Abstract
The dissemination of tumour cells to the lymph nodes is a complex process involving the formation of new lymph vessels, or lymphangiogenesis, produced by the tumour itself. The main growth factor involved in lymphangiogenesis is vascular endothelial growth factor C (VEGF-C), which is secreted by several different malignant tumours, including melanoma. Not only has VEGF-C expression been found in tumour cells, it has also been detected in tumour stromal cells like macrophages and fibroblasts. This study aimed to determine whether the expression of VEGF-C in tumour and stromal cells in cutaneous melanoma determines lymphangiogenesis and neoplastic dissemination to lymph nodes. We examined cases from 50 patients with melanoma who underwent selective biopsy of the sentinel lymph node. Immunohistochemical study was done with D2-40 to label lymph vessels, and the expression of VEGF-C was evaluated in tumour and stromal cells. Lymph vessel density was greater in sentinel lymph node-positive than in sentinel lymph node-negative cases, though the difference was not significant (P = 0.075). A significant correlation was seen between lymph vessel density and tumour thickness and the presence of ulceration. The main finding was that the expression of VEGF-C in fibroblasts was highly associated with the presence of metastasis in the sentinel node and with the Clark level. However, VEGF-C expression showed no relation in either tumour cells or macrophages with node status or other prognostic factors, such as the Breslow index or Clark level. Our results highlight the relevance of the stroma in tumour progression in cutaneous melanoma and its role in the spread to lymph nodes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The regional lymph node status is the most important prognostic factor in patients with melanoma. The state of lymph node involvement can be determined by selective sentinel lymph node biopsy. This node is considered to be the first node in the lymph chain into which lymph from an organ or body region drains. Since 1992, when Morton et al. [1] undertook the first sentinel node study in melanoma patients, this technique has proven of great use for the surgical treatment of these patients, avoiding unnecessary lymphadenectomies with the resulting effect on morbidity.
Tumours induce the formation of new blood vessels from already existing vessels that permit their growth in the primary site by a process known as angiogenesis [2]. These newly formed vessels also seem to play an important role in the production of distant metastasis [3]. In addition, the tumour can also induce the growth of new lymph vessels, a phenomenon called lymphangiogenesis [4].
The clinical importance of tumour lymphangiogenesis has recently been noted [5]. A relation exists between lymph vessel density (LVD) and the presence of lymph node metastasis in carcinomas of various sites, including the stomach [6], head and neck [7], breast [8], prostate [9] and colon [10]. However, research so far in malignant skin melanoma has provided contradictory findings. Whereas some authors found that lymphangiogenesis in primary melanoma was significantly increased in metastatic melanomas and that it could predict the status of the sentinel node [11–13], others reported that an increase in LVD was related with fewer lymph node metastases and a better prognosis [14, 15].
The vascular endothelial growth factors (VEGFs) comprise a family of five key factors (A, B, C, D and E) involved in the process of vascular growth. Of these, VEGF-C and VEGF-D have been directly implicated in the promotion of lymphangiogenesis, via their binding to the receptor VEGFR-3 that is present in the lymph vessel endothelium [16]. In cutaneous melanoma, VEGF-C expression in tumour cells has been related with the stage of progression [17], and some data suggest that VEGF-C expression in primary skin melanoma may be predictive of lymph node metastasis dissemination [18, 19]. However, VEGF-C is not only expressed in tumour cells; it is also expressed in tumour stroma cells, like macrophages and fibroblasts. The importance of the stroma in the development and progression of malignant neoplasia, via epithelial–mesenchymal interactions, is a concept that is acquiring greater scientific support [20].
The aim of this study was to determine whether VEGF-C expression is correlated with lymphangiogenesis in a group of patients with cutaneous melanoma and to assess whether this factor is determinant in the neoplastic dissemination to lymph nodes. We studied the immunohistochemical expression of VEGF-C in tumour cells and in macrophages and fibroblasts, comparing the results with the tumour-related LVD and the presence of sentinel lymph node metastasis.
Materials and methods
The study involved 50 cases of cutaneous melanoma collected retrospectively from the records of the Pathology Service of the Virgen de la Victoria University Hospital in Malaga, Spain, diagnosed between 2001 and 2004. All the patients gave informed consent, and the sentinel lymph node technique was performed in all. The node was resected and fully embedded in paraffin. In each tissue block, two consecutive sections were made each 40 μm until the tissue was consumed and stained with haematoxylin–eosin and immunohistochemically with HMB-45 antibody. Of the 50 cases in the study, the sentinel lymph node was negative (SLN negative) in 40 and positive (SLN positive) in 10.
