Abstract
The estrogen receptor (ER)/progesterone receptor (PR)-negative breast carcinomas (BCs) encompass three molecular subtypes: one with human epidermal growth factor receptor 2 (HER) overexpression, one normal like, and the triple negative. The androgen receptor (AR) is expressed in 70–90% of invasive BCs. The aim of our study is to detect the expression of AR in a series of ER/PR-negative BCs to ascertain if there is clinical significance in relation to BC molecular subtypes. A immunohistochemical study for all receptors and cytokeratin expression was performed in 232 cases of ER/PR-negative BCs. According to cytokeratin expression, BCs were classified into two groups: luminal-type BCs (44.2%) and basal-like-type BCs (55.8%). According to the expression of HER2, 59.3% were triple-negative BCs (when ER, PR, and HER2 were negative) and 40.7% were HER2-positive BCs. AR expression was observed in 128 tumors (56.6%). One hundred and ten cases (48.8%) had >10% and 18 (7.8%) had <10% of positively stained cells. AR immunoreactivity was found in 31.2% basal-like BCs, while in the luminal group 71.1% of cases were positive, showing highly significant correlation (p < 10−8). Regarding HER2 status, 76.7% of HER2-positive BC cases were AR positive compared with only 30.4% of triple-negative BC types, showing a strong statistically significant correlation. In conclusion, we show that AR is frequently expressed in ER/PR-negative BCs and that expression of HER2 and AR is highly correlated (p < 0.005). Our results point out the role of AR and HER2 in the pathogenesis of BCs and suggest the potential role of AR in clinical management of ER/PR-negative BCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The role of estrogen receptor (ER) and the progesterone receptor (PR) in human breast carcinomas is well established, but little is known about the significance of androgen receptor (AR). The androgen receptor is expressed in 70% to 90% of invasive breast cancers: a frequency comparable to, or higher than, that reported for ER (60–80%) and PR (50–70%) [1–11]. Although ER, PR, and AR are frequently co-expressed in breast cancers, a considerable proportion of AR-positive carcinomas, ranging from 9% to 50%, are negative for both ER and PR [2, 3, 5, 7, 9, 10, 12–15]. In addition, AR was identified as the only sex steroid receptor expressed in approximately 25% of breast cancer metastases [16].
The influence of AR expression on survival of breast cancer patients is unclear. AR seemed to predict a response to hormone therapy and a better overall survival, but AR expression was not predictive of disease-free survival in ER-positive neoplasms, while ER status was an independent prognostic factor for disease-free survival [5, 13, 17]. For ER-negative tumors, AR positivity was associated with significantly better disease-free survival and with better prognosis for metastatic disease [8] and with significantly better survival after disease recurrence compared with AR-negative tumors [18].
Gene expression profiles allow breast carcinoma (BC) classification into five main groups, two of them ER+ (luminal A and luminal B) and three ER− (human epidermal growth factor receptor 2 (HER)+, basal like, and normal breast like). The main immunophenotype of the basal-like group as defined by gene expression analysis is the lack of expression of ER, PR, and HER2 and the expression of basal-associated markers such as basal cytokeratins (CK5, CK17, and CK14) and EGFR. These are currently used as surrogate immunohistochemical markers for the identification of basal-like BCs in routine practice [15, 19–23]. The normal-like gene expression pattern was typified by the high expression of gene characteristic of adipose tissue and other non-epithelial cell types. This molecular subtype is not easily defined by immunohistochemical surrogates (ER−, PR−, HER2−, CK5/6−), and thus these tumors were designated as an “unclassified group.” Farmer et al. [24] have shown that AR expression level divides ER-negative tumors into two major gene expression clusters, including ER−/AR− (basal) and ER−/AR+ (molecular apocrine subtype).
