Abstract
Neuroblastoma (NB) accounts for 15% of all childhood cancer deaths. The majority of patients have widespread lymphatic and/or haematogenous metastases at diagnosis, but lymphangiogenesis has not been well documented. Sixty-seven NBs were immunostained for the lymphatic endothelial marker, LYVE-1, and the lymphatic density (LD) and lymphatic invasion (LI), were counted in LYVE-1-expressing lymphatics. LYVE-1-stained lymphatic vessels and LI were present in 26/67 (39%) and 14/67 (21%) of the NBs, respectively. Central LD (CLD) and LI were higher in NBs from stage 4 (p = 0.012, p = 0.004, respectively), high-risk group (p = 0.030, p = 0.002), NBs with high mitosis karyorrhexis index (MKI) (p = 0.011, p = 0.005), unfavourable histology group (p = 0.040, p = 0.017) and distant lymph node metastasis (LNM) (p < 0.001 for each). Marginal LD (MLD) was higher in patients with LNM (p < 0.001). CLD and MLD correlated with LI (p < 0.001 each). Total LYVE-1 protein levels, quantified by a sensitive enzyme-linked immunosorbent assay (n = 55), were also higher in NBs from patients with stage 4 disease (p = 0.046), high-risk group (p = 0.028), MYCN-amplified NBs (p = 0.034) and LNM (p = 0.038). Kaplan–Meier analysis showed that the presence of CLD was associated with both worse OS at 5 years (77% [95% CI: 62–87%] versus 60% [95% CI: 32–80%], p = 0.062) and EFS (74% [95% CI: 58–85%] versus 43% [95% CI: 15–69%], p = 0.070) and LI with OS (71% [95% CI: 57–81%] versus 56% [95% CI: 26–78%], p = 0.055). Significant upregulation of LYVE-1 and the presence of LI in patients with stage 4 and high-risk disease, MYCN-amplification and LNM suggests that LYVE-1 may have value as predictors of outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroblastoma (NB) is the most common extra-cranial solid paediatric cancer and is responsible for approximately 15% of all childhood cancer deaths, as reviewed by Maris [1]. NBs arise from tissue of the sympathetic nervous system and frequently originate in the adrenal medulla or the sympathetic abdominal ganglia. However, NBs can also arise in the neck, chest and pelvis. The International Neuroblastoma Staging System defines five stages of NBs: stages 1 through 4 and 4-special (4 s) [1]. Stage 4 s tumours occur in children <18 months old and are widely disseminated but have a good prognosis and are capable of spontaneous regression. Adverse prognostic factors include an age ≥18 months at diagnosis, an abdominal location of the primary tumour [2] and specific genetic abnormalities, such as MYCN oncogene amplification, the loss of chromosome 1p or the gain of chromosome 17q [1]. Pathology is also an important prognostic indicator [2, 3]. Differentiated ganglioneuromas (GNs) lie at the benign end of the spectrum, and undifferentiated or poorly differentiated NBs with a high mitosis karyorrhexis index (MKI) lie at the most malignant end of the spectrum [3]. The majority of patients with NBs have widespread lymphatic and/or haematogenous metastases at diagnosis. Whereas angiogenesis has been studied extensively (reviewed by Rossler) [4], few reports have examined lymphangiogenesis in NBs [5–11].
Increased lymphatic density (LD) and lymphatic invasion (LI) are correlated with the incidence of lymph node metastasis (LNM) and poor prognosis in some human cancers (comprehensively reviewed by Sleeman) [12]. Specific staining for lymphatic vessels has been facilitated by the discovery of several proteins that recognise lymphatic endothelial cells (LECs), such as podoplanin and the transcription factor prospero-related homeobox gene-1 (PROX1) [13, 14]. The hyaluronan receptor LYVE-1 has been widely used for the detection of tumour-associated lymphatic vessels in different types of tumours [12, 15, 16]. However, the only published investigation of LYVE-1 in NB reports on only a small number of samples [6]. Moreover, the presence or absence of LI was not commented on, and no correlative data on LI, clinicopathological or biological variables are available. We performed a comprehensive expression analysis of LYVE-1 in a series of malignant neuroblastic tumours to determine the following: (1) the sensitivity of LYVE-1 in detecting LD and LI by immunohistochemistry, (2) the potential role of LD and LI based on their associations with clinicopathological and biological features and (3) the value of LD and LI as predictors of outcome.