The clinical records of the patients were used to collect data on age, sex and location of the lesion. The histological slides were reviewed to confirm the diagnosis and establish the following parameters: histological type, tumour thickness or Breslow index, Clark level, presence of ulceration, signs of histological regression, vascular invasion and inflammatory infiltrate. The tumour thickness was divided into two ranges, ≤1.5 and >1.5 mm. The inflammatory infiltrate was evaluated as absent, moderate, or intense. Table 1 summarises the clinical and pathological characteristics of the series. The mean age was 49.68 years (range, 14–78 years); most of the patients were older than 35 years of age. The location of the lesions was mainly on the trunk and extremities, both in SLN-negative and in SLN-positive cases. In both groups, there were more women. The most common histological type in the SLN-negative cases was superficial spreading (77.5%), whereas in the SLN-positive cases, it was nodular melanoma (60%).
Immunohistochemical study
The immunohistochemical study was done with the most representative paraffin block of tumour tissue from the primary lesion and included both superficial and deep areas. Consecutive sections (5 μm in thickness) of paraffin-embedded cancer tissue were cut, mounted on xylan-coated slides, deparaffinised in xylene and rehydrated in a graded series of ethanol. Heat-induced epitope retrieval was done with the pressure cooker method in 0.1 mol/L sodium citrate buffer (pH 6). Endogenous peroxidase activity was blocked by incubation in 1% hydrogen peroxide in methanol for 30 min. Slides were incubated with primary antibody and stained according to the standard LSAB+, Dako REAL™ (K5005) method using an automatic immunostainer (TechMate, Dako, Copenhagen, Denmark). The chromogen used was a ready-to-use biotinylated link antibody and a ready-to-use streptavidin conjugated with alkaline phosphatase (Dako K5005) and slides were counterstained with haematoxylin. Sections of tumour tissue known to express the investigated antigens were used as positive controls. Negative controls were routinely carried out by substituting the primary antibody by non-specific IgG. The control sections were treated in parallel with the samples in the same run.
The mouse monoclonal antibody D2-40 (clone D2-40) (Signet Laboratories, Dedham, MA, USA) was used as a marker of lymphatic vessels. Incubation with the antibody was carried out for 20 min at a 1/200 dilution at room temperature. LVD was defined as the number of vessels per square millimetre. To determine LVD, we reviewed sections stained with D2-40 at low power (20×) seeking the areas of greater vessel density (hot spots) of the tumour. In each case, four hot spots, two intratumoral and two peritumoral, were selected. The quantification of the lymph vessels was done from digitalised images of 800 × 600 pixels obtained with a Polaroid I DMC camera and a Nikon Eclipse E400 microscope, with a magnification of ×100. The resulting image occupied an area of 0.68 mm2. The digitalised images were analysed with Visilog 6.3 software (Noesis, France), which provides the automatic quantification of the objects indicated. Isolated cells with no vascular lumen were not considered. The vessels in each previously selected hot spot were counted, and the number of vessels in each area was summed to obtain the total number of vessels per square millimetre.
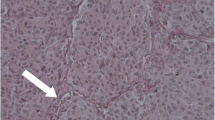
For VEGF-C immunohistochemical detection, a rabbit antibody (from Zymed Laboratories, South San Francisco, CA, USA) was used with incubation overnight at 4°C in a humidified chamber at a 1/300 dilution. VEGF-C expression was evaluated in the tumour cells, macrophages and stromal cells according to the overall staining intensity as: 1, negative (low or absent expression); 2, (moderate staining); or 3 (strong staining). Stromal cells were identified as cells with fine elongated nuclei, with elongated cytoplasms, generally situated among collagen bundles, in the tumour periphery, surrounding tumour nests (Fig. 1).
Immunohistochemical expression of VEGF-C in the peritumoral stroma: a stromal cells with very scant staining, valued as 1, the surrounding macrophages show a strong stain; b slight staining in some fibrocytes, also valued as 1; c moderate staining intensity, valued as 2; d VEGF-C negative melanoma with positive stroma, valued as 3
Statistical analysis
The statistical study was done with SPSS (version 17.0) for Windows. The study variables were first submitted to a basic descriptive analysis. The continuous variables were evaluated by their real numerical value, taking as representative values the arithmetic mean, range and standard deviation. The qualitative nominal variables, distributed in two or more categories depending on the case, were evaluated by studying the counts and corresponding frequencies. The Student’s t and ANOVA tests were used to examine the association of lymphatic vessel parameters with vascular vessel and clinicopathological parameters. Intratumoral and peritumoral lymphangiogenesis parameters were compared by means of the Spearman rho test. Associations between immunohistochemical parameters in tumour and stromal cells and clinicopathological parameters were assessed by the χ 2 test. A P < 0.05 was considered significant.