HER2 is a well-known prognostic and predictive parameter [25] and represents a biologically and clinically important feature. HER2 protein overexpression has been detected in 20–30% of invasive breast carcinoma [26, 27], and it has been found both in ER+ and in ER− breast cancer [28]. Some studies have investigated the relationship between AR and HER2 immunohistochemical status in breast carcinomas with conflicting results. Narita et al. [11], in agreement with other workers [6, 14], did not find any significant correlation, but other studies reported HER2 overexpression in grade 3, AR-positive carcinomas [8, 9, 29]. In addition, a functionally significant crosstalk between androgen receptor and the HER2 pathway has been identified in ER-negative breast cancer [30].
The prevalence and role of AR expression in triple-negative ER−/PR−/HER2 have not been clearly elucidated. Rakha et al. [31], investigating 282 triple-negative consecutive invasive breast cancers, showed AR expression in 13% of the cases.
The aim of this study was to evaluate the immunohistochemical expression of AR in a series of 232 ER− and PR− breast cancer and to correlate it with HER2 expression. In addition, the AR expression has been correlated with clinicopathological parameters, histopathological features, and follow-up data.
Materials and methods
Histological study
A total of 232 cases of ER- and PR-negative invasive BCs were selected among 1,301 consecutive invasive ductal BCs collected at the Department of Pathology, Ospedale di Circolo, and University of Insubria, Varese, Italy, from 1995 to 2000. Criteria for selection included the following: the cases were primary breast cancer, the histological type was ductal invasive carcinoma, NOS, excluding the apocrine and adenoid-cystic cancer types, and the immunohistochemical expression of ER and PR was considered negative (carcinomas were completely negative in 208 cases or present in less than 10% of tumor cells in 24 cases included in our series because clinical treatment followed guidelines of completely negative BCs). A complete and intense membrane staining for HER2 in >10% of tumor cells was considered as positive (94 cases). Of these BCs, 89 cases showed HER2 positivity in >30% of tumor cells (score 3+) while the remaining five cases were scored 2+. No FISH test for HER2 amplification was done for these cases. The tumor ER, PR, and HER2 status was available from pathology reports. Clinicopathological information concerning age, tumor site, size, and local extension were obtained from patient records. The tumor pathological stage was assessed according to the TNM sixth edition recommendations [32].
Formalin-fixed paraffin-embedded sections of tissues obtained from surgical samples (226 cases) and trucut core biopsies (six cases) were routinely stained with hematoxylin and eosin (H&E) for morphological assessment. Microscopic slides of all cases were reviewed by two senior pathologists (CR and FS) in order to assess the histological type, according to the World Health Organization’s recommendations [33], and the grade, applying the Elston–Ellis criteria [34].
Immunohistochemical study
For each case, immunohistochemical expression of AR was evaluated along with p53 and proliferative index (Ki-67, antibody MIB-1). In addition, basal and luminal differentiation of breast carcinomas was investigated using antibodies against CK5, CK14, CK8, and CK18 [35, 36]. In all cases, 4-μm-thick paraffin sections were deparaffinized in bioclear and rehydrated through a graded series of ethanol. Endogenous peroxidase activity was inhibited with 10% hydrogen peroxide (H2O2) in distilled water for 10 min. Antigen retrieval was provided using microwave for 10 min in 0.01 M sodium citrate buffer pH 6 and protease digestion. Sections were incubated overnight at 4°C with the primary antibodies and subsequently for 1 h with a 1:200 dilution of biotin-labeled secondary anti-mouse or anti-rabbit antibody followed by ABC complex (avidin, 1:100, and biotin-labeled peroxidase, 1:100) (Dako). Sections were then stained with a solution of 3,3′diaminobenzidine (Sigma) with H2O2 in Tris–HCl buffer, pH 7.4, lightly counterstained with Mayer’s hematoxylin, dehydrated, and mounted. (The source and dilution of primary antibodies used are listed in Table 1.)
In all cases, immunoreactivity was semiquantitatively evaluated and recorded as the percentage of positive cells. BCs were classified as basal like when at least 5% of cancer cells were immunoreactive for CK5 and/or CK14, independent of the intensity of the immunostaining [35, 36]. For AR, we considered nuclear labeling in more than 10% of neoplastic cells as the cutoff point for positivity, similar to standardized criteria used for other steroid hormone receptors [9].