Patients and methods
Patients
The study cohort consisted of 85 consecutive patients with NBs (including ganglioneuroblastomas, GNBs) treated at Bristol Children’s Hospital between January 1994 and June 2009. The study was approved by the South Bristol and North Somerset Research Ethics Committee (09/H0106/06). Written informed consent was obtained from parents, legal guardians or subjects for clinical information and use of tumour samples surplus to diagnostic requirements. Staging was based on the International Neuroblastoma Staging System [17]. Subjects were treated according to the appropriate clinical protocols based on clinicopathological and biological characteristics. Clinical information was extracted from the medical records, and all patient data were de-identified. The tumours were classified into prognostic groups according to the International Neuroblastoma Pathology Classification (INPC) system [3]: 79 NBs and 6 GNBs (4 intermixed and 2 nodular subtypes).
Tissue samples
Samples were obtained from the Paediatric Pathology files of the Bristol Royal Infirmary. All of the samples were obtained at primary resection (stages 1 and 2) or before chemotherapy was administered (stages 3 and 4). For immunohistochemistry, paraffin-embedded tissues were available for 67 NBs (including all GNBs). Paraffin-embedded sections were not available from 18 NBs because of insufficient viable tumour material remaining in the tissue blocks. For LYVE-1 protein assays, snap-frozen tissue samples, comprising at least 70% viable tumoural tissue, were available for 55 NBs (including the 18 NBs that did not have paraffin-embedded tissue sections).
Enzyme-linked immunosorbent assay (ELISA)
Snap-frozen tissue samples were homogenised in an ice-cold lysis buffer composed of 1% Triton X-100 buffer containing 1:100 dilutions of protease and phosphatase inhibitor cocktails (Sigma-Aldrich, Dorset, UK). After homogenisation, the samples were centrifuged at 12,000 × g for 15 min at 4°C. Total protein was quantified using the Bradford assay following the manufacturer’s instructions (Sigma-Aldrich, Dorset, UK).
The concentration of LYVE-1 total protein was measured using the human LYVE-1 Duoset ELISA kit (DY2089, R&D Systems, Abingdon, UK) according to the manufacturer’s instructions. Standard curves were constructed using two-fold serial dilutions of recombinant LYVE-1 protein (range 0–2,000 pg/ml). Optical densities were determined using a spectrophotometer at λ = 450 nm with a corrective reading at λ = 570 nm. Each test was performed in triplicate, and the mean values were used. The intraassay and interassay variation were both <10%.
Immunohistochemistry
Four-micrometer-thick consecutive sections from paraffin-embedded blocks were stained using a BOND-III automated immunostainer (Leica Biosystems, Milton Keynes, UK). Rabbit anti-human LYVE-1 polyclonal antibody (5 μg/ml, Abcam, Cambridge, UK) was applied to the sections for 1.5 h and detected using the BondTM Polymer Refine Detection system (DS9800, Leica Novocastra, Milton Keynes, UK). Omission of the primary antibody was used as a negative control.
Assessment of lymphatic vessel density (LD)
LYVE-1-stained sections were scanned at low magnification (×10 eyepiece, ×10 objective) to identify the ‘hot spots’ showing the maximum number of lymphatics. Three ‘hot spots’ within each tumour section were counted at ×200 magnification (×10 eyepiece, ×20 objective) with a surface area of 1.23 mm2. The sum of lymphatic vessels was divided by the sum of the surface area of all counted fields to obtain the mean lymphatic density (LD)/mm2. Vessels with a clearly defined lumen or well-defined linear vessel shape, but not single endothelial cells, were taken into account for LD determination. Intratumoural lymphatic hotspots from the central (CLD) and marginal (MLD) (<2 mm from the margin of tumour) regions of the tumour were counted as previously described [18] by two independent observers blinded to the clinical data. LYVE-1-stained lymphatic vessels were assessed for the presence or absence of lymphatic invasion (LI).