Results
D2-40 immunostaining
D2-40 stained endothelial cells of the lymph vessels but not of the blood vessels. The vessels had thin walls, covered by a single layer of poorly prominent endothelial cells. Occasional lymph vessels could be seen among the cell nests of the tumour mass, generally small, dispersed and with a collapsed lumen. The lymph vessels in the peritumoral areas were more frequent, larger and with dilated and tortuous lumina. The mean number of intratumoral and peritumoral lymph vessels in the whole series was 9.84 and 16.98/mm2, respectively. The great majority of cases had more lymph vessels in the periphery than in the intratumoral area (P < 0.001).
Although no significant association was seen between the LVD and sentinel node status, the mean LVD was greater in the SLN-positive cases than the SLN-negative cases in both intratumoral and peritumoral locations, with the corrected P for peritumoral location almost reaching significance (P = 0.075). A significant correlation was seen between LVD and tumour thickness (P = 0.003). Melanomas with a Breslow index of <1.5 mm had a lower intratumoral LVD than the melanomas with Breslow of >1.5 mm. Likewise, the presence of ulceration was significantly associated with an increase in intratumoral lymph vessels (P = 0.019), but not peritumoral vessels. The histological type and the intensity of the inflammatory infiltrate showed no relation with the number of lymph vessels within or around melanomas. The presence of regression phenomena, however, was associated with a lower LVD (P = 0.026) (Table 2).
VEGF-C immunostaining
VEGF-C immunostaining was studied in three cell types: melanoma cells, macrophages and fibroblastic stromal cells (Table 3). The VEGF-C staining was diffuse in the tumour cells from most cases, though a certain degree of heterogeneity was seen in the cell to cell staining intensity, and some cases even showed a clear difference in the staining intensity in a few tumour areas. The macrophages, both intratumoral and in the tumour periphery, generally stained stronger than in the melanoma cells. In fact, some melanoma-negative cases also showed strong staining in macrophages. In the tumour periphery, and occasionally among the tumour nests, fibroblastic stromal cells could be seen among the collagen bundles, generally with a concentric arrangement around the tumour, which also stained positively. As expected, VEGF-C expression in fibroblasts was almost absent in Clark II melanomas, in which the presence of fibroblasts around the tumour cells was minimal. The fibroblastic reaction was more evident in melanomas with levels of deeper infiltration into the dermis or the subcutaneous adipose tissue, though the fibroblast density was similar, independently of the level of invasion and the presence of lymph node involvement.
VEGF-C immunostaining of tumour cells was considered moderate or high in 29 cases and absent or low in 21 cases. Macrophages showed moderate or high staining in 33 cases and stromal cells in 16 cases. VEGF-C expression in tumour cells was of a similar intensity in the SLN-positive cases as in the SLN-negative cases. No significant differences were seen between metastatic and non-metastatic melanomas regarding macrophage staining. VEGF-C expression in the stroma, however, was clearly greater in those cases with a positive sentinel lymph node than those with a negative sentinel lymph node (P < 0.001). Nine SLN-positive cases (90%) showed moderate or high staining, whereas in 82.5% of the SLN-negative cases, the staining was null or slight in the stromal cells. In addition, stromal VEGF-C expression was also related with the Clark level of invasion (P = 0.05), mainly because most of the level V melanomas had a high expression of the marker, whereas all the level II cases were negative. No other significant differences were seen in VEGF-C expression in the tumour cells, macrophages, or stromal cells regarding histological type, thickness, inflammatory infiltrate, or regression signs. VEGF-C expression in the melanoma cells, the macrophages and the stroma showed no correlation with the intratumoral or peritumoral LVD.
Discussion
In most human cancers, including malignant cutaneous melanoma, the lymph vessels constitute the pathway of dissemination to the lymph nodes. In cutaneous melanoma, the first metastases to be detected affect the regional lymph nodes in at least 50% of patients [21]. In this case, the first lymph node in the lymph node drainage chain, known as the sentinel lymph node, is the first to be affected, and its status can predict that of the other lymph nodes [22]. Thus, the study of the sentinel lymph node provides important information on staging and prognosis, which in turn aid in treatment decisions [23]. Nonetheless, the sentinel lymph node procedure is not free from postoperative complications such as wound infection, seroma, haematoma, or lymphoedema [24].
Malignant tumours have recently been shown to induce the formation of new lymph vessels, a process known as lymphangiogenesis. Various clinicopathological studies have found a strong association between tumour lymphangiogenesis and metastatic involvement of the lymph nodes [6–9, 25–28].
