Abstract
Regional lymph node metastasis in gastric cancer is a definitive indicator of the patient’s prognosis. The goal of this study was to identify the predictors for lymph node metastasis among all the possible histopathological parameters, especially by conducting an objective discrimination of the lymphatic and blood vessels. A total of 210 resected primary gastric cancers with or without lymph node metastasis were evaluated based on the conventional histopathological parameters together with immunohistochemistry using antisera-recognizing lymphatic endothelial hyaluronan receptor-1 (LYVE-1), von Willebrand factor, and lymphangiogenesis promoter vascular endothelial growth factor-C (VEGF-C) antibodies. A multivariate regression analyses of the results indicated that only lymphatic invasion was a significant independent predictor of lymph node metastasis at any stage of cancer invasion. VEGF-C expression was partially related to lymph node metastasis in early gastric cancer. The identification of lymphatic invasion by LYVE-1 antibody is therefore useful to predict regional lymph node metastasis in gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Regional lymph node metastasis is an independent prognostic factor in patients with gastric cancer [1]. Among the routes by which cancer cells metastasize to the regional lymph nodes, metastasis through the lymphatics at the primary site is a major candidate. Conventional histopathological examinations reveal many small lymphatics and blood capillaries at the primary site of gastric cancer; however, the objective discrimination of these two types of vessels is often difficult when using only hematoxylin and eosin (HE) and Victoria blue staining. Recently, several immunohistochemical markers that recognize the endothelium of a lymphatic vessel have been successively developed, including LYVE-l [2], D2-40 [3], podoplanin [4], and prox-1 [5]. These markers are practically useful to demonstrate the relationship between cancer invasion and metastasis in early gastric cancer [6]. In addition, an immunochemical analysis with the lymphatic endothelial hyaluronan receptor-1 (LYVE-1) antibody in early gastric cancer reveals that lymphatic invasion by cancer cells predicts regional lymph node metastasis [7]. However, the relationship between the lymphatic invasion and lymph node metastasis should be universally explored at any stage of gastric cancer invasion because the characteristics of the local anatomical structure of the gastric wall may influence the route of gastric cancer metastasis.
Although angiogenesis as well as the dissemination of malignant cells through blood vessels play major roles in tumor growth [8, 9], lymphangiogenesis and the development of lymphatic vessels are directly responsible for the lymphatic spread of tumors [10, 11]. Various molecules belonging to the vascular endothelial growth factor (VEGF) family are thought to regulate the lymphatic vessel development [12, 13]. Some studies have demonstrated that VEGF factor C (VEGF-C) expression is closely associated with the prognosis of breast [14], lung [15], stomach [16], and colorectal [17] cancers. VEGF mediates angiogenesis and vascular permeability [18]. On the other hand, similar up-regulation of VEGF-C can lead to lymphangiogenesis, intralymphatic tumor growth, and lymph node metastasis [19]. However, in gastric cancer, there is no evidence confirming the relationship between VEGF-C expression and lymphatic invasion using immunohistochemistry.
The present study attempted to explore the relationship between lymphatic invasion and regional lymph node metastasis in conjunction with several histopathological parameters at any stage of gastric cancer invasion, focusing on the discrimination of the lymphatics and blood vessels, using immunohistochemistry. In addition, the possible role of VEGF-C-mediated lymphangiogenesis and the spread of gastric cancer were investigated.
Materials and methods
Materials
The surgically resected specimens of gastric cancer with the regional lymph nodes were obtained from a total of 210 patients. The patients were diagnosed and treated with a curative gastrectomy at Toho University Omori Medical Center, Saiseikai Kanagawa-Ken Hospital and Hiratsuka Municipal Hospital, Japan, between 1989 and 2005.
Curative surgery was defined as the removal of all gross cancer and the microscopic evaluation of the tumor-negative surgical margin. The patients underwent a lymph node dissection of group 1 and group 2 according to the Japanese Classification of Gastric Carcinoma [20], which were located at the nearest and the second nearest area of the primary cancer site. Lymph node metastasis was considered to be present when at least one lymph node invaded by cancer cells was observed. The patients were free from other types or degrees of invasion, distant visceral metastases, and complications due to other conditions. None of the patients underwent either preoperative chemotherapy or radiotherapy. Written informed consent to use the tissue specimens was provided by all patients at each medical institute.