Positive controls included normal breast tissue surrounding the neoplastic lesion (for all antibodies) and normal prostatic tissue (for AR). Negative controls included the omission of primary antibodies. All the immunoreactions were separately evaluated by two senior pathologists.
Statistical analysis
The statistical analysis was performed using the χ 2 test with Yates’ correction (SPSS 7.5 software). The survival analysis was performed by employing the Kaplan–Meier product limit estimate of probability of survival against time, producing a product limit survival curve for each of the following variables: age, stage, presence of lymph node metastases, and immunohistochemical expression of basal (CK5, CK14) and luminal (CK8, CK18) immunophenotype and AR.
Multivariate analysis was carried out using the Cox proportional hazard model. Variables with p < 0.15 in the univariate analysis were entered into the multivariate analysis. For all the survival analyses, the Survan XL program, version 1.14 (copyright © 1995–1997), was employed.
Results
Clinicopathological data and immunoprofile
The mean age of the 232 patients at diagnosis was 58.7 years (range 24–97 years). The most frequent location of tumors was the upper-external quadrant (37%), and the diameter ranged from 0.3 to 10.5 cm (mean 2.37 cm). The pathological stage at diagnosis was known in 224 patients: 112 tumors were in stage pT1 (50%), 91 in pT2 (40.6%), five in pT3 (2.2%), and 16 in pT4 (7.1%). Lymph node status was available for 175 cases in which axillary dissection was performed, and lymph node involvement was present in 83 cases (47.4%). The series of 232 cases included 216 (93.1%) poorly differentiated (G3) and 16 (6.8%) moderately differentiated (G2) ductal BCs.
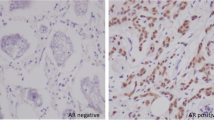
The mean percentage of Ki-67-positive nuclei was 55.7% (range 14–95). Overexpression of p53 in more than 10% of tumor cells was present in 168/231 (72.7%) cases with a mean percentage of p53-positive nuclei of 63.6%. The comprehensive results of the immunohistochemical study are reported in Table 2. According to luminal and basal cytokeratin expression, tumors were classified into two groups: 100 (44.2%) luminal-type BCs, expressing only CK8 and/or CK18, and 126 (55.8%) basal-like-type BCs positive for CK5 and/or CK14 in more than 5% of cancer cells, often also showing luminal cytokeratin expression; six cases failed. In addition, according to the expression of HER2, 137 (59.3%) cases were considered as triple-negative BCs (when ER, PR, and HER2 were negative) (Fig. 1) and 94 (40.7%) as HER2-positive BCs; one case failed.
AR immunohistochemistry
AR expression was evaluable in 226 cases, and AR positivity was observed in 128 BCs (56.6%). One hundred and ten cases (48.8%) had >10% positive cells, with an average of 59% of AR nuclear immunoreactivity (range 10–100%), and 18 (7.8%) showed <10% of positively stained cells, and 98 tumors (43.4%) were AR negative (Table 3).
Comparison of AR expression with other tumor clinicopathological features is shown in Table 4. While the pT did not show any statistically significant difference between T1 and T2–T3–T4 (45.8% of cases versus 51.8%; p = 0.38), the correlation of lymph node status with AR expression showed statistically significant results (p < 0.034). In fact, axillary lymph node metastases were present in 63.4% (52/82) of patients with AR-positive tumors, compared with 36.6% (30/82) of those with AR-negative lesions. On the contrary, 41 out 89 (46.1%) pN0 patients were AR positive and 48 out 89 (53.9%) were AR negative. Regarding tumor grade, AR positivity was seen in eight (50%) out of 16 G2 cases and in 102 (48.6%) out of 210 G3 cases.
