Abstract
Tumor-associated macrophages (TAMs) play a key role in cancer development. Especially, the immunosuppressive M2 phenotype is associated with increased tumor growth, invasiveness and metastasis. The differentiation of macrophages to the alternative phenotype M2 is mediated, inter alia, by macrophage colony-stimulating factor (M-CSF). Papillary renal cell carcinoma (RCC) represents a rare tumor type which, based upon histological criteria, can be subdivided into two subtypes (I and II), of which type II is associated with poor prognosis. In both subtypes, typically, a dense infiltrate of macrophages is found. In the present study, the expression of CD68, CD163, M-CSF, Ki-67, and CD31 was examined in 30 type I and 30 type II papillary RCCs (n = 60). Both types of papillary RCCs contained an equally dense infiltrate of CD68-positive macrophages. Nearly all macrophages in papillary RCC type II expressed CD163, a characteristic for M2 macrophages. In type I papillary RCC, less than 30 % of macrophages expressed CD163. Furthermore, tumor cells in type II papillary RCC expressed significantly more M-CSF and showed increased (Ki-67 expression defined) proliferative activity in comparison with type I papillary RCC. In addition, the (CD31 defined) capillary density was higher in type II than in type I papillary RCC. A dense infiltrate of M2 phenotype TAM and high M-CSF expression in tumor cells are key features of type II papillary RCC. These findings might explain why the prognosis of papillary RCC type II is worse than that of type I.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is widely accepted that inflammation, especially chronic inflammation, can induce cancer [1]. On the other hand, cancer can induce an inflammatory environment, which, in turn, promotes the development and progression of tumors [2]. In this process, macrophages, which display a remarkable phenotypic heterogeneity, play a key role.
Macrophages are derived from blood monocytes and differentiate in classically activated M1 or alternatively activated M2 macrophages [3, 4]. Interferon-γ induces macrophages of the M1 phenotype, which have proinflammatory activity, whereas interleukin-4/10/13 and macrophage colony-stimulating factor (M-CSF) recruit and activate macrophages of the anti-inflammatory M2 phenotype [5, 6]. M2 macrophages display tumor-promoting functions through secretion of pro-angiogenetic factors, induction of cell migration and invasion, as well as modulation of antitumor response [7, 8]. In many tumors, the presence of tumor-associated macrophages (TAMs) is associated with worse prognosis [9, 10]. Also in clear cell renal cell carcinoma (RCC), the presence of TAM has been found prognostically relevant [11].
Papillary RCC is a rare histological subtype of RCC, which comprises less than 10 % of all RCCs [12]. Based on histological criteria, papillary RCC can be divided in types I and II papillary RCC [13], of which type II shows worse prognosis [14, 15]. Typically, both types of papillary RCC are densely infiltrated with macrophages, especially foamy macrophages [13].
The aim of this study was to characterize TAM in papillary RCC, notably in regard of subtypes M1 and M2 and to assess whether the proliferation rate and capillary density are different between the two histological subtypes of papillary RCC. Furthermore, we investigated by immunohistochemistry M-CSF expression in papillary RCC.
Methods
Tissue samples
Tumor tissue of radical or partial nephrectomy specimens from 60 patients with a papillary RCC was included in this study and analyzed by morphology and immunohistochemistry. According to the WHO classification, all tumors were classified as papillary RCC subtypes I or II and staged. Clinical and histopathological data are summarized in Table 1.
Immunohistochemistry
Immunohistochemical reactions were performed on paraffin-embedded tissue sections of papillary RCC using the primary antibodies listed in Table 2. Antibody binding was detected using a biotinylated secondary antibody and streptavidin alkaline phosphatase (REAL, Dako, Hamburg, Germany). Fast red (Dako) was applied to visualize the sites of immunoreactivity. Staining results were read by light microscopy after counterstaining with Meyer’s hematoxylin.
Measurement of infiltrating macrophages, M-CSF expression, proliferation, and capillary density
Density of tumor-associated macrophages and M2 macrophages was assessed by counting all CD68- and CD163-positive cells in 10 high-power fields (HPFs, ×400 magnification).
The M-CSF expression was determined using an immunoreactive staining score (IRS) composed of the sum of the percentage of positive stained cells in a 0–5 scoring system (0 % stained cells, 0; <1 % stained cells, 1; 1–10 % cells, 2; 10–33 % stained cells, 3; 33–66 % stained cells, 4; and 66–100 % stained cells, 5) and of staining intensity in a 0–3 scoring system (0, negative; 1, weak; 2, intermediate; and 3, strong).
