Abstract
Ovarian clear cell carcinoma (OCCC) is a subtype of epithelial ovarian cancer with characteristic biological features and aggressive clinical behavior. OCCCs show a pattern of gene mutations different from other type I ovarian malignancies, notably a higher frequency of PIK3CA mutations. In low grade serous ovarian cancer, KRAS and BRAF mutations are frequent, but little data are available on the mutational status of these genes in OCCCs. To clarify this issue, we designed a clinicopathological study with the aim to establish the incidence of KRAS, NRAS, and BRAF hot spot mutations in OCCC. Between December 2006 and June 2012, 22 patients with a proven diagnosis of OCCC were admitted to our Institutions. In all cases, final diagnosis was established according to FIGO and WHO criteria. All women received complete surgical staging. The PyroMark Q24 system (Qiagen GmbH, Hilden, Germany) was used for pyrosequencing analysis of KRAS, NRAS, and BRAF hot spot regions on 2.5-μm sections of formalin-fixed paraffin-embedded tissue from primary OCCC. Pyrosequencing analysis of KRAS, NRAS, and BRAF hot spot regions revealed the presence of mutations only at codon 12 in exon 2 of KRAS in 3 of 22 (14 %) cases. We found no mutations in the hot spot regions of NRAF (exons 2, 3, 4) or BRAF (exon 15). The median age of women with a KRAS mutated OCCC was 74 years. These OCCC were unilateral FIGO stage IA lesions in two cases associated with foci of endometriosis. We conclude that in 14 % of OCCCs, a KRAS mutation occurs in codon 2 exon 2. NRAS and BRAF mutations were not found.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epithelial ovarian cancer (EOC) accounts for approximately 90 % of primary malignant ovarian tumors. It is a heterogeneous tumor category, classified in serous, mucinous, endometrioid, and clear cell histotypes, each with specific molecular characteristics and clinical outcome [1].
This classical histopathological classification of EOC has been recently revised, and currently, two broad categories are distinguished. Type I EOC (including low-grade serous, mucinous, endometrioid, and clear cell carcinoma) are confined to the ovary at diagnosis, grow slowly and are chemoresistant. Type II EOC (high-grade serous, undifferentiated carcinomas, carcinosarcomas) typically show poor histological differentiation, early extra-ovarian spread and respond well to platinum-based chemotherapy [2, 3]. Type I tumors often harbor somatic mutations of genes encoding protein kinases including KRAS, BRAF, PI3KCA, and ERRB2, along with other signaling molecules, such as CTNNB1 and PTEN. In contrast, Type II tumors generally lack these mutations but are characterized by chromosomal instability and high frequency of TP53 mutations [4–8].
In this complex scenario, ovarian clear cell carcinoma (OCCC) is considered an entity distinct from the above cited type I EOCs, due to its specific biological behavior. Recent findings have demonstrated that OCCCs typically show a higher frequency of PIK3CA mutations [9], which suggests that aberrations in telomere biology may play an important role in the pathogenesis of OCCC [10]. On the other hand, KRAS and BRAF mutations have been recognized as a frequent event in low grade serous ovarian cancer, but only few data are currently available on the mutational status of these genes in OCCCs. For these reasons, we designed a clinicopathological study with the aim to evaluate the incidence of KRAS, NRAS, and BRAF hot spot mutations in a consecutive single Institution series of patients with OCCC.
Material and methods
Patients
Between December 2006 and June 2012, 22 patients with a proven diagnosis of OCCC were admitted to the Gynecologic Oncology Unit of the Catholic University of the Sacred Heart. In all cases, histological diagnosis was established at the Department of Pathology of our Institution after an extensive and careful evaluation of tumor specimens by an experienced gynecological pathologist. In all cases, a final diagnosis of primary clear cell carcinoma of the ovary was made according to FIGO and WHO criteria [11]. All women received complete surgical staging, including the following: peritoneal washing, total hysterectomy, bilateral salpingo-oophorectomy, total omentectomy, peritoneal biopses, and pelvic/para-aortic lymphadenectomy up to the renal vessels. Patients gave written informed consent for clinical data to be collected and analyzed for research purposes. The study was approved by the local institutional review board.