Lymph vessel proliferation in malignant cutaneous melanoma exists not only in the periphery of the tumour but also within the tumour. Although the relevance of intratumoral versus peritumoral lymphatic vessels for cancer metastasis in lymph nodes remains unclear, some data suggest that the latter play a more active role. Whereas the intratumoral vessels are generally collapsed, the peritumoral vessels show wider, more dilated lumina, and tumour emboli within lymphatics are more common within the peritumoral vessels [13, 29]. As we found in our series, the density of lymphatic vessels is usually greater at the periphery than in the intratumoral location. This finding is similar to that of most studies on lymphangiogenesis in human cutaneous melanomas [11, 13, 29], though exceptions exist, with no significant differences being found [30]. However, the presence (as opposed to the absence) of intratumoral lymphatic vessels has been correlated with poor disease-free survival [11].
Comparison of LVD in SLN-positive versus SLN-negative cases showed a slightly increased number of lymphatic vessels in both intratumoral and peritumoral locations (intratumoral LVD, 9.02 ± 1.4 in SLN-negative and 13.10 ± 2.5 in SLN-positive cases; peritumoral LVD, 15.75 ± 1.4 in SLN-negative and 21.90 ± 3.9 in SLN-positive cases). However, the differences were not statistically significant, although peritumoral LVD reached levels close to significance (P = 0.075). Several authors have investigated the correlation between the degree of development of tumour lymphangiogenesis in cutaneous melanoma and the presence of metastases in the sentinel node, with conflicting results. Massi et al. [13], in a series of 15 melanomas with 30 matched controls, using immunochemistry for D2-40 and computer assisted morphometric analysis, found that the number and area of peritumoral and intratumoral lymphatic vessels were significantly higher in melanomas with SLN metastasis than in non-metastatic melanomas. Dadras et al. [11] reported that the number of peritumoral lymphatics and the average lymphatic vessel size were significantly increased in melanomas with early lymph node metastasis compared with non-metastatic melanomas. In this latter work, immunofluorescence staining for LYVE-1 on paraffin sections was used to recognise lymphatic vessels and computer-assisted morphometric analysis to obtain vessel density and size. These findings were subsequently validated in a prospective series [12] of 45 cutaneous melanomas with SLN biopsy, using similar methods. The results showed that the lymphatic vascular area was a highly sensitive and specific prognostic marker of sentinel node metastases, even more accurately than tumour thickness. Sahni et al. [30], on the other hand, found no significant difference in lymphatic counts between SLN-positive and SLN-negative cases using LYVE-1 and Chalkley point counting in 36 cases of cutaneous melanoma. Straume et al. [14] reported similar findings, but their series only included nodular melanoma, with LYVE-1 as a lymphatic marker. Surprisingly, in another report [15], lymphatic capillary density as assessed by immunohistochemical staining of VEGFR-3 showed an inverse correlation with SLN metastasis.
Methodological variability, particularly the antibody used as a marker of lymphatic vessels, may be the cause, at least in part, of disagreement in the results. In our study, we used D2-40, a specific marker of lymphatic vessels that recognises podoplanin, a mucin-type transmembrane glycoprotein [31]. Lyve-1 and D2-40 (podoplanin) are markers of lymphatic vessels commonly used to assess LVD in melanoma, and both have proved to be specific for lymphatic endothelial cells. However, in a comparative study on the usefulness of various immunohistochemical markers (Lyve-1, D2-40 and Prox-1) to identify lymphatic vessels in breast cancer, D2-40 proved to be the most sensitive marker [32]. In the “First international consensus on the methodology of lymphangiogenesis quantification in solid human tumors”, podoplanin, which is recognised by D2-40 antibody, was considered the most reliable marker of lymphatic vessels at present [33]. However, the consensus also recommended using a combination of markers that could vary depending on the type of tissue. It is necessary to standardise the methodology of lymphangiogenesis quantification in melanoma to determine its true prognostic significance.
