Abstract
Alpha-methylacyl-CoA racemase (AMACR, p504S), an enzyme involved in cellular energy metabolism by the oxidation of branched-chain fatty acids, is a biomarker that is known to be overexpressed in prostatic and colorectal carcinoma as well as in papillary renal cell carcinoma. We aimed to correlate its immunohistochemically detected expression with histopathological grading in noninvasive bladder cancer in order to hint at a so far unknown role of AMACR in the pathobiology of this tumor entity. Therefore, a cohort of 163 patients (mean age 65.3 years) diagnosed with noninvasive bladder cancer was immunohistochemically investigated in terms of AMACR expression. There was variable positive AMACR staining in 52 (31.9%) of the cases investigated. All tumors were graded by three independent clinical histopathologists according to the 1973 World Health Organization (WHO) and the 1998 WHO/International Society of Urological Pathology (ISUP) system. We found a significant positive correlation between AMACR expression and higher tumor grades using both histopathologic grading schemes. These novel findings clearly allow including high-grade noninvasive bladder carcinomas in the group of AMACR-positive neoplasms and might reflect a so far unknown role of AMACR racemase in the pathobiology and tumor cell energy metabolism of the latter tumor entity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Noninvasive bladder cancer is a common neoplasm with a male preponderance frequently encountered in histopathological practice. For 2008, 68,810 new cases of bladder cancer (noninvasive and invasive) are expected in the USA alone [4]. Typically, noninvasive bladder cancer is frequently seen to recur after surgical resection [17] and can progress to invasive neoplasms, which have a comparatively worse prognosis. Since it is difficult at present to reliably predict the individual course of the disease, several studies have recently addressed the issue of identifying new prognostic biomarkers.
Alpha-methyl-CoA racemase (AMACR, p504s), a biomarker with diagnostic potential in various solid tumors, is a peroxisomal and mitochondrial enzyme involved in the oxidation of branched-chain fatty acids and cholesterol metabolites [1]. AMACR is widely used as a positive marker of prostate cancer and has additionally been shown to bear prognostic significance in prostatic and in colorectal cancer [7, 10]. Lately, AMACR immunostaining has also been proposed as a diagnostically useful marker in terms of discriminating dysplastic from reactive epithelium in Barrett’s esophagus [2, 8].
So far, little is known about AMACR expression in noninvasive bladder cancer. This is the first comprehensive study aiming to correlate the immunohistochemically detected AMACR expression with both histopathologic grading schemes established in noninvasive bladder cancer (1973 World Health Organization and the 1998 WHO/International Society of Urological Pathology (ISUP) system) in order to shed light on the so far neglected role of AMACR in the pathobiology of noninvasive bladder cancer.
In order to compare AMACR staining results with other classic but not entirely validated markers, serial sections from all samples were additionally stained with monoclonal antibodies directed against p53 and Ki67. No attempt was made to correlate marker expression with the risk of tumor progression and/or recurrence during postsurgical follow-up.
Materials and methods
Selection of tumor samples
Retrospective computerized database analysis was performed in order to identify all patients with newly diagnosed noninvasive bladder cancer who underwent transurethral surgical resection (TUR) at the Carl-Thiem Klinikum Cottbus, Germany, between 1997 and 2004. This analysis yielded a total of 147 individuals with the following distribution: 102 tumors were graded as G1, 42 tumors were graded as G2, and only three tumors were graded as G3, respectively. In order to expand the latter “critical” group, a thorough retrospective medical chart review was performed at the archives of the HELIOS Klinikum Bad Saarow (the former HUMAINE Klinikum), Germany. Surveying the period of time between 1977 and 2007, another 27 archived wax-embedded noninvasive tumors graded as G3 were subsequently retrieved from the Department of Pathology, HELIOS Klinikum Bad Saarow to be also investigated in this study. Therefore, wax-embedded archived tissues sampled from a total of 174 patients diagnosed with noninvasive bladder cancer (102 G1, 42 G2, and 30 G3 tumors; 144 low-grade and 30 high-grade tumors according to WHO/ISUP 1998) were retrieved for this study. Notably, papillary urothelial neoplasms of low malignant potential (PUNLMP) and invasive bladder cancer were not assessed.