Statistical analysis
Patients were divided into high-risk and non-high-risk groups. Patients with non-amplified MYCN stage 1, 2 and 3 tumours and children <18 months at presentation with stage 4 and 4 s NBs that lacked MYCN amplification were classified as non-high-risk. Patients with stage 3 MYCN-amplified tumours and children ≥18 months of age with stage 4 disease were classified as high risk. Only two tumour-related deaths occurred among stages 1, 2 and 3, so we combined these stages.
Associations between the LYVE-1-LDs and LYVE-1 total protein levels and the clinicopathological and biological variables were compared using the Mann–Whitney or Cuzick test for trend (MKI and stage) tests. χ 2 tests were used to test whether the presence or absence of LI was independent of other variables. Spearman’s rank correlation was used to test for correlations between CLD, MLD and LYVE protein.
Overall survival (OS) was determined from the time of diagnosis to the last follow-up or tumour-specific-related death. Event-free survival (EFS) was calculated from the time of diagnosis until the time of first occurrence of relapse, progression, secondary malignancy or death or until the time of last contact if none of these occurred. Patients with poor or no response to therapy were excluded from the EFS analysis (n = 7, all stage 4). A patient with co-incidental stage 2 NB and stage 4 alveolar rhabdomyosarcoma died.
For comparison of groups in survival analyses, LYVE-1-LDs were categorised as absent versus present because the medians were zero. LYVE-1 total protein level was dichotomised at 2 pg/μg protein, which was close to the median value. OS and EFS were estimated using the Kaplan–Meier (KM) method, and the differences in survival of the LYVE-1 subgroups were compared using the log-rank test. We used Schoenfeld residuals to test the proportional hazards assumption for all Cox models fitted to the data and found no evidence that this assumption was not valid. Univariable Cox regression models were used to estimate the unadjusted hazard ratio for tumour-related death and relapse for each of the prognostic factors.
The statistical analyses were performed using the Stata 11.0 software package (Stata for Windows, StataCorp, Texas, US).
Results
Clinicopathological characteristics of the cohort
The clinicopathological characteristics of the 85 NB patients (including GNBs) are summarised in Table 1. The median age at presentation was 20.14 months (range 0.03–188.3 months), and the median follow-up time of all the survivors was 75 months (range 14.0–195.0 months).
LYVE-1 expression in neuroblastic tumours
Lymphatic density
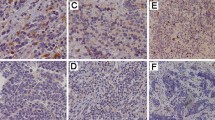
LYVE-1 expression was present mainly in the LECs but was also seen in scattered macrophages in the tumoural and stromal compartments. Lymphatics in the central part of the tumour were generally collapsed (Fig. 1a), and the marginal lymphatics were larger with wider lumens (Fig. 1b). However, lymphatics in the central part of the tumour were easily visualised when they contained tumour emboli (Fig. 1c).
Immunohistochemical expression of LYVE-1 in NBs. a Collapsed lymphatics (black arrow) in the central regions of the NB. b Large, dilated lymphatics in the marginal areas of NB containing tumour emboli. c LYVE-1-expressing lymphatics in the central region of NB show luminal occlusion by NB tumour emboli
The lymphatic count was 0 for 41/67 (61%) of patients. However, a significantly higher proportion of NBs exhibited MLD compared to NBs with CLD (39% vs. 26%, respectively, p < 0.001) (Table 2). The CLD could not be assessed in two NBs because the biopsies contained peripheral tumoural tissue only and the biopsies were 2 mm in depth.
LYVE-1 total protein expression
As a result of the relatively low LD counts, the number of NBs stained by immunohistochemistry and the commercial availability of a sensitive ELISA, LYVE-1 protein were quantified in tumour lysates. LYVE-1 protein was detected in all 55 NBs analysed (median 1.62 pg/μg, range 0.0005–61.59 pg/μg total protein).
Lymphatic invasion (LI)
Immunostaining with LYVE-1 highlighted the presence of lymphatic invasion (LI) by tumour emboli in 14/67 (21%) NBs and nodular GNBs (Table 2), but none of the intermixed GNBs were positive for LI. The lymphatics in the marginal as well as central regions showed tumour emboli (Fig. 1b, c).
The association of the LDs, LI and total protein with clinicopathological and biological variables
The relationships between LD and LI with clinicopathological and biological variables are shown in Table 2.