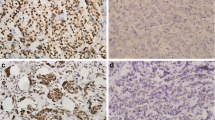
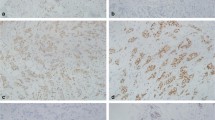
The basal-like-type BCs were AR positive in 31.2% (39/125) of cases, while in the luminal group 71.1% of cases (69/97) were positive, showing highly significant correlation (p < 10−8). Regarding AR immunoexpression and HER2 status, statistical analysis showed a strongly significant correlation: 76.7% (69/90) of HER2 positive BCs cases were AR positive (Fig. 2) compared with only 30.4% (41/135) of triple-negative BC types (p < 10−10).
Survival analysis
Information about overall and disease-free survival was available for 224 patients: 138 (61.6%) patients were alive after a median follow-up time of 116.4 months (range 83–173 months) while 86 (38.4%) out of 224 were dead of disease after a median follow-up time of 36.3 months (range 2–99 months). Regarding clinical evolution, 130 of the 138 alive patients (94.2%) were disease free after a median follow-up time of 116.2 months (range 83–173 months), while only eight (5.8%) experienced disease relapse after a median follow-up time of 68.3 months (range 6–119 months). For the group of the 86 dead women, 54 (62.8%) patients died with disease after a median follow-up of 33.63 months (range 2–99 months) while 32 (37.2%) developed a disease relapse after a median of 34.3 months (range 0–71 months).
No relationship between overall survival and AR expression was found (p = 0.16). In fact, among the still-living patients, 45.3% (62/137) harbored AR-positive BCs and 54.7% (75/137) harbored AR-negative BCs, while among the 84 deceased patients, 47 (56%) harbored AR-positive BCs and 37 (44%) harbored AR-negative ones.
Also, there was no significant correlation between AR status and disease-free survival (p = 0.44) since 22 (56.4%) of 39 patients with disease relapse harbored AR-positive BCs and 17 (43.6%) harbored AR-negative BCs. On the other hand, of the 183 patients with no evidence of distant metastasis, 86 (46.9%) women harbored AR-positive BCs while 93 (50.8%) harbored AR-negative BCs.
Survival analysis has been performed to determine a possible relationship between patient outcome and age at diagnosis, stage, presence of lymph node metastases, and immunohistochemical expression of basal (CK5, CK14) and luminal (CK8, CK18) markers and AR. Factors influencing survival in the univariate analysis were age at diagnosis (the probability of survival after a follow-up time of 144 months was 87.4% for patients ≤40 years old vs 57.5% for patients >40 years old, p = 0.006), pT stage (median survival time of 100 months for T1 vs 84 months for T2–T3–T4, p < 0.0001), and axillary lymph node status (median survival time of 101 months for lymph-node-negative tumors vs 70 months for lymph-node-positive tumors, p < 0.0001) (Figs. 3 and 4).
Kaplan–Meier survival curves illustrating overall survival for patients stratified by age at diagnosis (a), pT stage (b), and lymph node status (b). Significantly worse survival rates were noticed for patients with tumors occurred after the age of 40 years, advanced pT stage and presence of lymph node metastases
Multivariate analysis was performed using the Cox proportional hazard model including the three variables which were statistically significant in the univariate analysis. Results showed that stage and lymph node status are independent prognostic factors (p = 0.0261 and p = 0.0017 respectively, Table 5).
Discussion
AR as a target for the treatment of hormone-receptor-negative breast cancer is an uncharacterized territory and targeting AR with AR androgen-based hormonal therapy in ER and PR breast cancer should be taken into consideration as potential interactions between AR and other tumor-growth-related factors [37]. Recently, there has been renewed interest in alternative hormonal treatment, including progestin medroxyprogesterone acetate, for use both in early disease and in the advanced setting when conventional therapies fail [38–40]. These studies suggest AR as a marker for the efficiency of endocrine therapy and for new hormonal therapeutic strategies in women with estrogen-negative cancer.