Central review of histology
Hematoxylin and eosin (H&E)-stained tissue sections were reviewed for histopathologic stage and grade by three independent clinical histopathologists (S.G., G.K., and S.S.). The tumors, all pTa according to the latest TNM classification [11], were graded by the 1973 World Health Organization (WHO) and by the 1998 WHO/International Society of Urological Pathology (ISUP) systems. In equivocal cases, final judgement of grading to be used for statistical analyses was consistently performed by the reference histopathologist of this study (S.S.).
Tissue microarray construction
Formalin-fixed wax-embedded surgically resected tissues were retrieved from the surgical pathology archives at Carl-Thiem Klinikum Cottbus, Germany and from the HELIOS Klinikum Bad Saarow, Germany. Suitable areas for tissue retrieval were marked on H&E-stained sections, punched out of the paraffin block (1.5 mm punch diameter), and one punch from each tumor investigated was subsequently inserted into a recipient block as described elsewhere [6]. The tissue arrayer was purchased from Beecher Instruments (Woodland, USA). The tissue array was cut into 4 μm sections without any sectioning aids like tapes or additionally coated slides. Eleven samples (6.3%) were lost during tissue arraying and sectioning. Therefore, this study was finally conducted upon a total of 163 individuals (Table 1).
Immunohistochemistry
Immunohistochemical staining for AMACR was performed on the tissue microarray (TMA) slides using the standard streptavidin–biotin–peroxidase procedure. Freshly cut (4 μm) sections were mounted on superfrost slides (Menzel-Gläser, Germany), dewaxed with xylene, and gradually hydrated. The primary antibody was a rabbit monoclonal AMACR antibody (Biologo, Kronshagen, Germany; clone 13H4), which was diluted 1:200 using a background reducing dilution buffer from Zytomed Systems (Berlin, Germany). No other blocking agents were employed. The primary antibody was incubated at room temperature for 1 h, following IVIEW detection on the Ventana Benchmark XT (pretreatment “CC1 mild”).
In order to investigate the distribution pattern of AMACR expression within the tumor tissue (diffuse versus patchy/focal cytoplasmic expression), a total of 60 randomly selected tumor samples were stained prior to TMA generation. These selected samples also contained adjacent nonneoplastic urothelial mucosa for comparative assessment of AMACR expression.
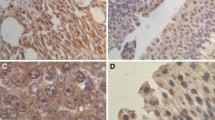
Semiquantification of AMACR immunostaining was performed at high power (×40 objective) according to the approach recently published by Lin et al. [7] with minor modifications. Briefly, positive staining was defined as to having more than 5% of tumor cells showing diffuse cytoplasmic staining. This cut-off was chosen in order to exclude possible nonspecific and/or artificial staining. The staining intensity was graded as negative (0), weak (1+), moderate (2+), or strong (3+). Examples are illustrated in Fig. 1. Since the distribution of AMACR immunoreactivity in the tumors was fairly homogeneous, evaluation of the staining intensity was straightforward, allowing for omittance to incorporate the percentage of positive tumor cells.
Using serial sections, all samples were additionally stained with monoclonal antibodies directed against p53 (DAKO; clone DO-7; dilution 1:50) and against Ki67 (DAKO; clone MiB-1; dilution 1:100), respectively. The latter two markers were semiquantified by recording the percentage of positively stained tumor cell nuclei considering all tumor cells depicted by means of each punch investigated.
In each experiment, a negative control was included in which the primary antibody was replaced by nonhuman reactive rabbit IgG (DAKO). Positive controls used in this study were sections from a prostate that contained both adenocarcinoma and benign prostatic glands. The slides were independently read by three clinical histopathologists (S.G., G.K., and K.S.) blinded with respect to the specimens. In order to assess reproducibility of the data, the slides were reassessed some weeks later.
