Abstract
It is often difficult to make a definitive diagnosis of papillary breast lesions using core needle biopsy (CNB) specimens. We studied loss of heterozygosity (LOH) on chromosome 16q in order to assess its diagnostic use for papillary breast lesions in CNB specimens. Of 25 patients with intraductal papillary breast tumors, we extracted DNA from paired samples of tumor cells from CNB specimens and non-tumor cells from subsequent excision specimens and analyzed LOH at the D16S419 and D16S514 loci on chromosome 16q. LOH analysis results were compared with final diagnoses based on pathological features of the resected specimens. On the CNB specimens, 21 tumors were histologically diagnosed as indeterminate or suspicious for malignancy, while four tumors were unambiguously malignant. Of the 21 indeterminate or suspicious tumors, 11 were finally diagnosed as benign and ten as malignant, and on these, LOH analyses were informative for 8 of the 11 benign tumors and 7 of the 10 malignant tumors. LOH was also informative on two of the four tumors unambiguously malignant on CNB. None of the eight informative benign tumors showed LOH on 16q. Six of the eleven informative malignant tumors showed LOH on 16q. LOH on 16q was significantly different between CNB specimens of benign and malignant intraductal papillary tumors (P = 0.007). Analysis of LOH on 16q may be helpful in making a definitive diagnosis in cases of papillary breast lesions, in both excised and CNB specimens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preoperative diagnosis of intraductal papillary tumors of the breast is challenging because of the difficulty of differentiating intraductal papillary carcinoma from intraductal papilloma. It is very difficult to diagnose the biological nature of these tumors based on mammography and ultrasonography, unless there is evidence of massive tumor invasion or rapid growth. Although image-guided core needle biopsy (CNB) is a highly reliable method of diagnosing breast lesions, it is often difficult to differentiate between intraductal papillary lesions based on routine pathological examination of CNB specimens. This difficulty arises because intraductal papillary carcinomas tend to be well differentiated, and CNB specimens do not always include a section with pathognomonic features. Therefore, a final diagnosis can often be made only by histological examination of the surgically resected specimen.
A number of genetic and chromosomal alterations have been identified in sporadic breast carcinomas, and their clinical implications have been investigated. Loss of heterozygosity (LOH) on chromosomes 16q and 17p are frequent in both invasive carcinoma and ductal carcinoma in situ (DCIS), irrespective of differences in the histological types and grades [1–8]. Several studies have reported a striking difference in the incidence of LOH on 16q between DCIS and intraductal papilloma [1, 5, 7] and have suggested that analysis of LOH on chromosome 16q could be helpful in the differential diagnosis of intraductal papillary tumors. In a previous study, we used Southern blot analysis to examine LOH on 16q in intracystic papillary tumors using DNA isolated from frozen, paired, surgically resected samples of tumor and non-tumor tissues [7]. More recently, we reported a polymerase chain reaction (PCR)-based LOH analysis technique using DNA isolated from paraffin-embedded tumor samples [9, 10]. In the study we report here, we used this PCR-based approach to assess its diagnostic utility on CNB specimens of indeterminate or suspicious intraductal papillary breast lesions.
Materials and methods
Samples
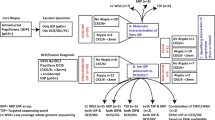
We selected tumor samples of 25 women with a preoperative diagnosis of intraductal papillary breast tumor by image-guided CNB, who had undergone surgical resection between 2005 and 2008, from the pathology computer database at the National Cancer Center Hospital, Japan. Image-guided CNB had been performed under sonographic guidance using either a 14-gauge needle or an 11-gauge vacuum-assisted biopsy probe. Twenty-one tumors had been diagnosed as indeterminate or suspicious for malignancy based on the pathological features of the CNB specimens and the lesions had been surgically resected for definitive histological diagnosis. The remaining four tumors had been unambiguously diagnosed as DCIS. The research protocol was approved by the Ethics Committee of the National Cancer Center Hospital, Japan. All patients gave written informed consent for use of their specimens in the study.
Histological criteria of intraductal papillary tumors
The diagnosis of intraductal papillary tumor was based on the presence of epithelial proliferations supported by fibrovascular stalks, with or without an intervening myoepithelial cell layer [11, 12]. All of the hematoxylin and eosin (H&E)-stained slides of the CNB and resected specimens were retrieved and reviewed for diagnostic consistency by the authors using published criteria.