In this study, as we considered that the route of gastric cancer metastasis may be influenced by the characteristics of the local anatomical structure of the gastric wall, we aimed to explore the relationship between the lymphatic invasion and lymph node metastasis. The patients were divided into six groups, including the following: cancer invasion confined to within the mucosa or submucosa (SM), with or without metastasis; tumor invasion up to the external rim of the propriate muscle layer (MP), with or without metastasis; and tumor invasion reaching subserosal layers (SS) with or without metastasis. We assigned the latest 35 consecutive cases that met the definition for each group from that surgically resected specimens previously described. We combined the cancer invasion confined within the mucosa or submucosa invasion as a single group according to the World Health Organization (WHO) classification with the Japanese modification [21]. The number of mucosal invasion cancers in the group with lymph node metastasis was 5 out of 35, while for the group without it, the number was 20 out of 35.
All of surgically resected stomachs were opened along the greater curvature, pinned on a cork board, and fixed in 10% formalin. After a careful gross inspection of the location of the primary cancer, the tumor size was measured on the length of its major axis. Each tumor was cut into 4-mm slices parallel to the major axis of the specimen and to the minor axis crossing the halfway point of the long axis. The slices were embedded in paraffin, cut into 3-μm-thick sections, and treated by double staining with Victoria blue and HE dyes to aid the identification of elastic fibers in the blood vessel structures, especially with regard to the veins.
Immunohistochemistry
The primary antibodies for immunohistochemistry used in this study were anti-LYVE-1, which was previously raised against a LYVE-1 polypeptide fragment [2], anti-human von Willebrand factor (vWF) antibody (Dako, Carpinteria, CA, USA), and anti-VEGF-C (Zymed Lab. Inc., South San Francisco, CA, USA). Immunohistochemistry with LYVE-1, vWF, and VEGF-C antibodies were carried out after deparaffinizing and dehydration of the thin-sectioned specimens. For LYVE-1 and VEGF-C, the sections were pretreated with 10 mM citrate buffer solution (pH 6.0) for 15 min at 95°C. They were then treated with 40 μg/ml proteinase K (Dako) for 3 min at room temperature.
After washing in distilled water, the sections were incubated with LYVE-1 (1:200 dilution) and VEGF-C (1:200 dilution) antibody for 1.5 h at room temperature, washed in Tris-buffered saline (TBS) containing Tween 20 and treated with the Catalyzed Signal Amplification II kit (Dako) according to the manufacturer’s instructions. For vWF immunohistochemistry, the sections were pretreated with 10 mM citrate buffer solution (pH 6.0) for 15 min at 95°C. After washing in TBS, they were treated with 3% hydrogen peroxide for 10 min and then with 3% non-fat dried milk in TBS containing Tween 20 for 30 min. The sections were then incubated with vWF antibody (1:25 dilution) for 2 h at room temperature. A further wash in TBS was followed by treatment with peroxidase-labeled polymer conjugated to goat and anti-rabbit or anti-mouse immunoglobulins (Envision+kit; Dako) for 30 min at room temperature. The immunostaining was visualized with diaminobenzidine tetrahydrochloride, followed by counterstaining with hematoxylin.
Histopathological variables
Each case was evaluated for the histopathological classification of primary cancer, the presence of vessel invasion, vessel density, and VEGF-C expression in cancer cells. The tumor type was assessed by examining sections that were stained with H&E and VB. The histopathological classification was determined according to the WHO classification with the Japanese modification. To determine the grade of differentiation, the major histopathological type showing at the primary site was categorized as papillary adenocarcinoma, well differentiated, moderately differentiated, poorly differentiated adenocarcinoma, or signet ring cell carcinoma. For the statistical analysis, the former three types were combined as a low-grade malignancy group and the latter two types as a high-grade malignancy group, according to the conventionally accepted relationship between cancer typing and biological behavior. All the histopathological specimens were separately reviewed by the three pathologists (HM, YI, and IF), and the results were finally determined after consensus readings when they were different among them.
