Abstract
Nephroblastoma prognosis has dramatically improved, but an unfavourable prognostic subgroup warrants development of novel therapeutic strategies. Selective KIT, PDGFRα and epidermal growth factor receptor (EGFR) tyrosine kinase inhibition evolved as powerful targeted therapy for gastrointestinal stromal tumours and non-small-cell lung cancer. To investigate a potential role for tyrosine kinase inhibition, we analyzed 209 nephroblastomas for immunohistochemical KIT and EGFR expression, 63 nephroblastomas for mutations in KIT exons 9, 11, 13, EGFR exons 18, 19, 20 and 21, and all 209 nephroblastomas for PDGFRα exons 12, 14 and 18. Twenty-two tumours (10.5%) expressed KIT, 31 (14.8%) EGFR, and 10 (4.8%) both KIT and EGFR, respectively. KIT expression was relatively more common among high-risk tumours (6/27; 22.3%) compared to low-/intermediate-risk tumours (26/181; 14.4%). Nine patients deceased, four of which had high-risk tumours with KIT expression in two of four and EGFR expression in one of four. There were no KIT, PDGFRα or EGFR mutations. Our results suggest no significant contribution of KIT, EGFR or PDGFRα mutations to nephroblastoma pathogenesis. Despite a trend towards association of immunohistochemical KIT and EGFR expression with poor outcome in high-risk nephroblastomas, statistical analysis did not yield significant correlations in this subgroup. Therefore, it remains open if KIT, PDGFRα or EGFR tyrosine kinase inhibition constitute a therapeutic target in nephroblastoma in the absence of KIT, PDGFRα or EGFR mutations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nephroblastoma is the most common malignant renal tumour in childhood and affects 1 in 8,000 children [11]. Over the past three decades, multimodal nephroblastoma therapy has been a story of success. Nephroblastoma prognosis has improved dramatically, and overall 10-year survival now approaches 85% [15]. Nevertheless, a poor prognostic subgroup of nephroblastomas remains. Diffuse anaplasia, persistent blastema after chemotherapy, allelic loss at 1p or 16q, TP53 mutations and advanced tumour stage have been identified as adverse prognostic factors [3, 10, 11, 31, 32]. Therefore, development of novel therapeutic strategies is mandatory.
Over recent years, receptor tyrosine kinases have evolved as focus of research for potential targeted tumour therapy [26]. Transmembrane receptor tyrosine kinases play an important role in the modulation of growth factor signalling [33]. Receptor tyrosine kinases KIT (v-kit Hardy-Zuckermann 4 feline sarcoma viral oncogene homologue), PDGFRα (platelet-derived growth factor receptor α) and EGFR (epidermal growth factor receptor) participate in cell growth and are implicated in malignant transformation and tumour progression. KIT is claimed to play an important role in kidney organogenesis. It is expressed during metanephrogenesis in blastema and immature epithelium and in the mature kidney in proximal and distal tubules [27]. KIT expression has been demonstrated in a subset of renal cell carcinomas, oncocytomas, mesoblastic nephromas, angiomyolipomas and a small series of nephroblastomas [22, 30]. The KIT internal tyrosine kinase component is structurally related to the platelet-derived growth factor receptor α [40]. PDGFRα overexpression has been described in carcinomas of colon, breast, lung, ovary and pancreas. Expression of activated PDGFRα in stromal cells of colon carcinomas has been associated with metastatic potential [23]. EGFR is expressed in most human tissues and plays an important role in normal cell biology. In the kidney, the human epidermal growth factor family members play a significant role in the mesenchymal to epithelial transition during renal tubulogenesis [41]. EGFR expression is found in a number of tumours, particularly squamous cell carcinomas, sarcomas, gliomas, breast, bladder and lung tumours. In nephroblastoma, EGFR expression has been described in a subset of tumours in one small series [14].