The Japanese reporting form for cytology and core needle biopsy [13] was used to review the CNB specimens. This reporting form records findings and a judgment of whether the specimen is adequate or inadequate. Adequate specimens are categorized as normal or benign, indeterminate, suspicious for malignancy, or malignant.
Intraductal papillary tumors were diagnosed as benign or malignant using the following histological criteria of cytological and structural features [11, 14]. Papillomas or benign papillary tumors were diagnosed in cases showing an arborescent structure composed of fibrovascular stalks covered by a layer of myoepithelial cells with overlying epithelial cells. Intraductal papillary carcinomas or malignant papillary tumors were usually large papillary lesions (mean 2 cm, range 0.4–10 cm) located within a large cystic duct, with thin fibrovascular stalks devoid of a myoepithelial cell layer and a neoplastic epithelial cell population with characteristics of low-grade DCIS. Cases of “papilloma with atypia” with focal atypical epithelial proliferation and low-grade nuclei [15] were categorized as indeterminate in CNB specimens and as benign in resected specimens. For cases in which it was difficult to distinguish between benign and malignant tumors, the diagnosis was made by assessing the architectural features and visualizing the myoepithelial cell layer with immunohistochemical staining. Final diagnosis was made by pathological examination of the excision specimens.
Microdissection of paraffin-embedded tissues and DNA extraction
For all 25 patients, we extracted DNA from paired samples of intraductal papillary tumor cells from CNB specimens and non-tumor cells (normal mammary glands or lymph nodes) from surgically resected specimens, as previously described [9, 10]. Formalin-fixed and paraffin-embedded tissue sections, 5 to 10 μm thick, were cut using a microtome. Sections mounted on PEN foil slides were deparaffinized in xylene for 5 min (twice) and rehydrated using a descending series of ethanol concentrations as follows: 100% for 30 s (twice), 95% for 30 s (twice), 70% for 10 s, and distilled water for 10 s. The sections were stained with Meyer’s hematoxylin, washed with water, and then stained with eosin for 1 min (H&E stain). The slides were dehydrated with 100% ethanol, placed in xylene for 10 min, and air-dried. Specific cells of interest were microdissected and selected using a Leica LMD 6000 system in accordance with the manufacturer’s instructions (Leica, Narishige Micromanipulator, Wetzlar, Germany). The microdissected cells were placed in 50 μl proteinase K solution (5 mg/ml proteinase K in 10 mM Tris–HCl, 1 mM EDTA, pH 8.0, 1% Tween 20) and incubated for 36–48 h at 55°C. The proteinase K was inactivated by incubating the samples at 95°C for 10 min, and then subjected to standard phenol-chloroform extraction and ethanol precipitation in the presence of glycogen. The pellets were resuspended in distilled water and the concentration was adjusted to 0.01 μg/μl. The extracted DNA samples were stored at 4°C until further use.
Selection of polymorphic markers
The chromosomal regions and markers used were D16S419 (16q12.2) and D16S514 (16q21). The following primer sequences were used for PCR amplification:
D16S419 | Forward | 5′-ATT TTT AAG GAA TGT AAA GNA CAC A-3′ |
Reverse | 5′-GAC GTT AGA CCA GGA GTC AG-3′ | |
D16S514 | Forward | 5′-CTA TCC ACT CAC TTT CCA GG-3′ |
Reverse | 5′-TCC CAC TGA TCA TCT TCT C-3′ |
We selected polymorphic markers located on chromosome 16q based on the following criteria: (1) the markers were localized to regions with frequent DNA polymorphisms and with frequent LOH events reported in intraductal papillary carcinomas, notably low-grade DCIS [1–5, 7, 16], and (2) the amplified fragments were <250 bp, indicating that they could be successfully amplified using DNA from formalin-fixed tissues. Forward and reverse primer pairs for oligonucleotide polymorphic markers corresponding to the sequences retrieved from the UniSTS database (http://www.ncbi.nlm.nih.gov/unists) were synthesized and purchased from Perkin-Elmer (Applied Biosystems, Foster City, CA, USA). The 5′ ends of the forward primers were labeled with 6-carboxyfluorescein (6-FAM).