Molecular analysis
The PyroMark Q24 system (Qiagen GmbH, Hilden, Germany) was used for pyrosequencing analysis of KRAS, NRAS, and BRAF hot spot regions on 2.5-μm sections of formalin-fixed paraffin-embedded tissue from primary OCCC.
In brief, all the slides were reviewed by a pathologist to evaluate the percentage of cancer cells (>50 % tumor cells was the base line requirement) before performing manual dissection of the tumor to avoid areas with tumor necrosis or few neoplastic cells. From these samples, DNA was extracted using QIAamp MinElute spin columns (Qiagen, GmbH, Hilden, Germany), according to the manufacturer’s protocol and the sequence of interest was amplified by PCR (Veriti 96 well Fast Thermal Cycler, Applied Biosystems Inc., Foster City CA). Using therascreen KRAS, NRAS and BRAF Pyro Kits CE (Qiagen GmbH, Hilden, Germany), all hot spot regions were analyzed and samples with a potential low-level mutation were reexamined in duplicates to arrive at a specificity of 0.98 and sensitivity of 0.99 [12–14]. The PyroMark TM Q24 software (Qiagen, GmbH, Hilden, Germany) was used for data analysis.
Results
Genomic profiling was conducted on a consecutive series of 22 women with OCCC. The clinicopathological characteristics of the study patients are summarized in Table 1. Our series confirms the trend toward presentation of OCCC at an early stage, with only 27 % of patients showing late stage disease at diagnosis. Furthermore, only one patient had bilateral ovarian lesions, and in 18 % of patients, OCCC occurred along with foci of endometriosis, an endometriotic ovarian cyst in one patient and pelvic endometrial lesions in another patient (Table 1).
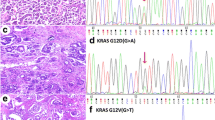
Pyrosequencing analysis of KRAS, NRAS, and BRAF hot spot regions revealed the presence of mutations only at codon 12, exon 2, of KRAS in 3 (14 %) cases. The following mutations were found: p.G12V (gly12 → val12), p.G12A (gly12 → ala12), and p.G12S (gly12 → cys12) (Table 2). Pyrogram traces demonstrating three hot spot KRAS mutations at codon 12 of exon 2 are presented in Fig. 1. No mutations in the hot spot regions of NRAF (exons 2, 3, 4) and BRAF (exon 15) genes were found. Hematoxylin/eosin stained histological images of the three KRAS mutated cases are presented in Fig. 2. Two of the KRAS mutated OCCC showed a tubulocystic pattern, and the remaining case a papillary pattern. The median maximum diameter of KRAS mutated OCCC was 110 mm. The median age was 74 years compared to 49 years in KRAS wild type OCCC. All women with a KRAS mutated OCCC the tumor was unilateral, staged IA according to FIGO classification.
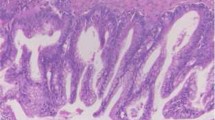
Hematoxylin-eosin stained histological images of the three OCCCs with a KRAS mutation and of an OCCC with wild type KRAS. a OCCC with KRAS mutation showing a tubulocystic pattern with clearcells, hyaline globules, oxyphilic cells, and abundant eosinophilic cytoplasm (magnification = 10×). b OCCC with KRAS mutation showing a papillary pattern with predominately hob-nail cells (magnification = 10×). c OCCC with KRAS mutation showing a tubulocystic pattern with clear cells and hob nail cells (magnification = 10×). d OCCC without KRAS mutation showing a tubulocystic pattern with clear cells and hobnail cells (magnification = 10×)
Discussion
OCCC accounts for 5 to 13 % of all epithelial ovarian malignancies [15]. From a clinical point of view, it usually occurs at a younger age than high-grade serous EOC [16]. It often presents as a pelvic mass confined to the ovary [17, 18], often arising in association with foci of endometriosis [17]. Moreover, the clinical behavior of OCCC is aggressive, with a low response rate to standard platinum-based chemotherapy [19–21] and poor prognosis compared to high-grade serous EOC [19].
OCCCs are generally p53 wild-type, with a low frequency of BRCA 1 and 2 germline mutations [22–25], and a low level of chromosomal instability. OCCCs express high levels of HIF1a (hypoxia inducible factor 1 alpha), and phosphoinositide 3-kinase catalytic alpha (PI3KCA) mutations are found in around 40 % of cases [26–29]. The proportion of HER2/neu expressing cases of OCCC is 2.5 to 10-fold higher than that of type I and II ovarian tumors, suggesting a potential role for HER2/neu inhibitors for treatment of this tumor [30].