Other studies have examined the significance of AR in breast carcinomas, usually in comparison with ER and PR status, but few immunohistochemical studies have investigated the expression of AR as the only sex steroid receptor expressed in ductal invasive breast carcinomas. In this context, our study focused on a large series of ER- and PR-negative ductal carcinomas, since most ER/PR-positive tumors are AR positive. It is clear from this study that AR expression is a frequent feature of ER- and PR-negative ductal breast carcinomas. Our results show a 56.6% incidence of AR positivity in ER- and PR-negative ductal invasive breast carcinomas, which is similar to the earlier reported percentage in previous investigations [8, 9, 41].
As is commonly expected in ER- and PR-negative ductal breast carcinomas, in the present investigation, almost all tumors (93.1%) were G3, showing a higher percentage of G3 cases than those (74%) reported by Scawn and Shousha [41]. AR positivity has been shown in 48.6% of poorly differentiated carcinomas (G3), in agreement with previous immunohistochemical studies examining a smaller series of cases of ER/PR-negative breast carcinomas [5, 8, 9, 11, 14].
Bryan et al. [17] did not find a significant correlation between AR and menopausal status in their patients, in contrast with Agoff and colleagues [8] who found that AR expression correlated with both increasing age and menopausal status. For our study, no information was available about menopausal status of the patients, but we could not find any tumor correlation of AR expression of BCs and the age of the patients.
Regarding the nodal status, which represents a reliable prognostic factor in breast carcinoma, there was a significant correlation between lymph node status and overall survival. In our study, we found that the percentage of axillary lymph node disease was 47.4%, in agreement with other investigations [41, 42] but higher than the general incidence of 20–30% commonly observed in the breast cancer [43]. One notable finding of the current study was the positive association between AR expression and lymph node metastases, in contrast with the results of other studies [6, 11]. On the other hand, we did not find a correlation of AR expression with the pT stage of the tumors, in agreement with recent studies [10].
An interesting finding of our study was the significant correlation between AR expression and HER2 status. This result is in agreement with the observations of other workers that have detected HER2 overexpression in G3-AR-positive carcinomas [8, 9, 29] but is in contrast with some other studies [6, 11, 14]. These data suggest that AR can be involved in the pathogenesis of breast carcinomas, indicating that HER2 may control the transcriptional activity of AR, the latter regulating its own transcription. The interaction of AR with the HER2-related pathway has been investigated in human prostate cancer cell lines, suggesting a crosstalk between AR and HER2 pathway and demonstrating that HER2 induces AR transactivation through the MAP kinase pathway [44–48]. These findings have potential clinical relevance since anti-androgens did not inhibit AR in the presence of HER2.
Doane et al. [28] point out the heterogeneity of AR expression as well as the complexity of AR signaling in breast cancer, showing that androgens inhibited the growth of some cell lines but stimulated the growth of other cell lines. Because of the clinical heterogeneity of ER-negative, PR-negative tumors, Doane et al. [28] speculated that molecular subsets exist in ER- and PR-negative tumors and postulated that AR may act in concert with other signal transduction pathway, and it is known that receptor tyrosine kinase pathway functions as a modulator of nuclear hormone receptor activity [49]. Very recently, similar results have been reported by Niemeier et al. [50] who, studying the AR expression in 189 consecutive BCs, outlined the close association between AR positivity and HER2 status in five out of eight HER2 BCs while Park et al. [51] reported the AR positivity in 32 out of 43 HER2 BCs. The authors suggest a potential target role for AR both in AR+ HER2 BC molecular class and in AR+ triple-negative BCs.
Carcinoma of the breast is recognized to be a heterogeneous disease consisting of phenotypically diverse population of cancer cells. Previous studies have demonstrated that BCs with basal-like phenotype is a special group of tumors that appear to be predominantly grade 3 ER and PR negative [52] and is characterized by a specific immunohistochemical profile and poor prognosis [51–53]. In our study, basal-like carcinomas represented more than half of the cases (55.8%), and they expressed AR receptors less frequently (31.2% of the cases) than luminal carcinomas (71.1% of the cases), in agreement with other investigations [15, 31, 54].