PCR
Genomic DNA was PCR amplified in a 25-μl reaction mixture containing 2 μl DNA solution corresponding to 20 ng genomic DNA, 0.4 pmol/μl of each primer, and 1× TaqMan Universal PCR Master Mix (Applied Biosystems) using a GeneAmp® PCR system 9600 (Applied Biosystems). The typical PCR cycling conditions included 2 min incubation at 50°C and 10 min denaturation at 95°C, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. An elongation step at 72°C for 10 min was added to the final cycle. Aliquots of the PCR products were then mixed with size standard and formamide, denatured, and run on an ABI 3130 automated capillary electrophoresis DNA sequencer (Applied Biosystems). The quantity and the quality of the DNA fragments amplified by PCR were confirmed by agarose gel electrophoresis. As a positive control, we used DNA isolated from formalin-fixed, paraffin-embedded tissues of five breast carcinomas in which LOH on 16q had already been detected by Southern blot analysis of fresh frozen tissues [17]. As a negative control, PCR was performed without template DNA.
Assessment of allele loss
The amplified products were assessed for peak height and area using Gene Mapper software (version 3.7; Applied Biosystems). Non-cancerous DNA samples with two different amplified bands were defined as informative cases for LOH analysis. The presence of LOH was determined in accordance with the manufacturer’s criteria. LOH was considered to exist if the ratio of the peak heights, which was calculated with the following formula, was <0.6 or >1.4: [peak height of the affected allele (allele A) of the tumor × peak height of the unaffected allele (allele B) of normal cells] / [peak height of allele A of normal cells × peak height of allele B of tumor cells] (Figs. 1 and 2) [17]. If the ratio of the peak height was 0.6 and 1.4 according to the formula, the case was judged to have retention of heterozygosity or absence of LOH.
Analysis of loss of heterozygosity (LOH) in an intraductal papillary tumor (case 4). a Based on the pathological features of the excised specimen, the tumor was diagnosed as intraductal papilloma. b Electrophoretogram showing constitutional heterozygosity (alleles 1 and 2) at the D16S514 locus in non-tumor DNA. The horizontal axis indicates the size of the DNA fragments (bp), and the vertical axis indicates signal intensity. c. Electrophoretogram showing retention of heterozygosity (alleles 1 and 2) at the D16S514 locus in tumor DNA. The axes are the same as in b
Analysis of loss of heterozygosity (LOH) in an intraductal papillary carcinoma (case 15). a Based on the pathological features of the excised specimen, the tumor was diagnosed as intraductal papillary carcinoma. b Electrophoretogram showing constitutional heterozygosity (alleles 1 and 2) at the D16S514 locus in non-tumor DNA. c Electrophoretogram showing loss of heterozygosity (loss of allele 2) at the D16S514 locus in tumor DNA
When the results were questionable, PCR amplification and LOH analysis were performed at least twice to obtain equivalent results. Results were considered non-informative when the normal tissue was constitutionally homozygous and were not evaluated when the tissue lysates were not amplified, that is, PCR was unsuccessful. When either D16S419 or D16S514 showed LOH, the tumor was considered to have LOH. The LOH analysis results were compared with the final diagnoses based on the pathological features of the surgically resected specimens.
Statistical analyses
The χ 2 test was used to determine differences between the benign and malignant groups of intraductal papillary tumors. Differences of P < 0.05 were considered statistically significant. PASW statistics 17 (SPSS, Inc., Chicago, IL, USA) was used for all statistical analyses.
Results
Of the 21 indeterminate or suspicious intraductal papillary tumors, 11 were finally diagnosed as benign and 10 as malignant by microscopic examination of surgically resected specimens (Table 1). The first clinical sign was nipple discharge in 8 (38%) and a palpable mass in 4 (19%) of the 21 cases. Sonographic findings of the papillary lesions included a well-defined solid mass in nine cases (43%), a cystic lesion with solid components in five (24%), and duct dilatation with solid components in seven (33%). Multiple papillary lesions were found in seven cases (33%). The median tumor size on imaging was 1.9 cm (range 0.6–4.0 cm). There were no significant differences in clinical or imaging findings between lesions finally diagnosed as malignant on excisional biopsy specimens and those finally diagnosed as benign (Table 1). Thirteen (62%) of the 21 lesions were biopsied using a 14-gauge needle, and 8 (38%) were biopsied using an 11-gauge vacuum-assisted biopsy probe. The type of percutaneous biopsy was not correlated with postoperative conversion of histopathological diagnosis.