The presence of ulceration could affect lymphangiogenesis. Our results show that intratumoral lymphatic vessels are significantly more numerous in ulcerated melanomas, whilst the density of peritumoral lymphatic vessels is similar in ulcerated and non-ulcerated lesions. Sahni et al. [30] found a significant difference when peritumoral and intratumoral lymphatic areas combined were considered but not when only intratumoral areas were taken into account. In the study of Massi et al. [13], the parameter associated with ulcerated melanoma was the average vessel area both in intratumoral and peritumoral locations. These results may be conflicting, but all agree that lymphangiogenesis is dependent on ulceration. The inflammation usually associated with ulceration may be one of the causes of the increase in lymph vessels. Both the inflammatory cells themselves as well as the proinflammatory cytokines secreted in this context have been implicated in the regulation of lymph vessel growth [34, 35]. We, too, found that tumour thickness was associated with significantly higher intratumoral LVD. However, ulceration and Breslow index are characteristics that are frequently associated as ulceration is more commonly present in thicker melanomas, as was the case in our series. Release of VEGFs mediated by hypoxia is proposed by Massi et al. [13] as the reason for the enlargement of lymphatic vessels in ulcerated melanomas. Likewise, primary melanomas in the vertical growth phase were reported to express more VEGF-C than those in the horizontal growth phase [17]. However, in our experience, VEGF-C is not increased in ulcerated melanomas nor is it associated with the Breslow index.
These findings, as well as the absence of a correlation between VEGF-C expression of the tumour or stromal cells and LVD, may be explained because lymphangiogenesis, just like angiogenesis, is a complex process in which multiple growth factors are involved, all inter-related. Factors other than VEGF-C, such as VEGF-A, hepatocyte growth factor, fibroblast growth factor-2, platelet-derived growth factors and insulin-like growth factors, may also contribute to promoting lymphangiogenesis during growth of the melanoma [36, 37]. In a large series of 175 melanomas, Straume et al. found no association between LVD and the immunohistochemical expression VEGF-C or other factors related with lymphangiogenesis [14].
Earlier data suggested that VEGF-C might be involved in the localisation of melanoma metastasis to the lymph nodes [18, 38]. In order to determine whether VEGF-C secreted by melanoma cells correlates with SLN involvement, we studied the immunohistochemical expression of VEGF-C in tumour cells from SLN-positive and SLN-negative patients. Of the 50 cases, 29 presented a moderate or intense stain, 23 (77%) of the SLN-negative and six (60%) of the SLN-positive cases. Thus, no significant association was seen between tumour VEGF-C and SLN involvement. These results agree with observations of both Dadras et al. [11] and Massi et al. [13], who reported that VEGF-C expression by tumour cells was detected in both metastatic and non-metastatic melanomas, with no correlation with lymph node status. Dadras et al. [12], on the other hand, detected moderate to high levels of cytoplasmatic VEGF-C in the majority of SLN-positive melanomas but in a minority of the SLN-negative melanomas (88.2% vs. 40.0%), and they found that tumour cell expression of VEGF-C may be useful to predict metastasis to the SLN. In addition, Bonne et al. [19], in a study of 113 cases, found that high VEGF-C expression in melanoma cells was positively associated with the presence of a positive SLN. These discordant results highlight the need for yet more research in this field to determine the usefulness of VEGF-C expression in tumour cells from cutaneous melanoma as a predictive factor of node status in the clinical setting.
This study focussed on VEGF-C secretion not only in tumour cells but also in stromal cells accompanying the cutaneous melanoma. The contribution of stromal cells to the formation of new lymphatic vessels or the appearance of lymph node metastasis is poorly understood. A great deal of evidence, both experimental and clinical, shows that the stroma and extracellular matrix play an important role in the development of human tumours [39]. The stromal cells around a tumour are mainly fibroblasts and inflammatory cells, like macrophages. The relevance of macrophages in angiogenesis has been determined in tumours from several different tumour locations [40, 41]. Macrophages are a source of antigenic factors, including VEGF-C and VEGF-D, that have also been associated with lymphangiogenesis, in both inflammatory and tumoral processes [42, 43].
Positive VEGF-C staining in cutaneous melanoma is usually more frequent in macrophages than in melanoma cells [12, 19]. Bonne et al. [19] observed that VEGF-C expression in tumour-associated macrophages (TAMs) was strongly associated with the sentinel lymph node status, just like VEGF-C expression in melanoma cells. This finding was not corroborated in our study, in which the SLN-negative and the SLN-positive cases showed a high level of positivity for VEGF-C in macrophages. However, like Bonne et al., we too failed to find an association with the pathological characteristics of the primary tumour, such as Breslow thickness, Clark level, inflammatory infiltrate, regression signs or ulceration.