The prognostic significance of AR expression in ER- and PR-negative breast cancer has been poorly investigated, while most studies have emphasized the prognostic value of AR status in ER+/PR+ BCs [5, 7, 13, 14, 17]. Our study focused on ER- and PR-negative tumors and did not find any association between AR expression with both the overall survival and the disease-free survival (Fig. 3). Our findings are only partially in agreement with previous investigations [8] that have found an association between AR expression and disease-free survival in the subset of patients with ER-negative BCs in univariate analysis but not in multivariate.
Our investigation has shown that, with multivariate analysis, only lymph node status and tumor size were independent prognostic variables, while AR status was not a significant predictor of disease-free survival and overall survival as well as the luminal, basal-like, and HER2 immunohistochemical categories. These results may be explained by the fact that our study was concentrated on ER- and PR-negative tumors, which tend to be high-grade ductal carcinomas with more advanced stage at diagnosis and a high rate of proliferation. On the other hand, this study was a retrospective investigation, and our survival curves may reflect innate characteristics of the tumors since that treatment has not been influenced by AR expression, and all patients had received standard therapies. In fact, the therapeutic approach to BCs in the middle of the 1990s was non-tailored as suggested by the more recent treatment guidelines. Although AR is expressed in almost 50% of ER-negative tumors, the functional role of AR in BC remains unclear; further exploration of this area could expand the repertoire of potential treatment for patient with ER-negative breast cancer [55].
We can conclude that AR is frequently expressed in ER- and PR-negative poorly differentiated (G3) BCs and that expression of HER2 and AR is highly correlated (p < 0.005). In addition, a notable finding was that the majority of AR-positive BCs were lymph node positive. The frequent expression of AR and common coexpression of AR and HER2 oncoprotein in ER- and PR-negative BCs raise the important question of the role of AR and HER2 in the pathogenesis and treatment of ER- and PR-negative BCs. Further investigations of AR in ER- and PR-negative BCs could provide more information concerning the involvement of AR in the pathogenesis of breast cancer and the potential role in clinical management of women with ER- and PR-negative breast carcinomas.
References
Ellis LM, Wittliff JL, Bryant MS et al (1989) Correlation of estrogen, progesterone, and androgen receptors in breast cancer. Am J Surg 157:577–581
Lea OA, Kvinnsland S, Thorsen T et al (1989) Improved measurement of androgen receptors in human breast cancer. Cancer Res 49:7162–7167
Kimura N, Mizokami A, Oonuma T et al (1993) Immunocytochemical localization of androgen receptor with polyclonal antibody in paraffin-embedded human tissue. J Histochem Cytochem 41:671–678
Hall RE, Aspinall JO, Horsfall DJ et al (1996) Expression of the androgen receptor and an androgen-responsive protein, apolipoprotein D, in human breast cancer. Br J Cancer 74:1175–1180
Kuenen-Boumeester V, Van Der Kwast TH, Claassen CC et al (1996) The clinical significance of androgen receptors in breast cancer and their relation to histological and cell biological parameters. Eur J Cancer 32A:1560–1565
Bièche I, Parfait B, Tozlu S et al (2001) Quantitation of androgen receptor gene expression in sporadic breast tumors by real-time RT-PCR: evidence that MYC is an AR-regulated gene. Carcinogenesis 22:1521–1526
Brys M, Wojcik M, Romanowicz-Makowska H et al (2002) Androgen receptor status in female breast cancer: RT-PCR and Western blot studies. J Cancer Res Clin Oncol 128:85–90
Agoff SN, Swanson PE, Linden H et al (2003) Androgen receptor expression in estrogen receptor-negative breast cancer. Immunohistochemical, clinical and prognostic associations. Am J Clin Pathol 120:725–731