Proliferation was analyzed by calculating a Ki-67 proliferation index (number of positive tumor cells/total number of tumor cells) in 10 HPFs. The capillary density (CD) was determined by counting all CD31+ microvessel profiles in 10 HPFs.
For the number of macrophages, the Ki-67 proliferation index and the CD a mean value over 10 HPFs were calculated. All sections were evaluated by two independent investigators.
Statistical analysis
Differences between papillary RCC (papRCC) subtypes were statistically evaluated using the t test (GraphPad Software, San Diego, CA, USA). A p value of <0.05 was considered significant. All data are presented as mean ± standard error of the mean (SEM).
Results
Morphology of papillary RCC
Histological examination of type II papillary RCC showed a characteristic pseudostratification of tumor cells with high nuclear pleomorphism and abundant eosinophilic cytoplasm. Type I papillary RCC showed monolayers of cuboidal tumor cells without pseudostratification and numerous foamy macrophages.
Characterization of macrophages
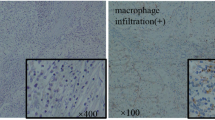
To investigate infiltrating macrophage density in papillary RCC, CD68 immunostaining was performed. Although, in particular, type I papillary RCC contained numerous foamy macrophages, density of CD68-positive macrophages was similar for types I and II papillary RCC (30.38 ± 2.9 vs. 37.05 ± 3.38; n. s., p > 0.05) (Figs. 1a, b and 2a). To determine whether the macrophages are M1 or M2, CD163 immunostaining was carried out. CD163, a member of the scavenger receptor cysteine-rich (SRCR) family, is typically expressed in M2-differentiated macrophages. Expression of CD163 was found in nearly all macrophages in type II papillary RCC (36.32 ± 3.43) (Figs. 1c and 2b). In contrast, in type I papillary RCC, fewer M2-differentiated macrophages were found (8.51 ± 0.8) (Figs. 1d and 2b). This difference was highly significant (p < 0.001) (Fig. 2b).
Immunohistochemistry of macrophages and M-CSF in papillary RCC. Types II and I papillary RCCs show equal numbers of CD68-expressing macrophages (a, b, ×20). A significantly higher number of CD163-positive M2 macrophages is found in type II papillary RCC (c, ×20) vs. type I papillary RCC (d, ×20). Stronger expression of M-CSF is detected in type II papillary RCC (e, ×20) than in type II papillary RCC (f, ×20)
Quantitative evaluation of macrophages, MCS-F expression, capillary density, and proliferation rate in both subtypes of papillary RCC. Between the subtypes of papillary RCC, no significant differences in CD68-positive macrophages (a) are found. A significantly higher number of CD163-positive M2 macrophages is detected in type II papillary RCC than in type I papillary RCC (b). Type II papillary RCC more strongly expresses M-CSF (c). The capillary density and the proliferation rate are significantly higher in type II than in type I papillary RCC (d, e)
Expression of M-CSF
Several interleukins and M-CSF are responsible for the differentiation of macrophages to the tumor-promoting M2 phenotype. To assess whether tumor cells express M-CSF, immunohistochemical staining for M-CSF was performed. In type II papillary RCC, M-CSF was strongly expressed (IRS 6.27 ± 0.29) (Figs. 1e and 2c), whereas in type I papillary RCC, expression of M-CSF was significantly lower (IRS 5.27 ± 0.27) (Figs. 1f and 2c). The difference between types II and I papillary RCCs was statistically significant (p = 0.028) (Fig. 2c).
Proliferation rate and capillary density
In type II papillary RCC, the percentage of Ki-67-immunoreactive cells was significantly higher (7.1 ± 1.09) than that in type I papillary RCC (1.13 ± 0.13) (Fig. 2d).
CD was evaluated by CD31 immunohistochemistry. In type II papillary RCC, CD (12 ± 0.83) was found to be higher than that in type I papillary RCC (6.58 ± 0.46). Statistical analysis indicated a highly significant difference (p < 0.001) (Fig. 2e).