Table 2 shows the final histological diagnoses and 16q LOH results of CNB specimens for each of the 25 intraductal papillary tumors. Eight of the 11 benign tumors were informative, and none of these cases showed LOH on 16q. Nine of the 14 malignant tumors were informative, and these showed frequent LOH on 16q. Out of the total of 25 papillary tumors, seven were considered non-informative (constitutional homozygosity) and one was not evaluated after PCR was unsuccessful. As representative results, case 4 in which 16q LOH was negative is shown in Fig. 1 and case 15 in which 16q LOH was positive is shown in Fig. 2. Case 4 was finally diagnosed as papilloma based on the pathological features of the resected specimen. Figure 1b, c show two peaks of alleles in both the non-tumor and tumor DNA. The ratio of allele 2 peak height to allele 1 peak height in the tumor DNA divided by the ratio in the normal DNA was 1.13. Therefore, this tumor was considered negative for LOH on 16q. On the other hand, case 15 (Fig. 2) was histologically diagnosed as low-grade DCIS or intraductal papillary carcinoma in the surgically resected specimen. Figure 2b, c shows a difference in the allele 2 peak heights between the normal and tumor DNA, and the ratio of allele 2 peak height to allele 1 peak height in the tumor DNA divided by the ratio in the normal DNA was 0.18. Therefore, this tumor was considered positive for LOH on 16q.
As shown in Table 3, 6 of the 11 (55%) informative malignant tumors showed LOH on 16q, whereas LOH was not detected in benign tumors. The incidence of 16q LOH in CNB specimens of intraductal papillary tumors was significantly different between benign and malignant tumors (P = 0.007). Of three malignant tumors which were negative for LOH on 16q, two were histologically diagnosed as intraductal papillary carcinoma associated with papilloma in the surgically resected specimens.
Discussion
The aim of this study was to evaluate the use of LOH on chromosome 16q to make a final diagnosis in case of an indeterminate or suspicous intraductal papillary tumor in a CNB specimen. We found a statistically significant difference in the incidence of 16q LOH between of benign and malignant intraductal papillary tumors on CNB specimens. The results of the present study suggest that analysis of LOH on 16q may be helpful for making a definitive diagnosis of an indeterminate or suspicious papillary breast lesion in CNB and surgically resected specimens.
In our previous studies, we examined LOH on 16q in intracystic papillary tumors by Southern blot analysis using frozen tissue samples [3, 5] and determined that the incidence of LOH on 16q is strikingly different between cases of DCIS and papilloma [1, 7]. In the present study, we performed PCR-based LOH analysis using DNA isolated from formalin-fixed, paraffin-embedded samples from CNB specimens of intraductal papillary tumors. Although we used a different technique and different type of samples than in previous studies, we show that the incidence of 16q LOH is significantly different between CNB specimens of benign and malignant intraductal papillary tumors.
In the present study, LOH was detected at either16q12.2 or 16q21 in 6 of 11 malignant tumors (55%), whereas LOH was not detected in histologically benign tumors. Similarly, our previous data on intracystic papillary breast tumors showed that 12 of 17 intracystic papillary adenocarcinomas (71%) had LOH on 16q, whereas none of 11 intraductal papillomas had this genetic alteration [1]. Di Cristofano et al. [5] documented LOH at locus 16q23.1–16q24.1 in 7 of 11 malignant samples (63.6%), whereas none of the four informative benign samples appeared to be altered. Taken together, LOH on 16q has high specificity and positive predictive value for the diagnosis of malignancy in intraductal papillary tumors of the breast.
None of the benign papillary lesions we examined in any of our studies, including the eight papillomas in the present study, revealed LOH on 16q. In contrast, Di Cristofano et al. [5] found LOH on 16q in benign papillary lesions, with LOH at locus 16q21.1–16q22.2 detected in both malignant and benign lesions, and at 16q23.3–16q24.1 detected only in malignant lesions. Based on these results, the authors concluded that these differences might be due to the use of the novel molecular marker D16S310 which targets 16q21.1–16q22.2, which putatively contains a tumor suppressor gene involved in the genesis/progression of breast carcinomas.
We propose that the differences between results can be explained by the cellular heterogeneity of the intraductal papillary lesions. Atypical proliferative breast lesions are thought to be precursors of breast carcinomas and have frequently been shown to have LOH on 16q [18, 19]. Atypical proliferative lesions and carcinomas are considered to be clones and probably originated from a field within these clones [19]. “Atypical papilloma” or “papilloma with atypia” is defined as papilloma with a proliferation of epithelial cells that have cytological and architectural features consistent with atypical ductal hyperplasia (ADH). Page et al. [15] further refined these terms and used atypical papilloma when the ADH focus involved 3 mm or less of the papillary lesion and the term minor DCIS lesion when the atypical focus involved more than 3 mm of the papillary lesion. These definitions were applied to the surgically resected specimens in the present study. In contrast, Tavassoli [20] suggested using the term atypical papilloma if the area of ADH occupies less than 33% of the papillary lesion, and the term carcinoma arising in a papilloma when the area of ADH occupies 33–90% of the papillary lesion. The ratio of atypical epithelial cells to total epithelial cells may have influenced the LOH analysis results.