LYVE-1-LD
CLD was higher in NBs from patients with stage 4 disease (p = 0.012), high-risk group NBs (p = 0.030), NBs with high MKI (p = 0.011), unfavourable histology (p = 0.040) and 1p deletion (p = 0.020) (Table 2). The data also show that CLD is increased in children ≥18 months at age of diagnosis, NBs from the primary abdominal site, MYCN amplification and chromosome 17q gain (Table 2). Similarly, MLD was higher in NBs with each of the adverse clinicopathological and biological variables, although p values indicated that there was insufficient power to exclude the possibility that differences may have been due to chance (Table 2).
LI
LI was higher in patients with stage 4 disease (p = 0.004), high-risk group (p = 0.002) and in NBs with high MKI (p = 0.005) and unfavourable histology (p = 0.017) (Table 2). LI also appeared to be increased in children ≥18 months at age of diagnosis, NBs from primary abdominal site, MYCN amplification and chromosome 17q gain (Table 2).
LYVE-1 total protein
The relationship between LYVE-1 protein expression in 55 NBs and the clinical, pathological and biological variables is shown in Fig. 2 and Supplementary File 1. There was an increase in LYVE-1 total protein levels in NB biopsies from stage 4 patients (Fig. 2a, p = 0.046), high-risk group (Fig. 2b, p = 0.028), LNM (Fig. 2c, p = 0.035) and those with MYCN amplification (Fig. 2d, p = 0.034). LYVE-1 total protein was higher in tumours with a high compared with intermediate or low MKI (median, 2.95 vs. 1.10 and 0.59 pg/μg total protein, respectively, p = 0.157), tumours with unfavourable compared with favourable histology NBs (median, 2.30 vs. 0.51 pg/μg total protein, respectively, p = 0.357), tumours with 1p deletion versus NBs without (median, 1.59 vs. 0.88 pg/μg total protein, respectively, p = 0.626) and tumours with a gain of the 17q chromosome versus NBs without (median, 3.56 vs. 0.44 pg/μg total protein, respectively, p = 0.126). There was no evidence of association between LYVE-1 protein levels and age at diagnosis, primary tumour location, INPC pathology prognostic group, MKI, deletion 1p or 17q gain (Supplementary File 1).
Increased LYVE-1 total protein levels in NBs from a stage 4 patients, b high-risk group, c LN metastasis and d those with MYCN amplification. Each data point (open circle) represents protein concentration for each sample, and the horizontal line represents the median values. All p-values calculated using Mann–Whitney U-test
Correlation between LD, LI and LNM
CLD and MLD were strongly correlated (n = 65, rho = 0.605, p < 0.001). CLD correlated with LI (rho = 0.611, p < 0.001) as did MLD (rho = 0.638, p < 0.001). Both CLD and MLD were higher in patients with LNM (p < 0.001 for each, Table 2). A higher proportion of patients with LI showed LNM (p < 0.001, Table 2).
Survival analysis
At the last follow-up, 26 of 85 patients (31%) had died, and 9 patients (10%) were alive with recurrent disease. All eight of the patients with poor or no response to therapy died.
In the univariable Cox proportional hazard regression analyses, age ≥18 months at diagnosis (p = 0.038), clinical stage 4 (p < 0.001), high-risk group (p < 0.001), high MKI value (p = 0.001), MYCN-amplification (p = 0.001), 1p deletion (p < 0.001) and 17q gain (p = 0.032) were associated with lower OS (Table 3).
Similarly, age ≥18 months at diagnosis (p = 0.015), clinical stage 4 (p < 0.001), high-risk group (p < 0.001), high MKI value (p = 0.004), INPC pathology unfavourable prognostic group (p < 0.001), 1p deletion (p < 0.001) and 17q gain (p = 0.022) were associated with worse EFS. There was a strong association between distant LNM and shorter EFS and a weaker association with worse OS (Table 3).