Discussion
TAMs play a key role in cancer-associated inflammation and influence the progression and prognosis of various tumor types [16]. The presence of a mononuclear inflammatory infiltrate in clear cell RCC is associated with decreased overall survival [17]. In breast cancer, a dense macrophage infiltrate is associated with increased nodal metastases and reduced recurrence-free survival [18]. Especially, an increased infiltration of the tumor by CD163-expressing M2 macrophages in breast cancer patients is associated with a higher risk of distant metastases and reduced survival [19]. The same observations have been made in pancreatic cancer [20]. In the current study, we confirm such findings for papillary RCC. Although overall macrophage density was not different between types I and II of papillary RCC, in type II papillary RCC, which is associated with more frequent nodal and distant metastases [14, 15], an increased number of CD163-positive M2 macrophages was found.
TAMs have been shown to induce tumor cell migration and invasion [8, 10]. Migration is regulated by M-CSF, also known as colony-stimulating factor 1 (CSF-1), which is secreted by tumor cells. M-CSF recruits macrophages and stimulates them to express epidermal growth factor (EGF), which, in turn, activates the migration of tumor cells [21]. M-CSF expression is associated with worse prognosis as has been shown, especially for tumors of the reproductive system [22–24]. Moreover, Nowicki et al. found decreased tumor growth in a M-CSF and macrophage-deficient mouse model [25]. Our investigations reveal a significantly higher expression of M-CSF in type II papillary RCC, which is in line with its worse prognosis in comparison to type I papillary RCC [15]. The stronger expression of M-CSF in papillary RCC type II might constitute a mechanism responsible for the significantly higher infiltration with M2 TAM, as was also shown for ovarian carcinoma [26].
It is widely accepted that TAMs enhance tumor progression by producing different growth factors such as EGF [27]. Moreover, TAMs activate the signal transducer and activator of transcription 3 (STAT3) through interleukin-10 [28], which is typically expressed by M2 TAM [29], which stimulates cell proliferation as shown in hepatocellular and ovarian carcinoma [30, 31]. Our data are in line with these observations: type II papillary RCC contained significantly more M2 TAM as well as a significantly higher proliferation rate than type I papillary RCC.
In various studies, TAM did not only affect tumor behavior through releasing growth factors, cytokines, and inflammatory mediators [32, 33] but also appeared to play a key role in angiogenesis by secreting cytokines like VEGF and hypoxia-inducible factors [34–36]. An important function in angiogenesis can also be deduced from the fact that macrophage infiltration is increased in aggressive tumors with necrosis and is upregulated by hypoxia [37, 38]. Furthermore, Leek et al. [18] found a positive correlation between high vascular density and dense macrophage infiltrates. Our results in papillary RCCs are in line with these observations: M2 TAM density and CD are significantly higher in type II than in type I papillary RCC. The reason for the higher CD in papillary RCC type II might be the higher level of angiogenic cytokines secreted by the infiltrating M2 TAM.
Taken together, our data suggest that the reduction of macrophage infiltration or of the secretion of macrophage-specific cytokines might influence the progression of cancer. Decreased macrophage infiltration through inhibition of M-CSF secretion has been shown to reduce tumor growth [39]. Alternative ways to reduce tumor progression might be to inhibit the secretion of angiogenic substances by TAM [40, 41] or to induce TAM to differentiate along the classically activated antitumor direction of the M1 phenotype [42].
In conclusion, a higher number of M2 TAM was found in type II than in type I papillary RCC, but the total number of macrophages was similar between the subtypes. Type II papillary RCC more strongly expressed M-CSF. These results suggest a positive feedback loop between cancer cells and TAM, which might explain the worse prognosis of type II papillary RCC as well as provide new therapeutic options.