Statistical analysis
The Spearman correlation was used to determine the magnitude and direction of the association between marker expression and histopathological tumor grade according to the 1973 World Health Organization (WHO) and the 1998 WHO/International Society of Urological Pathology (ISUP) systems. All differences were considered statistically significant if p < 0.05; p values are two-tailed. All calculations were performed using the statistical software package SPSS version 13.0.
Results
Reproducibility of staining evaluation
Intraobserver and interobserver variability of staining evaluation was found to be less than 2%. The rare equivocal cases were critically discussed among the clinical histopathologists involved in this study in order to establish consensus.
AMACR expression in tumor samples and in nonneoplastic urothelium
In all 60 randomly selected tumor samples immunohistochemically investigated prior to TMA construction, cytoplasmic expression of AMACR was found to be evenly distributed throughout the entire tumor tissue in a fairly homogeneous pattern. There was no focal and/or patchy staining pattern. The adjacent nonneoplastic urothelium either failed to show any AMACR expression at all or showed only patchy weak expression (Table 2). AMACR expression did not appear to be related to foci of mucosal inflammation.
Correlation between AMACR expression and histopathologic grading
Among the 163 tumor samples included in the final analysis, positive staining was detected in 52 (31.9%) carcinomas. Twenty-three cases (14.1%) showed weak, 16 cases (9.8%) showed moderate, and 13 cases (7.9%) showed strong immunostaining. The remaining 111 samples (68.1%) showed no immunoreactivity at all (Table 1).
There was a significant positive correlation between AMACR expression and grading by WHO 1973 (correlation coefficient ρ = 0.62; p < 0.01) and also between AMACR expression and grading by the WHO/ISUP system (correlation coefficient ρ = 0.48; p < 0.01).
Correlation of p53 and Ki67 with histopathologic grading
There was no significant correlation between p53 expression and grading by WHO 1973 (correlation coefficient ρ = 0.19; p = 0.35) or between p53 expression and grading by the WHO/ISUP system (correlation coefficient ρ = 0.13; p = 0.16).
Ki67 expression showed a significant positive correlation with tumor grading by WHO 1973 (correlation coefficient ρ = 0.25; p = 0.02) but failed to show any significant correlation with tumor grading by the WHO/ISUP system (correlation coefficient ρ = 0.06; p = 0.54), respectively.
Discussion
Among the limited number of studies reporting on AMACR expression in bladder cancer [12, 13], this is the first comprehensive study that correlates the immunohistochemically detected AMACR (racemase) expression with both established histopathologic grading schemes in a larger cohort of noninvasive urothelial carcinomas. So far, the rates of AMACR expression and its role in the pathobiology of noninvasive bladder cancer are essentially unknown.
Alpha-methylacyl-CoA racemase (AMACR, p504s), a peroxisomal and mitochondrial enzyme involved in the oxidation of branched-chain fatty acids and cholesterol metabolites, has recently emerged as a novel tumor biomarker [1]. Since AMACR is not tissue-specific, its diagnostic utility is restricted to specific clearly defined areas [14]. AMACR expression proved to be a sensitive and specific biomarker for the diagnosis of prostate cancer except for a few uncommon variants (e.g., atrophic, foamy gland, and pseudohyperplastic variants) [3] and was also suggested to bear clinical significance in colorectal carcinoma [7]. Our findings are in keeping with previous studies reporting on the insignificant association between p53 expression and both grading schemes [15, 16].
Evaluating a total of 20 specimens, Krüger et al. found Ki67 to be correlated with tumor grade in noninvasive bladder cancer [17]. Surveying a much larger cohort, our data support this notion in terms of tumor grading by WHO 1973 but failed to establish a significant correlation between Ki67 and tumor grading by the WHO/ISUP system. This discrepancy might be explained by the strikingly different total number of specimens assessed.
