultaneously denatured by placing the slides for 10 min at 80 °C. After overnight hybridization at 37 °C, slides were stringently washed. Hybrids were detected by FITC-conjugated sheep-anti-digoxigenin and CYE3-conjugated avidin. Results were studied with a LSM700 Zeiss microscope.
Results
SOX2 is equally expressed in mucinous and non-mucinous CRC
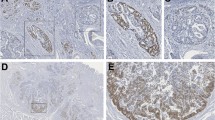
Since it was previously reported that SOX2 was specifically expressed in mucinous colorectal cancers [9], we performed SOX2 immunohistochemistry on 90 non-mucinous CRC, 57 mucinous CRC, and 15 samples of normal mucosa. Similar frequencies of nuclear SOX2 expression were detected in both subtypes of colorectal cancer, i.e., 18 of the 90 (20 %) non-mucinous and 12 of the 57 (21 %) mucinous CRC. Representative cases of both SOX2-negative and positive tumors are shown in Fig. 1. SOX2 positivity did not correlate with defects in mismatch repair, i.e., 24 out of 114 (21 %) microsatellite stable and 4 out of 21 (19 %) MSI-High/MSI-Low tumors were SOX2 positive.
SOX2 expression is observed predominantly in non-mucinous CRC. Immunohistochemistry for SOX2 in non-mucinous and mucinous CRC. Nuclear SOX2 expression was present in 18/90 non-mucinous and in 12/57 mucinous CRC. a Example of a SOX2-positive non-mucinous CRC. In the lower left corner, an enteric neural plexus is present serving as internal positive control for SOX2 staining. b Example of a SOX2-positive mucinous CRC. c Representative figure of a SOX2-negative mucinous CRC. In all cases, SOX2 IHC was only scored as positive when a clear nuclear staining was observed in at least 5 % of cells. Faint cytoplasmic staining was observed in several samples and was considered as unspecific background staining. Scale bars 200 μm
SOX2 was generally observed in patches of the tumor, next to SOX2-negative areas. Morphologically, the ectopically SOX2-expressing cells were indistinguishable from the SOX2-negative cells of the same tumor. As expected, SOX2 was absent in normal intestinal epithelium (data not shown). As an internal positive control for SOX2 expression, we used the submucosal and myenteric plexuses present in the intestine, which are known to express SOX2 (Fig. 1a, lower left corner) [23].
Increased SOX2 expression is not linked to genomic amplification of the SOX2 locus
Previously, enhanced SOX2 expression has been attributed to genomic amplification of the 3q26.33 locus for various tumor types [16, 19, 24–26]. However, SOX2 amplification appears to be confined to squamous cell carcinomas of various tissues, whereas it is rare/absent in adenocarcinomas [24]. To determine if genomic amplification possibly underlies the enhanced SOX2 expression that we observed in a subset of CRC, we performed fluorescence in situ hybridization on six samples that showed high levels of nuclear SOX2 expression. In all cases, we observed equal numbers of signals for the SOX2 and control probes, indicating that no amplification is present in the SOX2-expressing cells (data not shown). This is in line with a previous large genome-scale analysis of 257 CRC in which no amplification of the SOX2 locus was reported using SNP arrays to detect somatic copy-number alterations [27].
CDX2 is co-expressed in the SOX2-expressing CRC cells
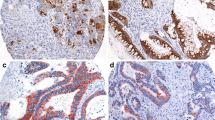
In the mature gastrointestinal tract, SOX2 is normally exclusively expressed in the rostral part of the digestive tract, i.e., in the esophagus and stomach epithelium, whereas it is completely excluded from the CDX2-positive intestine [12]. Since we detected ectopic SOX2 expression in intestinal tumors, we wondered whether there was simultaneous loss of CDX2, which would be suggestive of loss of intestinal identity. All samples, including the SOX2-positive patches, stained uniformly for CDX2 (Fig. 2), indicating that intestinal identity was retained. Thus, in contrast to the mutual exclusive expression pattern of SOX2 and CDX2 in the normal gastrointestinal tract, we observed co-expression of both proteins in a subset of CRC.