The location and number of vessel invasion was examined under the view field at a magnification of ×200. For this analysis, vascular invasion was defined as invasion and adherence of cancer cells to the inner surface of the lymphatic vessels determined by anti-LYVE-1 antibody or blood vessels determined by anti-vWF antibody with the use of Victoria blue-staining for the detection of the venous structure. Lymphatic and blood vessel invasion was considered to be present when at least one vessel invaded by cancer cells was observed in a given case. The location of the lymphatic and blood vessel invasion was also noted throughout the observation of the primary tumor.
To examine the lymphatic vessel density (LVD) by light microscopy, the immunostained sections were scanned along the tumor front margin at a low magnification of ×40, and the highly distinctive areas for lymphatic vessels with LYVE-1 antibody were selected. The number of lymphatic vessels lumens in these areas was counted in five view fields under a magnification of ×200. The average number of lymphatic vessels at each area was designated as the LVD. Blood vessel density (BVD) was determined with anti-vWF antibody, and their density was determined by the same procedures used for the LVD.
VEGF-C was evaluated according to the extent of staining as described by Siironen et al. [22]. Briefly, under the light microscope, when more than 10% of the primary cancer cells expressed VEGF-C in the cytoplasm of the cancer cells, they were recorded as positive.
Statistical analysis
Statistical analyses were performed using the chi-square test, Fisher’s exact test, and the Mann–Whitney’s U test to assess the significance of the impact of each subset of histopathological parameters on the lymph node status. Multivariate logistic regression analyses including these variables selected by backward elimination were also carried out to identify the independent predictors for lymph node metastasis. Any differences in the P value of less than 0.05 were considered to be statistically significant. All of the statistical analyses were examined using the StatView software program (SAS Institute, Raleigh, NC, USA).
Results
Comparison of each parameter between the node-negative and node-positive groups
A summary of the clinicopathologic characteristics of the patients in this study is shown in Table 1. The differences in the histopathological parameters between the node-negative and node-positive groups were indicated by the invasion depth in the gastric wall (Tables 2, 3, and 4). In all 15 cases on the SM invasion group with lymphatic invasion, cancer invasion reached the submucosa. The variables of age, sex, and the location of tumor were not always significant in conjunction with the lymph node status by the depth of cancer invasion in the following statistical treatments (data not shown). Therefore, those variables were omitted in the tables of the present study.
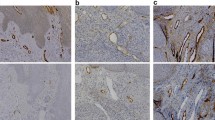
A significant difference was recognized between tumor size and regional lymph node metastasis in all the subsets by invasion depth using Mann–Whitney’s U test. The two varieties of vessel invasion were readily distinguished by LYVE-1 (Fig. 1) and vWF (Fig. 2) immunostaining. The frequency of lymphatic vessel invasion was significantly greater in the node-positive groups than the node-negative groups at any invasion depth, based on Fisher’s exact test. On the other hand, there was no significant relationship between blood vessel invasion and regional lymph node metastasis.
In all of the subsets of invasion depth, the low-grade malignancy group was predominant in the node-negative group, but there was no significant difference in cancer differentiation between the node-positive and node-negative groups as assessed by Fisher’s exact test. In addition, there was no significant difference in LVD and BVD between the node-positive and node-negative groups as assessed by Mann–Whitney’s U test. Although the frequency of VEGF-C expression in the node-positive groups tended to be greater than the node-negative groups, there was no significant difference for any invasion depth as assessed by Fisher’s exact test.
Logistic regression analyses of histopathological parameters for lymph node metastasis
The results of the multivariate analyses of the histopathological parameters for lymph node metastasis are shown in Table 5. In the SM invasion group, the size of tumor (odds ratio, 1.043; P = 0.0192), lymphatic invasion, and positive VEGF-C expression (odds ratio, 5.184;P = 0.0225) were significant independent predictors of lymph node metastasis by the multivariable analyses. In the MP invasion group, the size of the tumor and lymphatic invasion (odds ratio, 6.174; p = 0.0032) were significant independent predictors of lymph node metastasis by the multivariable analyses. In the SS invasion group, lymphatic invasion (odds ratio, 3.036; p = 0.0344) and LVD (odds ratio, 0.977; p = 0.0429) were significant independent predictors for lymph node metastasis by the multivariable analyses. In all the cases, the size of tumor (odds ratio, 1.044; p = 0.0038) and lymphatic invasion (odds ratio, 4.328; p = 0.0092) were significant independent predictors for lymph node metastasis based on multivariable analyses. Only lymphatic invasion was the consistent significant predictor for lymph node metastasis in any depth of tumor invasion based on the multivariate analysis.