Papillary lesions in CNB specimens are diagnosed as benign, atypical (indeterminate), suspicious for malignancy, or definitely malignant based on their pathologic features. Papillary lesions which are histologically diagnosed as definitely malignant must be treated as breast carcinomas. Papillary lesions with atypia, i.e., lesions that are histologically diagnosed as indeterminate or suspicious for malignancy in CNB specimens, need to be resected to determine if there is a more significant lesion [21]. Based on the results of our study, we propose that papillary lesions in CNB specimens that are histologically diagnosed as indeterminate or suspicious for malignancy and show LOH on 16q should also be treated as carcinoma. However, absence of LOH on 16q occurred in both papillomas and papillary carcinomas, and the predictive value of absence of LOH for a benign lesion was only 73%. In lesions in CNB judged as indeterminate or suspicious for malignancy, absence of LOH on 16q therefore has no diagnostic significance.
It is still controversial whether lesions diagnosed as papilloma without atypia by CNB need to be resected. From a pathological review of 19 papillary lesions with postoperative conversion from nonmalignant to malignant, Cheng et al. [22] concluded that the causes of diagnostic conversion were borderline atypical lesions (47%), sampling problems (32%), interpretation errors (16%), and an inadequate sample (5%). Based on the results of the present study, we cannot give clear guidelines for the management of papillomas without atypia based on LOH on 16q, but we consider that analysis of LOH on 16q in CNB specimens with an adequate amount of tumor tissue could reduce interpretation errors and be helpful in determining whether a papilloma without atypia needs to be resected.
The following limitations of the present study are worth discussing. First, results of analysis of LOH on 16q are not sufficiently sensitive for detection of malignancy. Absence of LOH cannot guarantee a benign lesion. Second, the number of cases examined in the present study is small. Third, we did not consider the possibility of intratumor heterogeneity, e.g., cases of carcinoma arising within papilloma. To our knowledge, this is nevertheless the first report which confirms that the incidence of LOH on 16q is significantly different between CNB specimens of benign and malignant intraductal papillary tumors. In conclusion, analysis of LOH on 16q may be helpful in making a definitive diagnosis in cases of papillary breast lesions, in both excised and CNB specimens.
References
Tsuda H, Uei Y, Fukutomi T, Hirohashi S (1994) Different incidence of loss of heterozygosity on chromosome 16q between intraductal papilloma and intracystic papillary carcinoma of the breast. Jpn J Cancer Res 85(10):992–996
Chen T, Sahin A, Aldaz CM (1996) Deletion map of chromosome 16q in ductal carcinoma in situ of the breast: refining a putative tumor suppressor gene region. Cancer Res 56(24):5605–5609
Fujii H, Szumel R, Marsh C, Zhou W, Gabrielson E (1996) Genetic progression, histological grade, and allelic loss in ductal carcinoma in situ of the breast. Cancer Res 56(22):5260–5265
Vos CB, ter Haar NT, Rosenberg C, Peterse JL, Cleton-Jansen AM, Cornelisse CJ, van de Vijver MJ (1999) Genetic alterations on chromosome 16 and 17 are important features of ductal carcinoma in situ of the breast and are associated with histologic type. Br J Cancer 81(8):1410–1418
Di Cristofano C, Mrad K, Zavaglia K, Bertacca G, Aretini P, Cipollini G, Bevilacqua G, Ben Romdhane K, Cavazzana A (2005) Papillary lesions of the breast: a molecular progression? Breast Cancer Res Treat 90(1):71–76
Radford DM, Fair KL, Phillips NJ, Ritter JH, Steinbrueck T, Holt MS, Donis-Keller H (1995) Allelotyping of ductal carcinoma in situ of the breast: deletion of loci on 8p, 13q, 16q, 17p and 17q. Cancer Res 55(15):3399–3405