The estimated overall survival (OS) at 5 years was 68% (95% CI: 56–77%) (Fig. 3a), and the estimated 5-year event-free survival (EFS) was 62% (95% CI: 50–72%) (Fig. 3b). Kaplan–Meier analysis showed that the presence of CLD was associated with both worse OS at 5 years (77% [95% CI: 62–87%] versus 60% [95% CI: 32–80%], p = 0.062) (Fig. 3c) and EFS (74% [95% CI: 58–85%] versus 43% [95% CI: 15–69%], p = 0.070) (Fig. 3d) and LI with OS (71% [95% CI: 57–81%] versus 56% [95% CI: 26–78%], p = 0.055). However, as the sample size is small and there are few events, confidence intervals for survival estimates are wide, and therefore, observed differences in survival may be due to chance. There was no evidence of an association between MLD or total protein and OS or EFS (Table 3).
Discussion
This study represents the first detailed expression analysis of the lymphatic endothelial marker LYVE-1 in biopsies of NB patients and is the first examination of the relationship between the expression levels of this marker, clinicopathological and biological features and the prognosis of the patients.
LYVE-1 detected intratumoural lymphatics in up to 39% of 67 NBs, in contrast to 72% reported in the only previous study of 25 NBs [6]. Variations in the sensitivity of antibodies from different sources may provide a possible explanation for this difference. However, similar to the previous study in NBs [6], we noted LYVE-1 positive intratumoural lymphatics in the central and peripheral regions of the NBs. Most of the LYVE-1-expressing lymphatics in the central, deeper parts of the tumour were small and collapsed, although they were easily visualised when tumour emboli were present. In contrast, the marginal lymphatics in the peripheral parts of the NBs were larger with widely patent lumina. There was also a significant difference in the lymphatic count between the central and marginal areas of the NBs, which is in line with previous studies of other cancers [15, 16, 18, 19].
In our cohort, CLD and MLD were highly correlated with each other, and all of the associations for MLD were similar to those with CLD, but a little less strong. Increased CLD was associated with key adverse prognostic factors, such as inclusion in the high-risk group. We also observed a significant increase in NBs from patients with stage 4 tumours, in line with those of the previous study of 25 NBs [6].
The primary significance of the increased number and size of lymphatic vessels is that they provide a possible conduit for the spread of NB cells. Intravasation into tumoural vessels is an essential step in the metastatic cascade, and the presence of LI is an important pathological feature for assessing the risk of recurrence and metastasis. Immunostaining by LYVE-1 confirmed the presence of LI by NB tumour emboli and also showed a strong relationship between LI and various adverse prognostic features. Moreover, increased CLD and MLD strongly correlated with LI and LNM. As expected, the presence of LI correlated with distant LNM. Furthermore, both LD and LI showed a trend towards a poorer OS, whereas LNM showed a trend towards poorer OS and EFS. Our results are in line with recent findings on melanomas [20], tumours that share an embryonic origin with NBs, and various epithelial cancers, as reviewed by Sleeman [12]. It is likely that a study including a larger cohort would show a significant relationship between LI and survival.
Quantification of total LYVE-1 protein using a sensitive ELISA assay also showed that LYVE-1 levels were significantly higher in the NBs of patients in an advanced stage, in the high-risk group or those with MYCN-amplified NBs. To our knowledge, LYVE-1 total protein, which measures expression in LEC and a subset of macrophages, has not been utilised in any other published clinical studies. The results obtained by immunohistochemistry were concordant with the data obtained by ELISA; increased LYVE-1 protein levels were also seen in NBs with similarly adverse prognostic features and distant LNM. However, LYVE-1 total protein levels did not show a significant association with OS or EFS in patients. Based on these findings, prospective studies including a larger number of samples from patients with NB may be useful to determine whether ELISA assays for LYVE-1 protein levels have clinical utility in providing prognostic data on the biological behaviour of NBs.
In summary, our results show for the first time that increased LYVE-1 (both LD and total protein levels) and LI are closely associated with key adverse risk factors and LNM. Distant LN involvement by NB is significantly correlated with EFS. These findings suggest that LYVE1 LD and LI may predict the outcome.