Moinfar F, Okcu M, Tsybrovskyy O et al (2003) Androgen receptors frequently are expressed in breast carcinomas. Potential relevance to new therapeutic strategies. Cancer 98:703–711
Riva C, Dainese E, Caprara G et al (2005) Immunohistochemical study of androgen receptors in breast carcinoma. Evidence of their frequent expression in lobular carcinoma. Virchows Arch 447:695–700
Narita D, Raica M, Suciu C et al (2006) Immunohistochemical expression of androgen receptor and prostate-specific antigen in breast cancer. Folia Histochem Cytobiol 44:165–172
Kuenen-Boumeester V, Van Der Kwast TH, Van Putten WLJ et al (1992) Immunohistochemical determination of androgen receptors in relation to oestrogen and progesterone receptors in female breast cancer. Int J Cancer 52:581–584
Soreide JA, Lea OA, Varhaug JE et al (1992) Androgen receptors in operable breast cancer: relation to other steroid hormone receptors, correlation to prognostic factors and predictive value for effect of adjuvant tamoxifen treatment. Eur J Surg Oncol 18:112–118
Isola JJ (1993) Immunohistochemical demonstration of androgen receptor in breast cancer and its relationship to other prognostic factors. J Pathol 170:31–35
Rakha EA, El-Sayed ME, Green AR et al (2007) Breast carcinoma with basal differentiation: a proposal for pathology definition based on basal cytokeratin expression. Histopathology 50:434–438
Bayer-Garner IB, Smoller B (2000) Androgen receptors: a marker of increase sensitivity for identifying breast cancer in skin metastasis of unknown primary site. Mod Pathol 13:119–122
Bryan RM, Mercer RJ, Bennett RC et al (1984) Androgen receptors in breast cancer. Cancer 54:2436–2440
Schippinger W, Regitnig P, Dandachi N et al (2006) Evaluation of the prognostic significance of androgen receptor expression in metastatic breast cancer. Virchows Arch 449:24–30
Perou CM, Sørlie T, Eisen MB et al (2000) Molecular portraits of human breast tumours. Nature 406:747–752
Sørlie T, Perou CM, Tibshirani R et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 98:10869–10874
Sørlie T, Tibshirani R, Parker J et al (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A 100:8418–8423
El-Rehim A, Ball G, Pinder SE et al (2005) High-throughput protein expression analysis using tissue microarray technology of a large well-characterised series identifies biologically distinct classes of breast cancer confirming recent cDNA expression analyses. Int J Cancer 116:340–350
Mattie MD, Benz CC, Bowers J et al (2006) Optimized high-throughput microRNA expression profiling provides novel biomarker assessment of clinical prostate and breast cancer biopsies. Mol Cancer 5:24
Farmer P, Bonnefoi H, Becette V et al (2005) Identification of molecular apocrine breast tumours by microarray analysis. Oncogene 24:4660–4671
Quenel N, Wafflart J, Bonichon F et al (1995) The prognostic value of c-erbB2 in primary breast carcinomas: a study on 942 cases. Breast Cancer Res Treat 35:283–291
Hubbard AL, Doris CP, Thompson AM et al (1994) Critical determination of the frequency of c-erbB-2 amplification in breast cancer. Br J Cancer 70:434–439
Ross JS, Fletcher JA (1999) HER-2/neu (c-erb-B2) gene and protein in breast cancer. Am J Clin Pathol 112:S53–S67
Doane AS, Danso M, Lal P et al (2006) An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene 25:3994–4008
Liegl B, Horn LC, Moinfar F (2005) Androgen receptors are frequently expressed in mammary and extramammary Paget’s disease. Mod Pathol 18:1283–1288
Naderi A, Hughes-Davies L (2008) A functionally significant cross-talk between androgen receptor and ErbB2 pathways in estrogen receptor negative breast cancer. Neoplasia 10:542–548
Rakha EA, El-Sayed ME, Green AR et al (2007) Prognostic markers in triple-negative breast cancer. Cancer 109:25–32
Greene FL, Page DL, Fleming ID et al (2002) The AJCC cancer staging manual, 6th edn. Springer, New York, pp 257–281