Co-expression of SOX2 and CDX2 in CRC. Immunohistochemistry for SOX2 (a, c) and CDX2 (b, d) in two examples of non-mucinous CRC. The upper tumor shows the reciprocal expression of SOX2 and CDX2, typically observed in the normal intestinal epithelium. Interestingly, SOX2 and CDX2 are clearly co-expressed in tumors with SOX2-positive patches. Scale bars a, b 200 μm; c, d 50 μm
MUC5AC is expressed independently from SOX2
Since it was previously suggested that the expression of SOX2 directly correlated with MUC5AC [10, 9], we performed immunohistochemistry for this gastric foveolar mucin on 10 mucinous and 22 non-mucinous colorectal cancers. Whereas MUC5AC expression was absent in the mucosa of normal intestinal tissue well separated from the tumor, specific staining was observed in both mucinous (Fig. 3a) and non-mucinous CRC (Fig. 3c). MUC5AC was detectable in 9 out of 10 (90 %) mucinous CRC, whereas only one of them was SOX2 positive. Likewise, 6 out of 22 (27 %) non-mucinous cancers expressed MUC5AC of which only three were also positive for SOX2. Moreover, MUC5AC was not exclusively expressed at sites with SOX2 expression, as regions within the tumors displayed both cells that were only positive for MUC5AC and cells that co-expressed SOX2 and MUC5AC (Fig. 3c, d; asterisk and arrowhead). Furthermore, expression of MUC5AC was also detected in some areas of morphologically unaffected mucosa adjacent to tumor tissue (Fig. 3e) rather than in the actual carcinoma. These sites did not display nuclear SOX2 expression (Fig. 3f). Thus, in our set, we observed numerous examples of cells aberrantly expressing MUC5AC independent of SOX2.
Aberrant expression of MUC5AC is independent of SOX2 expression. Immunohistochemistry for MUC5AC (left) and SOX2 (right) on CRC. a, b Example of a mucinous tumor showing positive MUC5AC staining despite the complete absence of nuclear SOX2. c, d Example of a non-mucinous tumor, partially showing co-expression of MUC5AC and SOX2 (asterisk) and a MUC5AC-positive section devoid of SOX2 (arrowhead). d also represents a clear example of the SOX2 tumor patches that we observe next to SOX2-negative areas. e, f Ectopic MUC5AC expression was also observed in histologically normal SOX2-negative epithelium (e; arrowhead) adjacent to non-mucinous tumor tissue (e; asterisk). Scale bars a–f 200 μm
Discussion
Colorectal cancer (CRC) can be subdivided into mucinous and non-mucinous CRC. A previous study suggested a putative link between SOX2 expression observed selectively in the mucinous subtype [9], which portends to have a worse clinical outcome [3]. In this study, we re-evaluated the expression behavior of SOX2, CDX2, and MUC5AC in both mucinous and non-mucinous CRC.
We observe ectopic SOX2 expression in approximately one fifth of the colorectal cancers. Ectopic expression appears not to be caused by genomic amplification of the SOX2 locus as determined by FISH analysis. This is in line with a recent observation by Maier et al. who showed that SOX2 amplification is confined to squamous cell carcinomas of various tissues, whereas it is rare/absent in adenocarcinomas [24]. It also fits with a large genome-scale analysis of 257 colorectal cancers in which no amplification of the SOX2 locus was reported using SNP arrays [27], suggesting that other mechanisms are at play to aberrantly express SOX2.
In stark contrast to the results of Park et al., we did not observe any correlation with mucinous differentiation [9]. Both subtypes of colorectal cancer showed SOX2 positivity in approximately 20 % of the lesions in our study, whereas this was the case in 74/90 (82 %) of the mucinous(-related) and 2/28 (7 %) of the non-mucinous lesions in the study of Park and co-workers. At present, we do not have a solid explanation for these different observations, but it may reside in the use of different antibodies (goat vs rabbit polyclonal) or differences in scoring of SOX2-positive cells. Given that SOX2 is a nuclear transcription factor, we only considered a distinct nuclear staining in at least 5 % of the cells as positive, whereas it is not clearly stated in the study of Park et al. whether cytoplasmic-only positive cases were excluded from counting. The latter is of relevance as in several of our samples we observed a faint but distinct cytoplasmic background staining in all cells present on the section, especially in the mucus-rich areas (see Fig. 3b as example), which we considered to be unspecific staining. In further support that SOX2 expression is not unique to mucinous CRC, others have also reported SOX2 expression in CRC without specifically linking it to mucinous differentiation [28–31].
In the normal gastrointestinal tract, SOX2 and CDX2 are expressed in a mutually exclusive manner, i.e., CDX2 marks all the intestinal epithelial cells, whereas SOX2 is expressed in the esophagus and stomach. Here, we report that the SOX2-positive patches in colorectal cancers retain normal expression of CDX2, meaning that both proteins are co-expressed in the same cells. Previously, we observed patchy co-expression of both proteins within gastric mucosa present in a subset of intestinal Meckel’s diverticula [21]. We also showed that induced co-expression of SOX2 and CDX2 in the developing embryonic mouse intestine resulted in the loss of CDX2 binding to its target sequences, leading to loss of intestinal identity and the acquisition of a gastric-like phenotype [14]. As such, the aberrant SOX2 expression could likewise affect the differentiation direction of the colorectal cancer cells. However, histological evaluation of the SOX2-positive cells did not provide any indication of differences in the cellular phenotype of SOX2-positive and negative cells, as both showed the typical dysplastic features of colorectal cancer.