Abbreviations
- NB:
-
Neuroblastoma
- LYVE-1:
-
Lymphatic vessel endothelial hyaluronan receptor-1
- LD:
-
Lymphatic density
- CLD:
-
Central lymphatic density
- MLD:
-
Marginal lymphatic density
- LNM:
-
Lymph node metastasis
- LI:
-
Lymphatic invasion
References
Maris JM, Hogarty MD, Bagatell R et al (2007) Neuroblastoma. Lancet 369:2106–2120
Cohn SL, Pearson AD, London WB et al (2009) The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol 27:289–297
Shimada H, Ambros IM, Dehner LP et al (1999) The International Neuroblastoma Pathology Classification (the Shimada system). Cancer 86:364–372
Rossler J, Taylor M, Geoerger B et al (2008) Angiogenesis as a target in neuroblastoma. Eur J Cancer 44:1645–1656
Lagodny J, Juttner E, Kayser G et al (2007) Lymphangiogenesis and its regulation in human neuroblastoma. Biochem Biophys Res Commun 352:571–577
Ribatti D, Nico B, Cimpean AM et al (2010) Podoplanin and LYVE-1 expression in lymphatic vessels of human neuroblastoma. J Neurooncol 100:151–152
Becker J, Wang B, Pavlakovic H et al (2010) Homeobox transcription factor Prox1 in sympathetic ganglia of vertebrate embryos: correlation with human stage 4 s neuroblastoma. Pediatr Res 68:112–117
Becker J, Pavlakovic H, Ludewig F et al (2010) Neuroblastoma progression correlates with downregulation of the lymphangiogenesis inhibitor sVEGFR-2. Clin Cancer Res 16:1431–1441
Eggert A, Ikegaki N, Kwiatkowski J et al (2000) High-level expression of angiogenic factors is associated with advanced tumor stage in human neuroblastomas. Clin Cancer Res 6:1900–1908
Komuro H, Kaneko S, Kaneko M et al (2001) Expression of angiogenic factors and tumor progression in human neuroblastoma. J Cancer Res Clin Oncol 127:739–743
Nowicki M, Konwerska A, Ostalska-Nowicka D et al (2008) Vascular endothelial growth factor (VEGF)-C—a potent risk factor in children diagnosed with stadium 4 neuroblastoma. Folia Histochem Cytobiol 46:493–499
Sleeman JP, Thiele W (2009) Tumor metastasis and the lymphatic vasculature. Int J Cancer 125:2747–2756
Baluk P, McDonald DM (2008) Markers for microscopic imaging of lymphangiogenesis and angiogenesis. Ann N Y Acad Sci 1131:1–12
Van der Auwera I, Cao Y, Tille JC et al (2006) First international consensus on the methodology of lymphangiogenesis quantification in solid human tumours. Br J Cancer 95:1611–1625
Beasley NJ, Prevo R, Banerji S et al (2002) Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res 62:1315–1320
Van der Auwera I, Van den Eynden GG, Colpaert CG et al (2005) Tumor lymphangiogenesis in inflammatory breast carcinoma: a histomorphometric study. Clin Cancer Res 11:7637–7642
Maris JM (2010) Recent advances in neuroblastoma. N Engl J Med 362:2202–2211
Gombos Z, Xu X, Chu CS et al (2005) Peritumoral lymphatic vessel density and vascular endothelial growth factor C expression in early-stage squamous cell carcinoma of the uterine cervix. Clin Cancer Res 11:8364–8371
Straume O, Jackson DG, Akslen LA (2003) Independent prognostic impact of lymphatic vessel density and presence of low-grade lymphangiogenesis in cutaneous melanoma. Clin Cancer Res 9:250–256
Dadras SS, Paul T, Bertoncini J et al (2003) Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival. Am J Pathol 162:1951–1960
Acknowledgments
The authors would like to thank Above and Beyond Charities of the University Hospitals Bristol Foundation Trust for their financial support and Ince & Co for their private donation. The authors are grateful to Mr. P.B. Savage for providing laboratory facilities.
Conflict of interest
The authors declare that there is no conflict of interest. No part of the article has been published or submitted elsewhere, and there are no financial relationships that may lead to conflict of interest. The manuscript has been read and approved by all authors.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary File 1
Relationship between total protein and variables (DOC 43 kb)
Rights and permissions
About this article
Cite this article
Ramani, P., Dungwa, J.V. & May, M.T. LYVE-1 upregulation and lymphatic invasion correlate with adverse prognostic factors and lymph node metastasis in neuroblastoma. Virchows Arch 460, 183–191 (2012). https://doi.org/10.1007/s00428-011-1190-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-011-1190-y