However, despite these interesting data, the specific pathways involved in driving the aggressive biological features of OCCC are still not completely defined [31–33]. For these reasons, we studied mutational status of KRAS, NRAS, and BRAF in OCCC. The RAS-RAF-MEK-ERK-MAP kinase pathway is often affected in human cancer. KRAS, NRAS, and BRAF are members of the RAS/RAF/MEK/extracellular signal-regulated kinase/mitogen-activated protein kinase pathway, a well-characterized signaling mechanism that mediates cellular responses to growth signals [34]. These three genes are upstream activators of the mitogen-activated protein kinase (MAPK) cascade [35]. KRAS gene shows a missense point mutation in 25 % of all cancers, generally located in codon 12, 13, and 61 [36]. These KRAS mutations lead to constitutive activation of the protein, increasing GDP/GTP exchange or decreasing GTPase activity of the protein, which ultimately increases cell proliferation [37].
Mutations of KRAS and BRAF genes in noninvasive and invasive carcinomas of the ovary have been reported previously, and KRAS mutations are found in around 75 % of mucinous ovarian tumors [6, 9, 35, 38–41]. Furthermore, activating mutations of KRAS and BRAF are present in over half of type I EOC and serous borderline tumors. In contrast, they are very uncommon in high-grade serous [6] and endometrioid carcinomas [40, 42–45]. As regards OCCCs, few and contrasting data have been reported until now regarding the mutational status of KRAS and BRAF [40, 46]. Jones S et al, and Auner V et al, reported KRAS mutations in 4.7 and 26 % of OCCCs, respectively [47, 48] while Rechsteiner M. et al failed to observe KRAS mutations in a large cohort of OCCCs [49].
We observed a KRAS gene mutation in 14 % of OCCCs which seems significantly lower than in other ovarian carcinoma subtypes, especially mucinous tumors. This frequency of KRAS mutation is similar to that reported for ovarian endometrioid carcinomas (7 %), which supports the hypothesis of a common origin of these two tumor types [2, 3, 6, 9, 50, 51]. Interestingly, we found KRAS mutations only in codon 12, exon 2, but not in codon 13, exon 2 nor in NRAS or BRAF. These mutations correspond to the following amino acid substitutions: p.G12V (gly12 → val12), p.G12A (gly12 → ala12), and p.G12S (gly12 → cys12), in agreement with other studies [12, 48, 49, 52–54]. Interestingly, the commonly described p.G12D mutation was not found in our series, which emphasizes the specific biological features of OCCCs. Finally, we confirmed the absence of BRAF hot spot mutations in OCCCs [40, 49, 55, 56], making BRAF alterations a very rare event in ovarian cancer.
In conclusion, we found a KRAS mutation in codon 2 exon 2 in 14 % of OCCCs. Our findings confirm that EOC, is a heterogeneous group of entities characterized by different molecular signatures.