In addition to macrophages, fibroblasts from the tumour stroma, also termed tumour-associated fibroblasts or carcinoma-associated fibroblasts (CAFs), play a relevant role in the development and progression of tumours. In vivo experiments mixing fibroblasts and epithelial cells from tumorigenic cell lines accelerated the growth and shortened the latency period of human epithelial tumours in athymic mice [44]. This protumorigenic action can be more clearly seen when CAFs are used in the models to study tumour stroma interaction, with the authors concluding that CAFs can direct tumour progression of initiated epithelial cells [45]. CAFs are able to secrete growth factors that contribute to the creation of a favourable microenvironment for tumour growth, promoting angiogenesis, migration and spread of tumour cells [46]. Fibroblasts can secrete VEGF, among other growth factors, and, together with inflammatory cells, are the principal source of host-derived VEGF in a tumour [47]. Interestingly, Koyama et al. [48], in an experimental study in breast carcinoma, also showed secretion of VEGF-D and VEGF-C by fibroblasts using immunohistochemistry and real-time quantitative RT-PCR. The authors conclude that lymphangiogenesis is probably also controlled by interaction between the tumour cells and the components of the stroma and that the stromal cells may serve as important inducers of tumour lymphangiogenesis in a suitable microenvironment.
Previous studies in cutaneous melanoma have shown VEGF-C expression in peritumoral dermal fibroblasts [11, 13]. However, a relation with the presence of metastasis in regional lymph nodes has not previously been reported. Surprisingly, in our study, the expression of VEGF-C in the fibroblasts was significantly associated with SLN status, more so than that of the epithelial cells or macrophages. Marked staining was seen in the vast majority of melanomas with lymphatic vessel metastasis, whereas in the SLN-negative cases, the VEGF-C staining was generally scarce or absent. Thus, evaluation of VEGF-C expression in the fibroblasts that are mainly found at the base of the tumour, around tumour nests of melanoma cells, may be useful as a predictive factor of SLN involvement.
The localisation of the fibroblasts in the periphery of the melanoma is coincident with that of the functionally active lymph vessels, which are mainly responsible for the dissemination to lymph nodes [49]. Thus, VEGF-C secreted by fibroblasts can act directly on local lymph vessels, creating a favourable microenvironment for metastatic dissemination. Although experimental studies have shown that overexpression of VEGF-C by tumour cells induces tumour-associated lymph vessel growth [50], a direct relation between a high immunohistochemical VEGF-C expression and a high LVD has not always been found in cutaneous melanomas [14]. This observation suggests the additional existence of a qualitative action of VEGF-C on lymph vessels, promoting tumour metastasis. This qualitative action may be exerted by facilitating the entry of tumour cells via the formation of intercellular gaps [51] or promoting the dilation of collecting lymph vessels, which would then cause an increase in the flow of lymph and the transport and dissemination of tumour cells [52]. Additionally, the lymphangiogenic factors secreted by the tumour may also act at a distance, provoking the appearance of areas of lymph vessel proliferation in draining lymph nodes and thus contributing to the settling and survival of tumour cells via the induction of a specific tumour microenvironment or “lymphvascular niche” [53].
Our findings underline the importance of tumour-associated stroma in the promotion of intratumoral lymphangiogenesis and show that it may also be a target for antineoplastic therapy. Given the results of this study, we consider that VEGF-C secretion by tumour-associated fibroblasts should be considered in future studies about prognostic factors in cutaneous melanoma.
References
Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, Foshag LJ, Cochran AJ (1992) Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg 127:392–399
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285:1182–1186
Folkman J (1995) Clinical applications of research on angiogenesis. N Engl J Med 333:1757–1762
Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Ylä-Herttuala S, Jäättelä M, Alitalo K (2001) Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res 61:1786–1790
Schacht V, Dadras SS, Johnson LA, Jackson DG, Hong YK, Detmar M (2005) Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am J Pathol 3:913–921
Kitadai Y, Kodama M, Cho S et al (2005) Quantitative analysis of lymphangiogenic markers for predicting metastasis of human gastric carcinoma to lymph nodes. Int J Cancer 115:388–392