References
Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357(9255):539–545. doi:10.1016/S0140-6736(00)04046-0
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454(7203):436–444. doi:10.1038/nature07205
Gordon S (2003) Alternative activation of macrophages. Nat Rev Immunol 3(1):23–35. doi:10.1038/nri978
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23(11):549–555
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25(12):677–686. doi:10.1016/j.it.2004.09.015
Mosser DM (2003) The many faces of macrophage activation. J Leukoc Biol 73(2):209–212
Qian BZ, Pollard JW (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141(1):39–51. doi:10.1016/j.cell.2010.03.014
Condeelis J, Pollard JW (2006) Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124(2):263–266. doi:10.1016/j.cell.2006.01.007
Mantovani A, Sica A (2010) Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol 22(2):231–237. doi:10.1016/j.coi.2010.01.009
Pollard JW (2004) Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 4(1):71–78. doi:10.1038/nrc1256
Komohara Y, Hasita H, Ohnishi K, Fujiwara Y, Suzu S, Eto M, Takeya M (2011) Macrophage infiltration and its prognostic relevance in clear cell renal cell carcinoma. Cancer Sci 102(7):1424–1431. doi:10.1111/j.1349-7006.2011.01945.x
Mydlo JH, Bard RH (1987) Analysis of papillary renal adenocarcinoma. Urology 30(6):529–534
Delahunt B, Eble JN (1997) Papillary renal cell carcinoma: a clinicopathologic and immunohistochemical study of 105 tumors. Mod Pathol 10(6):537–544
Moch H, Gasser T, Amin MB, Torhorst J, Sauter G, Mihatsch MJ (2000) Prognostic utility of the recently recommended histologic classification and revised TNM staging system of renal cell carcinoma: a Swiss experience with 588 tumors. Cancer 89(3):604–614
Pignot G, Elie C, Conquy S, Vieillefond A, Flam T, Zerbib M, Debre B, Amsellem-Ouazana D (2007) Survival analysis of 130 patients with papillary renal cell carcinoma: prognostic utility of type 1 and type 2 subclassification. Urology 69(2):230–235. doi:10.1016/j.urology.2006.09.052
Solinas G, Germano G, Mantovani A, Allavena P (2009) Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol 86(5):1065–1073. doi:10.1189/jlb.0609385
Webster WS, Lohse CM, Thompson RH, Dong H, Frigola X, Dicks DL, Sengupta S, Frank I, Leibovich BC, Blute ML, Cheville JC, Kwon ED (2006) Mononuclear cell infiltration in clear-cell renal cell carcinoma independently predicts patient survival. Cancer 107(1):46–53. doi:10.1002/cncr.21951
Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL (1996) Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res 56(20):4625–4629
Shabo I, Stal O, Olsson H, Dore S, Svanvik J (2008) Breast cancer expression of CD163, a macrophage scavenger receptor, is related to early distant recurrence and reduced patient survival. Int J Cancer 123(4):780–786. doi:10.1002/ijc.23527
Yoshikawa K, Mitsunaga S, Kinoshita T, Konishi M, Takahashi S, Gotohda N, Kato Y, Aizawa M, Ochiai A (2012) Impact of tumor-associated macrophages on invasive ductal carcinoma of the pancreas head. Cancer Sci 103(11):2012–2020. doi:10.1111/j.1349-7006.2012.02411.x
Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, Graf T, Pollard JW, Segall J, Condeelis J (2004) A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res 64(19):7022–7029. doi:10.1158/0008-5472.CAN-04-1449
Kacinski BM (1995) CSF-1 and its receptor in ovarian, endometrial and breast cancer. Ann Med 27(1):79–85
Smith HO, Anderson PS, Kuo DY, Goldberg GL, DeVictoria CL, Boocock CA, Jones JG, Runowicz CD, Stanley ER, Pollard JW (1995) The role of colony-stimulating factor 1 and its receptor in the etiopathogenesis of endometrial adenocarcinoma. Clin Cancer Res 1(3):313–325
Scholl SM, Pallud C, Beuvon F, Hacene K, Stanley ER, Rohrschneider L, Tang R, Pouillart P, Lidereau R (1994) Anti-colony-stimulating factor-1 antibody staining in primary breast adenocarcinomas correlates with marked inflammatory cell infiltrates and prognosis. J Natl Cancer Inst 86(2):120–126