Sites of lymphatic vessel invasion within the gastric wallby the invasion depth of cancer
Although the lymphatic and blood vessels were readily distinguished by LYVE-1 and vWF immunostaining in the normal part of the resected stomach, they frequently appeared to be affected and destroyed within the tumors. The spatial distribution of lymphatic vessel invasion within the gastric wall by the invasion depth of all the 78 lymphatic invasion-positive cases is indicated in Table 6. For this analysis, the submucosal layer was arbitrarily subdivided into two groups: SM1, in which cancer invaded up to the upper half of the submucosa, and SM2, in which cancer invaded the whole layer of the submucosa. In the SM invasion group, lymphatic invasion was detected in the mucosa (6.7%, 1/15), the SM1 (93.3%, 14/15), and the SM2 (26.7%, 4/15). In the MP invasion group, lymphatic invasion was detected in the SM1 (79.1%, 19/24), the SM2 (16.7%, 4/24), and the muscularis propria (8.3%, 2/24). In the SS invasion group, lymphatic invasion was detected in the mucosa (5.1%, 2/39), SM1 (66.7%, 26/39), SM2 (17.9%, 7/39), and the muscularis propria (41.0%, 16/39). Accordingly, in any depth of cancer invasion, lymphatic invasion was most frequently detected in the SM1 layer.
Comparison of each parameter with the expression of VEGF-C
In the stomach, VEGF-C expression was mainly present at the tumor front (Fig. 3) and usually detected as small heterogeneous nodules in the cytoplasm of the cancer cells. In addition, it was also weakly present in the cytoplasm of some normal ductal cells. In the SM invasion group, the VEGF-C expression was positive in 20 cases (28.6%, 20/70). As for the PM invasion group, VEGF-C expression was detected in 29 cases (41.4%, 29/70). In the SS invasion group, it was detected in 29 cases (41.4%, 29/70). Although the frequency of the positive expression of VEGF-C in the node-positive cases was higher than in node-negative groups in any group of invasion depth, there was no statistical significance between them.
A further comparison of histopathological variables, such as the location of the primary cancer, the tumor size, grade of cancer differentiation, blood vessel invasion, LVD, and BVD, was made with the expression of VEGF-C. However, none of the variables defined were significant. In addition, the depth of cancer invasion was also not significantly related to the frequency of VEGF-C expression.
Discussion
The present study demonstrated the significance of lymphatic vessel invasion on regional lymph node metastasis in gastric cancer using LYVE-1 and vWF antibodies for the objective discrimination of the lymphatic and blood vessels. The results of a multivariate analysis indicated that only the lymphatic invasion by cancer cells was consistently recognized as an independent histopathological predictor of regional lymph node metastasis at any depth of tumor invasion. The macroscopic issue of tumor size and microscopic VEGF-C expression in cancer cells were also significant; however, their roles seemed to differ with regard to the stage of cancer invasion. Since the lymphatic vessels are a route to lymph node, it would be reasonable to conclude that only lymphatic invasion is a notable predictor for lymph node metastasis. In the tumor, node, metastasis system, the extent of lymph node metastasis is one of the essential factors for assessing the extent of disease, determining prognosis, and establishing the therapy strategies in gastric cancer patients.
Recently, several immunohistochemical markers recognizing the lymphatic vessel endothelium have been successively identified, including LYVE-l [2], D2-40 [3], podoplanin [4], prox-1[5], desmoplakin [23], and D6 [24]. In addition, a more specific LYVE-1 antibody has been raised from the epitope that is a part of the polypeptide-fragment of the original LYVE-1 [2]. This antibody has enabled the objective detection of lymphatics in studies of the normal prostate [25], atherosclerosis [26], early-stage gastric cancer [7], and early-stage colorectal cancer [27]. In this study, it also enabled the distinctive discrimination of the lymphatic vessels from the blood vessels at any stage of gastric cancer.