Franchi A, Gallo O, Massi D, Baroni G, Santucci M (2004) Tumor lymphangiogenesis in head and neck squamous cell carcinoma. A morphometric study with clinical correlations. Cancer 5:973–978
Nakamura Y, Yasuoka H, Tsujimoto M, Imabun S, Nakahara M, Nakao K, Nakamura M, Mori I, Kakudo K (2005) Lymph vessel density correlates with nodal status, VEGF-C expression, and prognosis in breast cancer. Breast Cancer Res Treat 91:125–132
Zeng Y, Opeskin K, Horvath LG, Sutherland RL, Williams ED (2005) Lymphatic vessel density and lymph node metastasis in prostate cancer. Prostate 9999:1–9
Yan G, Zhou XY, Cai SJ, Zhang GH, Peng JJ, Du X (2008) Lymphangiogenic and angiogenic microvessel density in human primary sporadic colorectal carcinoma. World J Gastroenterol 14:101–107
Dadras SS, Paul T, Bertoncini J, Brown LF, Muzikansky A, Jackson DG, Ellwanger U, Garbe C, Mihm MC, Detmar M (2003) Tumor lymphangiogenesis. A novel prognostic indicador for cutaneous melanoma metastasis and survival. Am J Pathol 162:1951–1960
Dadras SS, Lange-Asschenfeldt B, Velasco P, Nguyen L, Vora A, Muzikansky A, Jahnke K, Hauschild A, Hirakawa S, Mihm MC, Detmar M (2005) Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph node. Mod Pathol 18:1232–1242
Massi D, Puig S, Franchi A, Malvehy J, Vidal-Sicart S, González-Cao M, Baroni G, Ketabchi S, Palou J, Santucci M (2006) Tumour lymphangiogenesis is a possible predictor of sentinel lymph node status in cutaneous melanoma: a case–control study. J Clin Pathol 59:166–173
Straume O, Jackson DG, Akslen LA (2003) Independent prognostic impact of lymphatic vessel density and presence of low-grade lymphangiogenesis in cutaneous melanoma. Clin Cancer Res 9:250–256
Wobser M, Siedel C, Schrama D, Bröcker EB, Becker JC, Vetter-Kauczok CS (2006) Expression pattern of the lymphatic and vascular markers VEGFR-3 and CD31 does not predict regional lymph node metastasis in cutaneous melanoma. Arch Dermatol Res 297:352–357
Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K (1996) A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J 15:1751
JS Goydos, Gorski DH (2003) Vascular endothelial growth factor-C mRNA expression correlates with stage of progression in patients with melanoma. Clin Cancer Res 9:5962–5967
Schietroma C, Cianfarani F, Lacal PM, Odorisio T, Orecchia A, Kanitakis J, D'Atri S, Failla CM, Zambruno G (2003) Vascular endothelial growth factor-C correlates with lymph node localization of human melanoma metastases. Cancer 98:789–797
Boone B, Blokx W, De Bacquer D, Lambert J, Ruiter D, Brochez L (2008) The role of VEGF-C staining in predicting regional metastasis in melanoma. Virchows Arch 453:257–265
Shekhar MP, Pauley R, Heppner G (2003) Host microenvironment in breast cancer development: extracellular matrix-stromal cell contribution to neoplastic phenotype of epithelial cells in the breast. Breast Cancer Res 5:130–135
Meier F, Will S, Ellwanger U, Schlagenhauff B, Schittek B, Rassner G, Garbe C (2002) Metastatic pathways and time courses in the orderly progression of cutaneous melanoma. Br J Dermatol 147:62–70
Reintgen D, Cruse CW, Wells K et al (1994) The orderly progression of melanoma nodal metastases. Ann Surg 220:759–767
Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Elashoff R, Essner R, Nieweg OE, Roses DF, Hoekstra HJ, Karakousis CP, Reintgen DS, Coventry BJ, Glass EC, Wang HJ (2006) Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med 355:1307–1317
Kretschmer L, Thoms KM, Peeters S, Haenssle H, Bertsch HP, Emmert S (2008) Postoperative morbidity of lymph node excision for cutaneous melanoma-sentinel lymphonodectomy versus complete regional lymph node dissection. Melanoma Res 18:16–21
Sleeman JP, Thiele W (2009) Tumor metastasis and the lymphatic vasculature. Int J Cancer 125:2747–2756
Tobler NE, Detmar M (2006) Tumor and lymph node lymphangiogenesis—impact on cancer metastasis. J Leukoc Biol 80:691–696
Choi WW, Lewis MM, Lawson D, Yin-Goen Q, Birdsong GG, Cotsonis GA, Cohen C, Young AN (2005) Angiogenic and lymphangiogenic microvessel density in breast carcinoma: correlation with clinicopathologic parameters and VEGF-family gene expression. Mod Pathol 18:143–152
Ueda M, Hung YC, Terai Y, Kanda K, Kanemura M, Futakuchi H, Yamaguchi H, Akise D, Yasuda M, Ueki M (2005) Vascular endothelial growth factor-C expression and invasive phenotype in ovarian carcinomas. Clin Cancer Res 11:3225–3232
Skobe M, Hamberg LM, Hawighorst T, Schirner M, Wolf GL, Alitalo K, Detmar M (2001) Concurrent induction of lymphagiogenesis, angiogénesis and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol 159:893–903