References
Kurman RJ, Shih IM (2010) The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol 34:433–443
Shih IM, Kurman RJ (2004) Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol 164:1511–1518
Shih IM, Kurman RJ (2005) Molecular pathogenesis of ovarian borderline tumors: new insights and old challenges. Clin Cancer Res 11:7273–7279
Bonome T, Lee JY, Park DC, Radonovich M, Pise-Masison C, Brady J, Gardner GJ, Hao K, Wong WH, Barrett JC, Lu KH, Sood AK, Gershenson DM, Mok SC, Birrer MJ (2005) Expression profiling of serous low malignant potential, low-grade, and high-grade tumors of the ovary. Cancer Res 65:10602–10612
Zorn KK, Bonome T, Gangi L, Chandramouli GV, Awtrey CS, Gardner GJ, Barrett JC, Boyd J, Birrer MJ (2005) Gene expression profiles of serous, endometrioid, and clear cell subtypes of ovarian and endometrial cancer. Clin Cancer Res 11:6422–6430
Singer G, Oldt R 3rd, Cohen Y, Wang BG, Sidransky D, Kurman RJ, Shih IM (2003) Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst 95:484–486
Obata K, Morland SJ, Watson RH, Hitchcock A, Chenevix-Trench G, Thomas EJ, Campbell IG (1998) Frequent PTEN/MMAC mutations in endometrioid but not serous or mucinous epithelial ovarian tumors. Cancer Res 58:2095–2097
Wu R, Zhai Y, Fearon ER, Cho KR (2001) Diverse mechanisms of beta-catenin deregulation in ovarian endometrioid adenocarcinomas. Cancer Res 61:8247–8255
Despierre E, Yesilyurt BT, Lambrechts S, Johnson N, Verheijen R, van der Burg M, Casado A, Rustin G, Berns E, Leunen K, Amant F, Moerman P, Lambrechts D, Vergote I, EORTC GCG and EORTC GCG Translational Research Group (2014) Epithelial ovarian cancer: rationale for changing the one-fits-all standard treatment regimen to subtype-specific treatment. Int J Gynecol Cancer 24:468–77
Wu RC, Ayhan A, Maeda D, Kim KR, Clarke BA, Shaw P, Chui MH, Rosen B, Shih IM, Wang TL (2014) Frequent somatic mutations of the telomerase reverse transcriptase promoter in ovarian clear cell carcinoma but not in other major types of gynaecological malignancy. J Pathol 232:473–81
Gilks CB, Bell DA, Huntsman D, Longacre TA, Oliva E, Soslow R, Tsuda H, Zannoni GF, Zhao C, Zhou X (2014) Ovarian tumors: clear cell tumors. In: Kurman R, Carcangiu ML, Young R (eds) World Health Organization classification of tumours: pathology and genetics of tumours of female genital organs. IARC, Lyon
Heublein S, Grasse K, Hessel H, Burges A, Lenhard M, Engel J, Kirchner T, Jeschke U, Mayr D (2013) KRAS, BRAF genotyping reveals genetic heterogeneity of ovarian borderline tumors and associated implants. BMC Cancer 13:483
Modest DP, Jung A, Moosmann N, Laubender RP, Giessen C, Schulz C, Haas M, Neumann J, Boeck S, Kirchner T, Heinemann V, Stintzing S (2012) The influence of KRAS and BRAF mutations on the efficacy of cetuximab-based first-line therapy of metastatic colorectal cancer: an analysis of the AIO KRK-0104-trial. Int J Cancer 131:980–6
Neumann J, Zeindl-Eberhart E, Kirchner T, Jung A (2009) Frequency and type of KRAS mutations in routine diagnostic analysis of metastatic colorectal cancer. Pathol Res Pract 205:858–62
Cho KR, Shih IM (2009) Ovarian cancer. Annu Rev Pathol 4:287–313
Chan JK, Teoh D, Hu JM, Shin JY, Osann K, Kapp DS (2008) Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol Oncol 109:370–376
Anglesio MS, Carey MS, Köbel M, Mackay H, Huntsman DG, Vancouver Ovarian Clear Cell Symposium Speakers (2011) Clear cell carcinoma of the ovary: a report from the first ovarian clear cell symposium. Gynecol Oncol 121:407–415
Pectasides D, Pectasides E, Psyrri A, Economopoulos T (2006) Treatment issues in clear cell carcinoma of the ovary: a different entity? Oncologist 11:1089–1094
Pectasides D, Fountzilas G, Aravantinos G, Kalofonos C, Efstathiou H, Farmakis D, Skarlos D, Pavlidis N, Economopoulos T, Dimopoulos MA (2006) Advanced stage clear-cell epithelial ovarian cancer: the Hellenic Cooperative Oncology Group experience. Gynecol Oncol 102:285–291
Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, Kita T, Suzuki M, Sato I, Taguchi K (2000) Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer 88:2584–2589
Rauh-Hain JA, Winograd D, Growdon WB, Schorge JO, Goodman AK, Boruta DM, Berkowitz RS, Horowitz NS, Del Carmen MG (2012) Prognostic determinants in patients with uterine and ovarian clear carcinoma. Gynecol Oncol 125:376–380
Risch HA, McLaughlin JR, Cole DE, Rosen B, Bradley L, Fan I, Tang J, Li S, Zhang S, Shaw PA, Narod SA (2006) Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J Natl Cancer Inst 98:1694–1706
Köbel M, Reuss A, Ad B, Kommoss S, Kommoss F, Gao D, Kalloger SE, Huntsman DG, Gilks CB (2010) The biological and clinical value of p53 expression in pelvic high-grade serous carcinomas. J Pathol 222:191–198
Salani R, Kurman RJ, Giuntoli R 2nd, Gardner G, Bristow R, Wang TL, Shih IM (2008) Assessment of TP53 mutation using purified tissue samples of ovarian serous carcinomas reveals a higher mutation rate than previously reported and does not correlate with drug resistance. Int J Gynecol Cancer 18:487–491
Ahmed AA, Etemadmoghadam D, Temple J, Lynch AG, Riad M, Sharma R, Stewart C, Fereday S, Caldas C, Defazio A, Bowtell D, Brenton JD (2010) Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J Pathol 221:49–56
Itamochi H, Kigawa J, Terakawa N (2008) Mechanisms of chemoresistance and poor prognosis in ovarian clear cell carcinoma. Cancer Sci 99:653–658
Köbel M, Kalloger SE, Boyd N, McKinney S, Mehl E, Palmer C, Leung S, Bowen NJ, Ionescu DN, Rajput A, Prentice LM, Miller D, Santos J, Swenerton K, Gilks CB, Huntsman D (2008) Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med 5:e232
Kuo KT, Mao TL, Jones S, Veras E, Ayhan A, Wang TL, Glas R, Slamon D, Velculescu VE, Kuman RJ, Shih IM (2009) Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma. Am J Pathol 174:1597–1601
Campbell IG, Russell SE, Choong DY, Montgomery KG, Ciavarella ML, Hooi CS, Cristiano BE, Pearson RB, Phillips WA (2004) Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res 64:7678–7681
Zannoni GF, Morassi F, Prisco MG, De Stefano I, Vellone VG, Arena V, Scambia G, Gallo D (2012) Clinicopathologic and immunohistochemical features of ovarian clear cell carcinomas in comparison with type I and type II tumors. Int J Gynecol Pathol 31:507–516
Kajihara H, Yamada Y, Kanayama S, Furukawa N, Noguchi T, Haruta S, Yoshida S, Sado T, Oi H, Kobayashi H (2010) Clear cell carcinoma of the ovary: potential pathogenic mechanisms. Oncol Rep 23:1193–1203
Yamaguchi K, Mandai M, Oura T, Matsumura N, Hamanishi J, Baba T, Matsui S, Murphy SK, Konishi I (2010) Identification of an ovarian clear cell carcinoma gene signature that reflects inherent disease biology and the carcinogenic processes. Oncogene 29:1741–1752
Mandai M, Matsumura N, Baba T, Yamaguchi K, Hamanishi J, Konishi I (2011) Ovarian clear cell carcinoma as a stress-responsive cancer: influence of the microenvironment on the carcinogenesis and cancer phenotype. Cancer Lett 310:129–133
Olson JM, Hallahan AR (2004) p38 MAP kinase: a convergence point in cancer therapy. Trends Mol Med 10:125–129
McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA (2007) Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta 1773:1263–1284
Gemignani ML, Schlaerth AC, Bogomolniy F, Barakat RR, Lin O, Soslow R, Venkatraman E, Boyd J (2003) Role of KRAS and BRAF gene mutations in mucinous ovarian carcinoma. Gynecol Oncol 90:378–381
Downward J (2003) Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer 3:11–22
Sieben NL, Macropoulos P, Roemen GM, Kolkman-Uljee SM, Jan Fleuren G, Houmadi R, Diss T, Warren B, Al Adnani M, De Goeij AP, Krausz T, Flanagan AM (2004) In ovarian neoplasms, BRAF, but not KRAS, mutations are restricted to low-grade serous tumours. J Pathol 202:336–340
Pohl G, Ho CL, Kurman RJ, Bristow R, Wang TL, Shih IM (2005) Inactivation of the mitogen-activated protein kinase pathway as a potential target-based therapy in ovarian serous tumors with KRAS or BRAF mutations. Cancer Res 65:1994–2000