Nowicki A, Szenajch J, Ostrowska G, Wojtowicz A, Wojtowicz K, Kruszewski AA, Maruszynski M, Aukerman SL, Wiktor-Jedrzejczak W (1996) Impaired tumor growth in colony-stimulating factor 1 (CSF-1)-deficient, macrophage-deficient op/op mouse: evidence for a role of CSF-1-dependent macrophages in formation of tumor stroma. Int J Cancer J Int cancer 65(1):112–119. doi:10.1002/(SICI)1097-0215(19960103)65:1<112::AID-IJC19>3.0.CO;2-I
Kawamura K, Komohara Y, Takaishi K, Katabuchi H, Takeya M (2009) Detection of M2 macrophages and colony-stimulating factor 1 expression in serous and mucinous ovarian epithelial tumors. Pathol Int 59(5):300–305. doi:10.1111/j.1440-1827.2009.02369.x
O’Sullivan C, Lewis CE, Harris AL, McGee JO (1993) Secretion of epidermal growth factor by macrophages associated with breast carcinoma. Lancet 342(8864):148–149
Murray PJ (2007) The JAK-STAT signaling pathway: input and output integration. J Immunol 178(5):2623–2629
Sica A, Schioppa T, Mantovani A, Allavena P (2006) Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer 42(6):717–727. doi:10.1016/j.ejca.2006.01.003
Mano Y, Aishima S, Fujita N, Tanaka Y, Kubo Y, Motomura T, Taketomi A, Shirabe K, Maehara Y, Oda Y (2013) Tumor-associated macrophage promotes tumor progression via STAT3 signaling in hepatocellular carcinoma. Pathobiology 80(3):146–154. doi:10.1159/000346196
Takaishi K, Komohara Y, Tashiro H, Ohtake H, Nakagawa T, Katabuchi H, Takeya M (2010) Involvement of M2-polarized macrophages in the ascites from advanced epithelial ovarian carcinoma in tumor progression via Stat3 activation. Cancer Sci 101(10):2128–2136. doi:10.1111/j.1349-7006.2010.01652.x
Chen JJ, Lin YC, Yao PL, Yuan A, Chen HY, Shun CT, Tsai MF, Chen CH, Yang PC (2005) Tumor-associated macrophages: the double-edged sword in cancer progression. J Clin Oncol 23(5):953–964. doi:10.1200/JCO.2005.12.172
Lewis CE, Pollard JW (2006) Distinct role of macrophages in different tumor microenvironments. Cancer Res 66(2):605–612. doi:10.1158/0008-5472.CAN-05-4005
Lewis JS, Landers RJ, Underwood JC, Harris AL, Lewis CE (2000) Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J Pathol 192(2):150–158. doi:10.1002/1096-9896(2000)9999:9999<::AID-PATH687>3.0.CO;2-G
Valkovic T, Dobrila F, Melato M, Sasso F, Rizzardi C, Jonjic N (2002) Correlation between vascular endothelial growth factor, angiogenesis, and tumor-associated macrophages in invasive ductal breast carcinoma. Virchows Arch 440(6):583–588. doi:10.1007/s004280100458
Leek RD, Talks KL, Pezzella F, Turley H, Campo L, Brown NS, Bicknell R, Taylor M, Gatter KC, Harris AL (2002) Relation of hypoxia-inducible factor-2 alpha (HIF-2 alpha) expression in tumor-infiltrative macrophages to tumor angiogenesis and the oxidative thymidine phosphorylase pathway in human breast cancer. Cancer Res 62(5):1326–1329
Murdoch C, Giannoudis A, Lewis CE (2004) Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood 104(8):2224–2234. doi:10.1182/blood-2004-03-1109
Leek RD, Landers RJ, Harris AL, Lewis CE (1999) Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. Br J Cancer 79(5–6):991–995. doi:10.1038/sj.bjc.6690158
Aharinejad S, Abraham D, Paulus P, Abri H, Hofmann M, Grossschmidt K, Schafer R, Stanley ER, Hofbauer R (2002) Colony-stimulating factor-1 antisense treatment suppresses growth of human tumor xenografts in mice. Cancer Res 62(18):5317–5324
De Palma M, Murdoch C, Venneri MA, Naldini L, Lewis CE (2007) Tie2-expressing monocytes: regulation of tumor angiogenesis and therapeutic implications. Trends Immunol 28(12):519–524. doi:10.1016/j.it.2007.09.004
Huang H, Lai JY, Do J, Liu D, Li L, Del Rosario J, Doppalapudi VR, Pirie-Shepherd S, Levin N, Bradshaw C, Woodnutt G, Lappe R, Bhat A (2011) Specifically targeting angiopoietin-2 inhibits angiogenesis, Tie2-expressing monocyte infiltration, and tumor growth. Clin Cancer Res 17(5):1001–1011. doi:10.1158/1078-0432.CCR-10-2317
Balkwill F, Mantovani A (2010) Cancer and inflammation: implications for pharmacology and therapeutics. Clin Pharmacol Ther 87(4):401–406. doi:10.1038/clpt.2009.312
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Behnes, C.L., Bremmer, F., Hemmerlein, B. et al. Tumor-associated macrophages are involved in tumor progression in papillary renal cell carcinoma. Virchows Arch 464, 191–196 (2014). https://doi.org/10.1007/s00428-013-1523-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-013-1523-0