Compared with the latter classic but not entirely validated markers, our immunostaining data suggest AMACR to be implicated in the pathobiology of noninvasive bladder cancer. One reasonable interpretation of this novel observation is based on the notion that endogenous fatty acids represent an exploitable storage of energy for human bladder cancer [18]. Fatty acid synthase (FAS), a key lipogenic enzyme, is involved in the biological activities of bladder cancer in which it has been reported to be overexpressed [19]. Since both established histopathological grading systems of noninvasive bladder cancer conventionally reflect cellular differentiation, one might suggest cellular dedifferentiation occurring in the latter tumor entity to be accompanied by a possible change in tumor cell energy metabolism characterized by increased oxidation of branched-chain fatty acids which might be brought about by AMACR [20]. The observation that AMACR expression appears to be evenly distributed throughout the entire tumor tissue while being absent or only weakly expressed in the adjacent urothelium strongly supports the concept of intrinsic genetic alterations rather than aberrant epiphenomenon. The lacking association between AMACR expression and foci of mucosal inflammation also supports this hypothesis. However, it should be kept in mind that immunohistochemically detected AMACR expression at the cellular protein level does not necessarily reflect enzymatic activity. Therefore, further in vitro studies are clearly needed. The elucidation of a so far unknown possible implication of AMACR racemase in the pathobiology of invasive bladder cancer might also be an interesting focus to be targeted in the future.
Our observations contrast with the inverse correlation which has been reported between AMACR expression and histopathologic grading in colorectal cancer [5]. This discrepancy might be attributable to a completely different pivotal biological role of AMACR which might be involved in the tumorigenesis of colon cancer by bringing about the oxidation of branched-chain fatty acids from red meat and certain dairy products, the consumption of which increases the risk of colorectal cancer [5, 9]. With respect to noninvasive bladder cancer, there is currently no scientific data that might suggest any link between AMACR and the carcinogenesis behind the development of the latter tumor entity. However, our findings might hint at a so far neglected role of AMACR in the pathobiology of noninvasive bladder cancer, and—according to our immunostaining data—might suggest a possible implication in tumor cellular energy metabolism. Thus, further studies are clearly needed to back this up and to clarify whether this observation might provide the basis for novel targeted therapy strategies applicable in a subset of noninvasive bladder cancer.
However, given the relatively low rate of its expression in low-grade tumors observed in our study, it appears unlikely that AMACR might become a diagnostic biomarker in low-grade bladder cancer contrasting with its possible role as a diagnostic marker in high-grade noninvasive tumors. Further studies including detailed follow-up data in terms of tumor progression and recurrence are needed to address the issue of a possible prognostic value of AMACR in bladder cancer which might be suggested by its positive correlation to higher tumor grades.
References
Daugherty SE, Platz EA, Shugart YY, Fallin MD, Isaacs WB, Chatterjee N, Welch R, Huang WY, Hayes RB (2007) Variants in the alpha-methylacyl-CoA racemase gene and the association with advanced distal colorectal adenoma. Cancer Epidemiol Biomark Prev 16:1536–1542
Dorer R, Odze RD (2006) AMACR immunostaining is useful in detecting dysplastic epithelium in Barrett’s esophagus. Am J Surg Pathol 30:871–877
Hameed O, Humphrey PA (2005) Immunohistochemistry in diagnostic surgical pathology of the prostate. Semin Diagn Pathol 22:88–104
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ (2008) Cancer statistics, 2008. CA Cancer J Clin 58(2):71–96
Jiang Z, Fanger GR, Banner BF, Woda BA, Algate P, Dresser K, Xu J, Reed SG, Rock KL, Chu PG (2003) A dietary enzyme: alpha-methylacyl-CoA racemase/P504S is overexpressed in colon carcinoma. Cancer Detect Prev 27:422–426
Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP (1998) Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 4:844–847
Lin A, Weiser MR, Klimstra DS, Paty PB, Tang LH, Al-Ahmadie H, Hoo Park S, Guillem JG, Temple L, Wong WD, Gerald WL, Shia J (2007) Differential expression of alpha-methylacyl-coencyme A racemase in colorectal carcinoma bears clinical and pathologic significance. Hum Pathol 38:850–856
Lisovsky M, Falkowski O, Bhuiya T (2006) Expression of alpha-methylacyl-coencyme A racemase in dysplastic Barrett’s epithelium. Hum Pathol 37:1601–1606
Sesink AL, Termont DS, Kleibeuker JH, Van der Meer R (2000) Red meat and colon cancer: dietary haem, but not fat, has cytotoxic and hyperproliferative effects on rat colonic epithelium. Carcinogenesis 21:1909–1915
Skinnider BF, Oliva E, Young RH, Amin MB (2004) Expression of alpha-methylacyl-CoA racemase (P504S) in nephrogenic adenoma: a significant immunohistochemical pitfall compounding the differential diagnosis with prostatic adenocarcinoma. Am J Surg Pathol 28:701–705
Sobin LH, Wittekind CH (1997) TNM classification of malignant tumors, 5th edn. Wiley-Liss, New York, pp 187–190
Suh N, Yang XJ, Tretiakova MS, Humphrey PA, Wang HL (2005) Value of CDX2, villin, and alpha-methylacyl coencyme A racemase immunostains in the distinction between primary adenocarcinoma of the bladder and secondary colorectal adenocarcinoma. Mod Pathol 18:1217–1222
Jiang Z, Fanger GR, Woda BA, Banner BF, Algate P, Dresser K, Xu J, Chu PG (2003) Expression of alpha-methylacyl-CoA racemase (P504s) in various malignant neoplasms and normal tissues: a study of 761 cases. Hum Pathol 34:792–796
Went PT, Sauter G, Oberholzer M, Bubendorf L (2006) Abundant expression of AMACR in many distinct tumour types. Pathology 38:426–432
Karakök M, Aydin A, Bakir K, Ucak R, Korkmaz C (2001) AgNOR/P53 expression compared with different grades in bladder carcinomas. Int Urol Nephrol 33:353–355
Vatne V, Maartmann-Moe H, Hoestmark J (1995) The prognostic value of p53 in superficially infiltrating transitional cell carcinoma. Scand J Urol Nephrol 29:491–495
Krüger S, Müller H (1995) Correlation of morphometry, nucleolar organizerregions, proliferating cell nuclear antigen and Ki67 antigen expression with grading and staging in urinary bladder carcinomas. Br J Urol 75:480–484
Visca P, Sebastiani V, Pizer ES, Botti C, De Carli P, Filippi S, Monaco S, Alo PL (2003) Immunohistochemical expression and prognostic significance of FAS and GLUT1 in bladder carcinoma. Anticancer Res 23:335–339
Turyn J, Schlichtholz B, Dettlaff-Pokora A, Presler M, Goyke E, Matuszewski M, Kmiec Z, Krajka K, Swierczynski J (2003) Increased activity of glycerol 3-phosphate dehydrogenase and other lipogenic enzymes in human bladder cancer. Horm Metab Res 35:565–569
Zha S, Ferdinadusse S, Hicks JL, Denis S, Dunn TA, Wanders RJ, Luo J, De Marzo AM, Isaacs WB (2005) Peroxisomal branched chain fatty acid beta-oxidation pathway is upregulated in prostate cancer. Prostate 63:316–323
Acknowledgements
The authors gratefully thank Inna Spivak, Ph.D., Department of applied Mathematics, Faculty of Mathematics, Natural Sciences and Computer Science, Brandenburg University of Technology Cottbus (B T U), Germany, for her excellent assistance in the statistical evaluation of the data. We also gratefully thank Olaf Kaufmann, M.D., for allowing us to investigate the archived specimens assessed in this study. We are also greatly indebted to the Sonnenfeld Stiftung (Berlin, Germany) for sponsoring the tissue micro arrayer to the uropathological working group of G.K. We declare that the experiments comply with the current laws of the country in which they were performed.
Disclosure of Conflict of Interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gunia, S., May, M., Scholmann, K. et al. Expression of alpha-methylacyl-CoA racemase correlates with histopathologic grading in noninvasive bladder cancer. Virchows Arch 453, 165–170 (2008). https://doi.org/10.1007/s00428-008-0638-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-008-0638-1