The lymphatic spread of cancer is assumed to occur through the permeating of cancer cells into the peritumorous lymphatics, eventually reaching the regional lymph nodes. The close contact between tumor cells and lymphatics is thought to be an initial step in lymphatic metastasis. It is reasonable that cancer cells invading the submucosal layer easily penetrate the lymphatic lumen because most of the lymphatics are present just beneath the lamina muscularis mucosae in the normal stomach. The depth and total volume of submucosal invasion by the gastric cancer are closely correlated with lymph node metastasis [28–30]. In the current study, lymphatic invasion by cancer cells was most frequently found in the submucosal layer, and its frequency was highest in gastric cancer invading within the submucosal layer. The current results suggest that lymphatic vessels in the submucosal layer provide the main entry source for lymph node metastasis through lymphatic invasion even in advanced gastric cancers.
The lymphatic vessels at the tumor margin probably play a functional role in the drainage to the lymph nodes [31]. Therefore, in this study, the number of lumens in the lymphatic vessels was counted at the peripheral part of the tumor. The results, however, indicated no significant difference in the LVD between the node-positive and node-negative groups in the SM and MP invasion groups. On the other hand, in the SS invasion group, the results of a multivariate analysis demonstrated the LVD to be a significant independent predictor for lymph node metastasis. One reason why there are some differences between the current results and those in the previous studies [32–34] is due to the fact that the number of lymphatic vessels tend to decrease in the central portion of the primary cancer. The cancer cells may invade or destroy vessels in the central portion, while newly proliferating lymphatic vessel may provide the entry to cancer invasion at the peripheral area of the tumor, especially in the submucosal layer. Such differences in the decrease or increase of the number of the lymphatic vessels at each site may collectively result in the determination of the value.
In conjunction with the LVD, vascular or lymphatic metastasis may also be regulated through angiogenesis and lymphangiogenesis mediated by the growth factors expressed by the cancer cells. Among these, VEGF-C has been recently examined with regard to the relationship of the lymphatic vessels with cancer cell invasion. VEGF-C expression from cancer cells promotes lymphangiogenesis [35], which may provide the route for cancer invasion. Despite a significant correlation between lymph node metastasis and VEGF-C expression in esophageal [36], gastric, and colorectal cancers [37], such a relationship was not evident in the present study under the objective definitions of the location and estimation for VEGF-C expression. This indicates that VEGF-C expression from cancer cells does not seem to always promote lymphangiogenesis in the stroma, while cancer cells actively invade and damage the stroma.
Apart from the potentiality of lymphatic invasion through lymphangiogenesis, VEGF-C expression from cancer cells has been directly considered in conjunction with positive lymph node metastasis in cancers of the breast [38], thyroid [39], lung [40], and prostate [41]. In gastric cancer, based on a similar correlation of VEGF-C with lymph node metastasis, patients with high expression of VEGF-C show a poor prognosis [42, 43]. The present results indicated that significant up-regulation of VEGF-C from cancer cells was recognized in the patients with regional lymph node metastasis, but only in early gastric cancer. This finding is in agreement in part with that described in a previous report [44], thus suggesting the possibility that VEGF-C up-regulation may promote lymph node metastasis via lymphatic vessels in patients with gastric cancer.
In the present study, as lymphatic invasion is usually found in the submucosal layer at any stage of gastric cancer, such detection therefore considered to be essential to predict regional lymph node metastasis in gastric cancer. It is especially important for the detection of lymphatic invasion to observe the submucosa around the primary tumor in patients with gastric cancer. The application of an endoscopic resection for early gastric cancer has been extended to the larger and more deeply invaded cases on the basis of guideline criteria [45]. Even in such cases, we cannot rule out the possibility of regional lymph node metastasis when we encounter the lymphatic invasion in the gastric mucosa or submucosa. Apart form these situations, when lymphatic invasion by gastric cancer is demonstrated in the gastrectomied cases of PM and SS invasions without any regional lymph node metastasis, we will carefully follow the possibility of any regional lymph node metastasis in those patients. If we can predict metastasis to the lymph node from a lymphatic vessel invasion in the primary site, it may be an important tool for choosing the optimal treatment protocol for cancer recurrence. Furthermore, the early identification of patients who is such at high risk and the appropriate treatment may significantly improve the survival rate.
In this study, LYVE-1 immunohistochemistry is therefore considered to be useful in predicting lymph node metastasis from gastric cancer with various depths of invasion.