Tavassoli FA, Devilee P (2003) Pathology & genetics tumours of the breast and female genital organs. WHO, Albany
Elston CW, Ellis IO (2002) Pathological prognostic factors in breast cancer I The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 41(3):151–153, Histopathology 1991;403–410
Laakso M, Loman N, Borg A, Isola J et al (2005) Cytokeratin 5/14-positive breast cancer: true basal phenotype confined to BRCA1 tumors. Mod Pathol 18:1321–1328
Laakso M, Tanner M, Nilsson J et al (2006) Basoluminal carcinoma: a new biologically and prognostically distinct entity between basal and luminal breast cancer. Clin Cancer Res 12:4185–4191
Nahleh Z (2008) Androgen receptor as a target for the treatment of hormone receptor-negative breast cancer: an unchartered territory. Future Oncol 4:15–21
Zaucha R, Sosinska-Mielcarek K, Jassem J (2004) Long-term survival of a patient with primarily chemo-resistant metastatic breast cancer treated with Medroxyprogesterone acetate. Breast 13:321–324
Otani S, Toyota N, Nozaka K et al (2004) Successful combination therapy with 5′-DFUR and MPA for breast cancer with spinal and vertebral metastases. Gan To Kagaku Ryoho 31:2151–2153
Focan C, Beauduin M, Majos F et al (2004) High-dose oral medroxyprogesterone acetate or tamoxifen as adjuvant hormone therapy for node-negative early-stage breast cancer: randomized trial with 7-year update. Clin Breast Cancer 5:136–141
Scawn R, Shousha S (2002) Morphologic spectrum of estrogen receptor-negative breast carcinoma. Arch Pathol Lab Med 126:325–330
Putti TC, El-Rehim DM, Rakha EA et al (2005) Estrogen receptor-negative breast carcinomas: a review of morphology and immunophenotypical analysis. Mod Pathol 18:26–35
Allred DC, Harvey JM, Berardo M et al (1998) Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 11:155–168
Yeh S, Lin HK, Kang HY et al (1999) From HER/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. Proc Natl Acad Sci U S A 96:5458–5463
Meng TC, Lee MS, Lin MF (2000) Interaction between protein tyrosine phosphatase and protein tyrosine kinase is involved in androgen-promoted growth of human prostate cancer cells. Oncogene 19:2664–2677
Liu Y, Majumder S, McCall W et al (2005) Inhibition of HER-2/neu kinase impairs androgen receptor recruitment to the androgen responsive enhancer. Cancer Res 65:3404–3409
Guo Z, Dai B, Jiang T et al (2006) Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell 66(10):309–319
Mellinghoff IK, Vivanco I, Kwon A et al (2004) HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell 6:517–527
Shao D, Lazar MA (1999) Modulating nuclear receptor function: may the phos be with you. J Clin Invest 103:1617–1618
Niemeier LA, Dabbs DJ, Beriwal S et al (2010) Androgen receptor in breast cancer: expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod Pathol 23:205–212
Park S, Koo J, Park HS et al (2010) Expression of androgen receptors in primary breast cancer. Ann Oncol 21:488–492
Jones C, Nonni AV, Fulford L et al (2001) CGH analysis of ductal carcinoma of the breast with basaloid/myoepithelial cell differentiation. Br J Cancer 85:422–427
Nielsen TO, Hsu FD, Jensen K et al (2004) Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 10:5367–5374
Rakha EA, Putti TC, Abd El-Rehim DM et al (2006) Morphological and immunophenotypic analysis of breast carcinomas with basal and myoepithelial differentiation. J Pathol 208:495–506
Moe RE, Anderson BO (2007) Androgens and androgen receptors: a clinically neglected sector in breast cancer biology. J Surg Ong 95:437–439
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Research support
This study has been in part supported by a grant from the University of Insubria, Varese, Italy.
Rights and permissions
About this article
Cite this article
Micello, D., Marando, A., Sahnane, N. et al. Androgen receptor is frequently expressed in HER2-positive, ER/PR-negative breast cancers. Virchows Arch 457, 467–476 (2010). https://doi.org/10.1007/s00428-010-0964-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-010-0964-y