Sahni D, Robson A, Orchard G, Szydlo R, Evans AV, Russell-Jones R (2005) The use of LYVE-1 antibody for detecting lymphatic involvement in patients with malignant melanoma of known sentinel node status. J Clin Pathol 58:715–721
Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D (1999) Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol 154:385–394
Van der Auwera I, Van den Eynden GG, Colpaert CG, Van Laere SJ, van Dam P, Van Marck EA, Dirix LY, Vermeulen PB (2005) Tumor lymphangiogenesis in inflammatory breast carcinoma: a histomorphometric study. Clin Cancer Res 11:7637–7642
Van der Auwera I, Cao Y, Tille JC, Pepper MS, Jackson DG, Fox SB, Harris AL, Dirix LY, Vermeulen PB (2006) First international consensus on the methodology of lymphangiogenesis quantification in solid human tumours. Br J Cancer 95:1611–1625
Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D'Amore PA, Dana MR, Wiegand SJ, Streilein JW (2004) VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest 113:1040–1050
Ristimaki A, Narko K, Enholm B, Joukov V, Alitalo K (1998) Proinflammatory cytokines regulate expression of the lymphatic endothelial mitogen vascular endothelial growth factor-C. J Biol Chem 273:8413–8418
Rinderknecht M, Detmar M (2008) Tumor lymphangiogenesis and melanoma metastasis. J Cell Physiol 216:347–354
Alitalo K, Tammela T, Petrova TV (2005) Lymphangiogenesis in development and human disease. Nature 438:946–953
Vihinen PP, Hilli J, Vuoristo MS, Syrjänen KJ, Kähäri VM, Pyrhönen SO (2007) Serum VEGF-C is associated with metastatic site in patients with malignant melanoma. Acta Oncol 46:678–684
Angeli F, Koumakis G, Chen MC, Kumar S, Delinassios JG (2009) Role of stromal fibroblasts in cancer: promoting or impeding? Tumour Biol 30:109–120
Vicioso L, Gonzalez FJ, Alvarez M, Ribelles N, Molina M, Marquez A, Perez L, Matilla A, Alba E (2006) Elevated serum levels of vascular endothelial growth factor are associated with tumor-associated macrophages in primary breast cancer. Am J Clin Pathol 125:111–118
Coffelt SB, Hughes R, Lewis CE (2009) Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochim Biophys Acta 1796:11–18
Yamashita M, Iwama N, Date F, Shibata N, Miki H, Yamauchi K, Sawai T, Sato S, Takahashi T, Ono M (2009) Macrophages participate in lymphangiogenesis in idiopathic diffuse alveolar damage through CCL19-CCR7 signal. Hum Pathol 40:1553–1563
Schoppmann SF, Birner P, Stöckl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D (2002) Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol 161:947–956
Camps JL, Chang SM, Hsu TC, Freeman MR, Hong SJ, Zhau HE, von Eschenbach AC, Chung LW (1990) Fibroblast-mediated acceleration of human epithelial tumor growth in vivo. Proc Natl Acad Sci USA 87:75–79
Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR (1999) Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res 59:5002–5011
Kalluri R, Zeisberg M (2006) Fibroblasts in cancer. Nat Rev Cancer 6:392–401
Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK, Seed B (1998) Tumor induction of VEGF promoter activity in stromal cells. Cell 94:715–725
Koyama H, Kobayashi N, Harada M, Takeoka M, Kawai Y, Sano K, Fujimori M, Amano J, Ohhashi T, Kannagi R, Kimata K, Taniguchi S, Itano N (2008) Significance of tumor-associated stroma in promotion of intratumoral lymphangiogenesis: pivotal role of a hyaluronan-rich tumor microenvironment. Am J Pathol 172:179–193
Tammela T, Alitalo K (2010) Lymphangiogenesis: molecular mechanisms and future promise. Cell 140:460–476
Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M (2001) Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med 7:192–198
Tammela T, Saaristo A, Holopainen T, Lyytikka J, Kotronen A, Pitkonen M, Abo-Ramadan U, Yla-Herttuala S, Petrova TV, Alitalo K (2007) Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat Med 13:1458–1466
He Y et al (2005) Vascular endothelial cell growth factor receptor 3-mediated activation of lymphatic endothelium is crucial for tumor cell entry and spread via lymphatic vessels. Cancer Res 65:4739–4746
Hirakawa S (2009) From tumor lymphangiogenesis to lymphvascular niche. Cancer Sci 100:983–989
Acknowledgements
This study was supported by the Consejería de Salud de la Junta de Andalucía, Spain (grant number PI-0322/2007). We are very grateful to María José Lozano, from the Malaga University Pathology Department laboratory, for her technical collaboration in this study.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gallego, E., Vicioso, L., Álvarez, M. et al. Stromal expression of vascular endothelial growth factor C is relevant to predict sentinel lymph node status in melanomas. Virchows Arch 458, 621–630 (2011). https://doi.org/10.1007/s00428-011-1044-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-011-1044-7