References
Bando E, Yonemura Y, Taniguchi K et al (2002) Outcome of ratio of lymph node metastasis in gastric carcinoma. Ann Surg Oncol 9:775–784
Akishima Y, Ito K, Zhang L et al (2004) Immunohistochemical detection of human small lymphatic vessels under normal and pathological conditions using the LYVE-1 antibody. Virchows Arch 444:153–157
Kahn HJ, Marks A (2002) A new monoclonal antibody, D2-40, for detection of lymphatic invasion in primary tumors. Lab Invest 82:1255–1257
Wigle JT, Oliver G (1999) Prox1 function is required for the development of the murine lymphatic system. Cell 98:769–778
Ebata N, Nodasaka Y, Sawa Y et al (2001) Desmoplakin as a specific marker of lymphatic vessels. Microvasc Res 61:40–48
Sako A, Kitayama J, Ishikawa M et al (2006) Impact of immunohistochemically identified lymphatic invasion on nodal metastasis in early gastric cancer. Gastric Cancer 9:295–302
Fujimoto A, Ishikawa Y, Akishima-Fukasawa Y et al (2007) Significance of lymphatic invasion on regional lymph node metastasis in early gastric cancer using LYVE-1 immunohistochemical analysis. Am J Clin Pathol 27:82–88
Shore VH, Wang TH, Wang CL et al (1997) Vascular endothelial growth factor, placenta growth factor and their receptors in isolated human trophoblast. Placenta 18:657–665
Cao Y, Linden P, Shima D et al (1996) In vivo angiogenic activity and hypoxia induction of heterodimers of placenta growth factor/vascular endothelial growth factor. J Clin Invest 98:2507–2511
Duff SE, Li C, Jeziorska M et al (2003) Vascular endothelial growth factors C and D and lymphangiogenesis in gastrointestinal tract malignancy. Br J Cancer 89:426–430
Pepper MS, Tille JC, Nisato R et al (2003) Lymphangiogenesis and tumor metastasis. Cell Tissue Res 314:167–177
Joukov V, Pajusola K, Kaipainen A et al (1996) A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J 15:290–298
Jeltsch M, Kaipainen A, Joukov V et al (1997) Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 276:1423–1425
Jin Q, Hemminki K, Enquist K et al (2005) Vascular endothelial growth factor polymorphisms in relation to breast cancer development and prognosis. Clin Cancer Res 11:3647–3653
Koukourakis MI, Papazoglou D, Giatromanolaki A et al (2004) VEGF gene sequence variation defines VEGF gene expression status and angiogenic activity in non-small cell lung cancer. Lung Cancer 46:293–298
Liu DH, Zhang XY, Fan DM et al (2001) Expression of vascular endothelial growth factor and its role in oncogenesis of human gastric carcinoma. World J Gastroent 7:500–505
Yamamori M, Sakaeda T, Nakamura T et al (2004) Association of VEGF genotype with mRNA level in colorectal adenocarcinomas. Biochem Biophys Res Commun 325:144–150
Koukourakis MI, Papazoglou D, Giatromanolaki A et al (2004) VEGF gene sequence variation defines VEGF gene expression status and angiogenic activity in non-small cell lung cancer. Lung Cancer 46:293–298
Ferrara N (2002) VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer 2:795–803
Japanese Research Society for Gastric (1995) Japanese classification of gastric carcinoma, 1st edn. Kanehara, Tokyo
Sugano H, Nakamura K, Kato Y (1982) Pathological studies of gastric carcinoma. Acta Pathol Jpn 32(suppl 2):329–347
Siironen P, Ristimäki A, Narko K et al (2006) VEGF-C and COX-2 expression in papillary thyroid cancer. Endocr Relat Cancer 13:465–473
Nibbs RJ, Kriehuber E, Ponath PD et al (2001) The beta-chemokine receptor D6 is expressed by lymphatic endothelium and a subset of vascular tumors. Am J Pathol 158:867–877
Irjala H, Johansson EL, Grenman R et al (2001) Mannose receptor is a novel ligand for L-selectin and mediates lymphocyte binding to lymphatic endothelium. J Exp Med 194:1033–1042
Soh S, Ishii T, Sato E et al (2005) Topographic distribution of lymphatic vessels in the normal human prostate. Prostate 63:330–335
Nakano T, Nakashima Y, Yonemitsu Y et al (2005) Angiogenesis and lymphangiogenesis and expression of lymphangiogenic factors in the atherosclerotic intima of human coronary arteries. Hum Pathol 36:330–340