Tsuda H, Fukutomi T, Hirohashi S (1995) Pattern of gene alterations in intraductal breast neoplasms associated with histological type and grade. Clin Cancer Res 1(3):261–267
Tsuda H, Callen DF, Fukutomi T, Nakamura Y, Hirohashi S (1994) Allele loss on chromosome 16q24.2-qter occurs frequently in breast cancers irrespectively of differences in phenotype and extent of spread. Cancer Res 54(2):513–517
Yamamoto S, Tsuda H, Takano M, Hase K, Tamai S, Matsubara O (2008) Clear-cell adenofibroma can be a clonal precursor for clear-cell adenocarcinoma of the ovary: a possible alternative ovarian clear-cell carcinogenic pathway. J Pathol 216(1):103–110
Yoshida M, Mouri Y, Yamamoto S, Yorozuya K, Fujii K, Nakano S, Fukutomi T, Hara K, Tsuda H (2010) Intracystic invasive papillary carcinoma of the male breast with analyses of loss of heterozygosity on chromosome 16q. Breast Cancer 17(2):146–150
Tavassoli FA, Devilee P (eds) (2003) Pathology and genetics of tumours of the breast and female genital organs. World Health Organization Classification of Tumours. IARC Press, Lyon
Rosen PP (2009) Papillary Carcinoma. In: Rosen PP (ed) Rosen’s breast pathology, 3rd edn. Lippincott Williams & Wilkins, Philadelphia, pp 423–448
Tsuchiya S, Akiyama F, Moriya T, Tsuda H, Umemura S, Katayama Y, Ishihara A, Inai Y, Itoh H, Kitamura T (2009) A new reporting form for breast cytology. Breast Cancer 16(3):202–206
Kraus FT, Neubecker RD (1962) The differential diagnosis of papillary tumors of the breast. Cancer 15:444–455
Page DL, Salhany KE, Jensen RA, Dupont WD (1996) Subsequent breast carcinoma risk after biopsy with atypia in a breast papilloma. Cancer 78(2):258–266
Cleton-Jansen AM, Moerland EW, Kuipers-Dijkshoorn NJ, Callen DF, Sutherland GR, Hansen B, Devilee P, Cornelisse CJ (1994) At least two different regions are involved in allelic imbalance on chromosome arm 16q in breast cancer. Genes Chromosome Cancer 9(2):101–107
Niederacher D, Picard F, van Roeyen C, An HX, Bender HG, Beckmann MW (1997) Patterns of allelic loss on chromosome 17 in sporadic breast carcinomas detected by fluorescent-labeled microsatellite analysis. Genes Chromosome Cancer 18(3):181–192
Lakhani SR, Collins N, Stratton MR, Sloane JP (1995) Atypical ductal hyperplasia of the breast: clonal proliferation with loss of heterozygosity on chromosomes 16q and 17p. J Clin Pathol 48(7):611–615
Tsuda H, Takarabe T, Akashi-Tanaka S, Fukutomi T, Hirohashi S (2001) Pattern of chromosome 6q loss differs between an atypical proliferative lesion and an intraductal or invasive ductal carcinoma occurring subsequently in the same area of the breast. Mod Pathol 14(5):382–388
Tavassoli FA (1999) Papillary lesions. In: Tavassoli FA (ed) Pathology of the breast, 2nd edn. Appleton and Lange, Stamford, pp 325–372
Ibarra JA (2006) Papillary lesions of the breast. Breast J 12(3):237–251
Cheng TY, Chen CM, Lee MY, Lin KJ, Hung CF, Yang PS, Yu BL, Yang CE, Tsai TJ, Lin CW (2009) Risk factors associated with conversion from nonmalignant to malignant diagnosis after surgical excision of breast papillary lesions. Ann Surg Oncol 16(12):3375–3379
Acknowledgments
We thank Ms. Kozue Suzuki (Basic Pathology, National Defense Medical College), Sachiko Miura, M.T. and Chizu Kina, M.T. (Department of Pathology and Clinical Laboratories, National Cancer Center Hospital) for technical assistance. This work was presented at the 7th Biennial Meeting of the Asian Breast Cancer Society held on October 8 to 10, 2009 in Seoul, Korea.
Funding
This work was supported in part by a Grant-in-Aid for Cancer Research [5] and in part by a Grant-in-Aid for Scientific Research from the Ministry of Health, Labour and Welfare of Japan.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshida, M., Tsuda, H., Yamamoto, S. et al. Loss of heterozygosity on chromosome 16q suggests malignancy in core needle biopsy specimens of intraductal papillary breast lesions. Virchows Arch 460, 497–504 (2012). https://doi.org/10.1007/s00428-012-1200-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-012-1200-8