Previously, a second link between SOX2 and the acquisition of gastric features was made by showing within colorectal cancers concordant expression of SOX2 with the gastric foveolar mucin MUC5AC [9]. In support of this, SOX2 was also shown to activate MUC5AC reporter constructs and to increase MUC5AC expression in COS-7 cells [10, 9]. MUC5AC expression is however observed in a large proportion of colorectal cancers, far exceeding the fraction of SOX2-positive tumors [7, 8]. Also in our case, we observed between 27 and 90 % of MUC5AC-positive tumors. Moreover, in our sample set, we observed numerous examples of cells aberrantly expressing MUC5AC independent of SOX2, indicating that although SOX2 may contribute to MUC5AC expression, it is not absolutely required. Taken together, our data do not support a role for aberrant SOX2 in the induction of gastric features within colorectal cancer.
In conclusion, we show that SOX2 is not specifically expressed by mucinous CRCs and does not correlate with MUC5AC expression, as was previously suggested. Moreover, we show that SOX2 coincides in all cases with CDX2, suggesting that intestinal identity is not lost in the SOX2-expressing cells.
References
Jass JR (2007) Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology 50(1):113–130
Byrd JC, Bresalier RS (2004) Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev 23(1–2):77–99
Verhulst J, Ferdinande L, Demetter P, Ceelen W (2012) Mucinous subtype as prognostic factor in colorectal cancer: a systematic review and meta-analysis. J Clin Pathol 65(5):381–388
Moniaux N, Andrianifahanana M, Brand RE, Batra SK (2004) Multiple roles of mucins in pancreatic cancer, a lethal and challenging malignancy. Br J Cancer 91(9):1633–1638
Mukhopadhyay P, Chakraborty S, Ponnusamy MP, Lakshmanan I, Jain M, Batra SK (2011) Mucins in the pathogenesis of breast cancer: implications in diagnosis, prognosis and therapy. Biochim Biophys Acta 1815(2):224–240
Kufe DW (2009) Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer 9(12):874–885
Biemer-Huttmann AE, Walsh MD, McGuckin MA, Ajioka Y, Watanabe H, Leggett BA, Jass JR (1999) Immunohistochemical staining patterns of MUC1, MUC2, MUC4, and MUC5AC mucins in hyperplastic polyps, serrated adenomas, and traditional adenomas of the colorectum. J Histochem Cytochem 47(8):1039–1048
Biemer-Huttmann AE, Walsh MD, McGuckin MA, Simms LA, Young J, Leggett BA, Jass JR (2000) Mucin core protein expression in colorectal cancers with high levels of microsatellite instability indicates a novel pathway of morphogenesis. Clin Cancer Res 6(5):1909–1916
Park ET, Gum JR, Kakar S, Kwon SW, Deng G, Kim YS (2008) Aberrant expression of SOX2 upregulates MUC5AC gastric foveolar mucin in mucinous cancers of the colorectum and related lesions. Int J Cancer 122(6):1253–1260
Li XL, Eishi Y, Bai YQ, Sakai H, Akiyama Y, Tani M, Takizawa T, Koike M, Yuasa Y (2004) Expression of the SRY-related HMG box protein SOX2 in human gastric carcinoma. Int J Oncol 24(2):257–263
Jaenisch R, Young R (2008) Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell 132(4):567–582
Sherwood RI, Chen TY, Melton DA (2009) Transcriptional dynamics of endodermal organ formation. Dev Dyn 238(1):29–42
Gao N, White P, Kaestner KH (2009) Establishment of intestinal identity and epithelial-mesenchymal signaling by Cdx2. Dev Cell 16(4):588–599
Raghoebir L, Bakker ER, Mills JC, Swagemakers S, Kempen MB, Munck AB, Driegen S, Meijer D, Grosveld F, Tibboel D, Smits R, Rottier RJ (2012) SOX2 redirects the developmental fate of the intestinal epithelium toward a premature gastric phenotype. J Mol Cell Biol 4(6):377–385
Sanada Y, Yoshida K, Ohara M, Oeda M, Konishi K, Tsutani Y (2006) Histopathologic evaluation of stepwise progression of pancreatic carcinoma with immunohistochemical analysis of gastric epithelial transcription factor SOX2: comparison of expression patterns between invasive components and cancerous or nonneoplastic intraductal components. Pancreas 32(2):164–170
Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, Kim SY, Wardwell L, Tamayo P, Gat-Viks I, Ramos AH, Woo MS et al (2009) SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet 41(11):1238–1242
Leis O, Eguiara A, Lopez-Arribillaga E, Alberdi MJ, Hernandez-Garcia S, Elorriaga K, Pandiella A, Rezola R, Martin AG (2012) Sox2 expression in breast tumours and activation in breast cancer stem cells. Oncogene 31(11):1354–1365
Basu-Roy U, Seo E, Ramanathapuram L, Rapp TB, Perry JA, Orkin SH, Mansukhani A, Basilico C (2012) Sox2 maintains self renewal of tumor-initiating cells in osteosarcomas. Oncogene 31(18):2270–2282
Alonso MM, Diez-Valle R, Manterola L, Rubio A, Liu D, Cortes-Santiago N, Urquiza L, Jauregi P, Lopez de Munain A, Sampron N, Aramburu A, Tejada-Solis S et al (2011) Genetic and epigenetic modifications of Sox2 contribute to the invasive phenotype of malignant gliomas. PLoS ONE 6(11):e26740
van Lier MG, Leenen CH, Wagner A, Ramsoekh D, Dubbink HJ, van den Ouweland AM, Westenend PJ, de Graaf EJ, Wolters LM, Vrijland WW, Kuipers EJ, van Leerdam ME et al (2012) Yield of routine molecular analyses in colorectal cancer patients ≤70 years to detect underlying Lynch syndrome. J Pathol 226(5):764–774
Raghoebir L, Biermann K, Buscop-van Kempen M, Wijnen RM, Tibboel D, Smits R, Rottier RJ (2013) Disturbed balance between SOX2 and CDX2 in human vitelline duct anomalies and intestinal duplications. Virchows Arch 462(5):515–522
Ochieng JK, Schilders K, Kool H, Boerema-de Munck A, Buscop-van Kempen M, Gontan C, Smits R, Grosveld FG, Wijnen RM, Tibboel D, Rottier RJ (2014) Sox2 regulates the emergence of lung basal cells by directly activating the transcription of Trp63. Am J Respir Cell Mol Biol
Arnold K, Sarkar A, Yram MA, Polo JM, Bronson R, Sengupta S, Seandel M, Geijsen N, Hochedlinger K (2011) Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell 9(4):317–329
Maier S, Wilbertz T, Braun M, Scheble V, Reischl M, Mikut R, Menon R, Nikolov P, Petersen K, Beschorner C, Moch H, Kakies C et al (2011) SOX2 amplification is a common event in squamous cell carcinomas of different organ sites. Hum Pathol 42(8):1078–1088
Rudin CM, Durinck S, Stawiski EW, Poirier JT, Modrusan Z, Shames DS, Bergbower EA, Guan Y, Shin J, Guillory J, Rivers CS, Foo CK et al (2012) Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet 44(10):1111–1116
Pham DL, Scheble V, Bareiss P, Fischer A, Beschorner C, Adam A, Bachmann C, Neubauer H, Boesmueller H, Kanz L, Wallwiener D, Fend F et al (2013) SOX2 expression and prognostic significance in ovarian carcinoma. Int J Gynecol Pathol 32(4):358–367
Comprehensive molecular characterization of human colon and rectal cancer (2012). Nature 487 (7407):330–337
Neumann J, Bahr F, Horst D, Kriegl L, Engel J, Luque RM, Gerhard M, Kirchner T, Jung A (2011) SOX2 expression correlates with lymph-node metastases and distant spread in right-sided colon cancer. BMC Cancer 11:518
Ong CW, Kim LG, Kong HH, Low LY, Iacopetta B, Soong R, Salto-Tellez M (2010) CD133 expression predicts for non-response to chemotherapy in colorectal cancer. Mod Pathol 23(3):450–457
Saigusa S, Tanaka K, Toiyama Y, Yokoe T, Okugawa Y, Ioue Y, Miki C, Kusunoki M (2009) Correlation of CD133, OCT4, and SOX2 in rectal cancer and their association with distant recurrence after chemoradiotherapy. Ann Surg Oncol 16(12):3488–3498
Han X, Fang X, Lou X, Hua D, Ding W, Foltz G, Hood L, Yuan Y, Lin B (2012) Silencing SOX2 induced mesenchymal-epithelial transition and its expression predicts liver and lymph node metastasis of CRC patients. PLoS ONE 7(8):e41335
Acknowledgments
This work was supported by an Erasmus MC grant (LR).
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ron Smits and Robbert J. Rottier contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Raghoebir, L., Biermann, K., Kempen, M.Bv. et al. Aberrant SOX2 expression in colorectal cancers does not correlate with mucinous differentiation and gastric mucin MUC5AC expression. Virchows Arch 465, 395–400 (2014). https://doi.org/10.1007/s00428-014-1638-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-014-1638-y