Ishikawa Y, Akishima-Fukasawa Y, Ito K et al (2008) Histopathologic determinants of regional lymph node metastasis in early colorectal cancer. Cancer 112:924–933
Cai J, Ikeguchi M, Maeta M et al (2000) Micrometastasis in lymph nodes and microinvasion of the muscularis propria in primary lesions of submucosal gastric cancer. Surgery 127:32–39
Kurihara N, Kubota T, Otani Y et al (1998) Lymph node metastasis of early gastric cancer with submucosal invasion. Br J Surg 85:835–839
Matsuzaki H, Kikuchi S, Kakita A (2003) Evaluation of the morphology of submucosal tumor invasion and its volume in early gastric cancer. In Vivo 17:41–44
Padera TP, Kadambi A, di Tomaso E et al (2002) Lymphatic metastasis in the absence of functional intratumor lymphatics. Science 296:1883–1886
Wang TB, Deng MH, Qiu WS et al (2007) Association of serum vascular endothelial growth factor-C and lymphatic vessel density with lymph node metastasis and prognosis of patients with gastric cancer. World J Gastroenterol 28:1794–1797
Yuanming L, Feng G, Lei T et al (2007) Quantitative analysis of lymphangiogenic markers in human gastroenteric tumor. Arch Med Res 38:106–112
Nakamura Y, Yasuoka H, Tsujimoto M et al (2006) Importance of lymph vessels in gastric cancer: a prognostic indicator in general and a predictor for lymph node metastasis in early stage cancer. J Clin Pathol 59:77–82
Hachisuka T, Narikiyo M, Yamada Y et al (2005) High lymphatic vessel density correlates with overexpression of VEGF-C in gastric cancer. Oncol Rep 13:733–737
Kitadai Y, Amioka T, Haruma K et al (2001) Clinicopathological significance of vascular endothelial growth factor (VEGF)-C in human esophageal squamous cell carcinomas. Int J Cancer 93:662–666
Parr C, Jiang WG (2003) Quantitative analysis of lymphangiogenic markers in human colorectal cancer. Int J Oncol 23:533–539
Kurebayashi J, Otsuki T, Kunisue H et al (1999) Expression of vascular endothelial growth factor (VEGF) family members in breast cancer. Jpn J Cancer Res 90:977–981
Fellmer PT, Sato K, Tanaka R et al (1999) Vascular endothelial growth factor-C gene expression in papillary and follicular thyroid carcinomas. Surgery 126:1056–1062
Ohta Y, Nozawa H, Tanaka Y et al (2000) Increased vascular endothelial growth factor and vascular endothelial growth factor-c and decreased nm23 expression associated with microdissemination in the lymph nodes in stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 119:804–813
Tsurusaki T, Kanda S, Sakai H et al (1999) Vascular endothelial growth factor-C expression in human prostatic carcinoma and its relationship to lymph node metastasis. Br J Cancer 80:309–313
Yonemura Y, Endo Y, Fujita H et al (1999) Role of vascular endothelial growth factor C expression in the development of lymph node metastasis in gastric cancer. Clin Cancer Res 5:1823–1829
Takahashi A, Kono K, Itakura J et al (2002) Correlation of vascular endothelial growth factor-C expression with tumor-infiltrating dendritic cells in gastric cancer. Oncology 62:121–127
Kitadai Y, Kodama M, Cho S et al (2005) Quantitative analysis of lymphangiogenic markers for predicting metastasis of human gastric carcinoma to lymph nodes. Int J Cancer 115:388–392
Gotoda T (2007) Endoscopic resection of early gastric cancer. Gastric Cancer 10:1–11
Acknowledgment
The authors are grateful to Ms. E. Ohnishi and Ms. T. Sato for their skillful technical assistance throughout the study. This work was partly supported by the Grants-in-Aid for Scientific Research (no. 19590368) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morita, H., Ishikawa, Y., Akishima-Fukasawa, Y. et al. Histopathological predictor for regional lymph node metastasis in gastric cancer. Virchows Arch 454, 143–151 (2009). https://doi.org/10.1007/s00428-008-0717-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-008-0717-3