Mayr D, Hirschmann A, Löhrs U, Diebold J (2006) KRAS and BRAF mutations in ovarian tumors: a comprehensive study of invasive carcinomas, borderline tumors and extraovarian implants. Gynecol Oncol 103:883–887
Ichikawa Y, Nishida M, Suzuki H, Yoshida S, Tsunoda H, Kubo T, Uchida K, Miwa M (1994) Mutation of K-ras protooncogene is associated with histological subtypes in human mucinous ovarian tumors. Cancer Res 54:33–35
Wu R, Hendrix-Lucas N, Kuick R, Zhai Y, Schwartz DR, Akyol A, Hanash S, Misek DE, Katabuchi H, Williams BO, Fearon ER, Cho KR (2007) Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/beta-catenin and PI3K/Pten signaling pathways. Cancer Cell 11:321–333
Enomoto T, Weghorst CM, Inoue M, Tanizawa O, Rice JM (1991) K-ras activation occurs frequently in mucinous adenocarcinomas and rarely in other common epithelial tumors of the human ovary. Am J Pathol 139:777–785
Caduff RF, Svoboda-Newman SM, Bartos RE, Ferguson AW, Frank TS (1998) Comparative analysis of histologic homologues of endometrial and ovarian carcinoma. Am J Surg Pathol 22:319–326
Amemiya S, Sekizawa A, Otsuka J, Tachikawa T, Saito H, Okai T (2004) Malignant transformation of endometriosis and genetic alterations of K-ras and microsatellite instability. Int J Gynaecol Obstet 86:371–376
Willner J, Wurz K, Allison KH, Galic V, Garcia RL, Goff BA, Swisher EM (2007) Alternate molecular genetic pathways in ovarian carcinomas of common histological types. Hum Pathol 38:607–613
Jones S, Wang TL, Shih IM, Mao TL, Nakayama K, Roden R, Glas R, Slamon D, Diaz LA Jr, Vogelstein B, Kinzler KW, Velculescu VE, Papadopoulos N (2010) Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science 330:228–231
Auner V, Kriegshäuser G, Tong D, Horvat R, Reinthaller A, Mustea A, Zeillinger R (2009) KRAS mutation analysis in ovarian samples using a high sensitivity biochip assay. BMC Cancer 9:111
Rechsteiner M, Zimmermann AK, Wild PJ, Caduff R, von Teichman A, Fink D, Moch H, Noske A (2013) TP53 mutations are common in all subtypes of epithelial ovarian cancer and occur concomitantly with KRAS mutations in the mucinous type. Exp Mol Pathol 95:235–241
Kurman RJ, Seidman JD, Shih IM (2005) Serous borderline tumours of the ovary. Histopathology 47:310–315
Russell SE, McCluggage WG (2004) A multistep model for ovarian tumorigenesis: the value of mutation analysis in the KRAS and BRAF genes. J Pathol 203:617–619
Nodin B, Zendehrokh N, Sundström M, Jirström K (2013) Clinicopathological correlates and prognostic significance of KRAS mutation status in a pooled prospective cohort of epithelial ovarian cancer. Diagn Pathol 8:106
Tsang YT, Deavers MT, Sun CC, Kwan SY, Kuo E, Malpica A, Mok SS, Gershenson DM, Wong KK (2013) KRAS (but not BRAF) mutations in ovarian serous borderline tumor are associated with recurrent low-grade serous carcinoma. J Pathol 231:449–56
Ardighieri L, Zeppernick F, Hannibal CG, Vang R, Cope L, Junge J, Kjaer SK, Kurman RJ, Shih IM (2014) Mutational analysis of BRAF and KRAS in ovarian serous borderline (atypical proliferative) tumours and associated peritoneal implants. J Pathol 232:16–22
Wong KK, Tsang YT, Deavers MT, Mok SC, Zu Z, Sun C, Malpica A, Wolf JK, Lu KH, Gershenson DM (2010) BRAF mutation is rare in advanced-stage low-grade ovarian serous carcinomas. Am J Pathol 177:1611–1617
Ross JS, Ali SM, Wang K, Palmer G, Yelensky R, Lipson D, Miller VA, Zajchowski D, Shawver LK, Stephens PJ (2013) Comprehensive genomic profiling of epithelial ovarian cancer by next generation sequencing-based diagnostic assay reveals new routes to targeted therapies. Gynecol Oncol 130:554–559
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zannoni, G.F., Improta, G., Chiarello, G. et al. Mutational status of KRAS, NRAS, and BRAF in primary clear cell ovarian carcinoma. Virchows Arch 465, 193–198 (2014). https://doi.org/10.1007/s00428-014-1599-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-014-1599-1