Selective inhibition of receptor tyrosine kinases has evolved as a powerful, targeted therapeutic tool in a number of tumours, and the spectrum of small molecule receptor tyrosine kinase inhibitors is rapidly expanding [26]. Imatinib/Gleevec® and Gefitinib/Iressa® are now well established as therapeutic inhibitors of KIT and EGFR, respectively. In particular, gastrointestinal stromal tumours and leukemias have been shown to be amenable to receptor tyrosine kinase inhibitor therapy [16, 17, 21, 34]. KIT iuxtamembranous exon 9, 11 and 13 mutations correspond with susceptibility to tyrosine kinase inhibition and improved survival in gastrointestinal stromal tumours and myeloic leukemia [8, 9, 13, 36, 38]. Up to 90% of gastrointestinal stromal tumours are KIT-mutated, and exon 11 mutations are most common [20, 25]. Approximately 7–12% of gastrointestinal stromal tumours without KIT mutations show PDGFRα mutations instead and are susceptible, though to a lesser degree, to imatinib treatment [7, 20, 38]. Interestingly, KIT and PDGFRα are located in the same chromosomal region 4q12 [35]. PDGFRα mutations are most often located in exon 18, and rarely in exons 12 and 14. In clinical phase 3 studies, a new generation of KIT/PDGFR receptor tyrosine kinase inhibitors for resistant tumours, e.g. sunitinib/Sutent®, are currently being tested [6]. Furthermore, activating EGFR gene mutations of exons 18, 19, 20 or 21 in non-small-cell lung carcinoma result in inhibition of apoptosis, in tumour proliferation, angiogenesis, invasion and metastasis [29, 33]. EGFR gene mutations confer susceptibility to gefitinib/Iressa® EGFR receptor tyrosine kinase inhibition [19].
In nephroblastomas, immunohistochemical KIT and EGFR expression has only been investigated in a small number of tumours [14], and neither KIT, PDGFRα nor EGFR mutations have been reported in these tumours so far. In a separate small set of 12 KIT-positive nephroblastomas, we detected mutations of KIT exon 11 in two tumours (Bruder et al., manuscript submitted). Moreover, no information is available so far with regard to tyrosine kinase expression and outcome. To provide a rationale for potential targeted small molecule receptor tyrosine kinase inhibition in nephroblastomas, we screened 209 nephroblastomas for immunohistochemical KIT and EGFR expression. Tumours with KIT expression were analysed for mutations of KIT exons 9, 11, 13. Similarly, tumours with EGFR expression were submitted to sequence analysis of EGFR exons 18, 19, 20 and 21. All 209 tumours were analysed for PDGFRα exons 12, 14 and 18 mutations.
Materials and methods
Nephroblastomas
From the archives of the Kiel Paediatric Tumour Registry, 209 nephroblastomas were compiled (1993–2002). Patient data were available on gender, age and stage at diagnosis, tumour subtype, therapy mode and patient outcome. Male to female ratio was 1:1.27. Median patient age was 4.3 years. Preoperative chemotherapy had been administered to 181 patients according to the current International Paediatric Oncology Society (SIOP) protocol, and 28 patients underwent primary tumour resection. All nephroblastomas were classified according to the current SIOP classification [39] (Fig. 1). This current nephroblastoma classification accounts for the differential prognostic impact of blastema before and after chemotherapy and newly categorises residual blastema after chemotherapy in tumours with more than one third viable tumour tissue and more than two thirds of the viable tumour tissue of blastemal type (i.e. blastema predominant) as high-risk histology [39]. Tumours were reviewed by IL, DH, EB and SCW as well as by the SIOP nephroblastoma panel.
For tumour diagnosis, at least one block per centimeter tumour diameter was embedded. A mean of five blocks were available for tumour classification at the Kiel Paediatric Tumour Registry.
A paraffin tumour tissue microarray was constructed for efficient expression screening as previously described [24] (Fig. 2). For adult tumours, like urothelial carcinoma, one single punch cylinder per tumour was previously shown to be representative of each tumour [28]. For the present study of nephroblastoma, the paraffin tissue microarray construction technique was modified and specifically adapted to account for nephroblastoma as a heterogeneous embryonal tumour. Therefore, each histological tumour component of all 209 nephroblastomas was sampled, the respective tumour area circled on an H&E slide, and the corresponding paraffin block area marked before punching, resulting in five to six punch cylinders (mean 5.4, median 5) for each tumour on a total of three recipient blocks with a total of 1,178 punch cylinders. Normal renal parenchyma was included whenever present.
Criteria for tumour components for inclusion in the paraffin tissue microarray comprised all known histological nephroblastoma components, i.e. blastemal, epithelial, stromal and anaplastic tumour areas [11]. To carefully account for tumour heterogeneity and regional tumour variability, each histological tumour component was diligently selected for punching and was represented on the paraffin tissue microarray. Tumour punch samples originated from one or multiple blocks (median 3 blocks, mean 2.7 blocks). Punch core needle diameter was 0.6 mm as described previously [24, 28].
Immunohistochemistry
Paraffin tissue microarray sections were cut at 4 μm according to a protocol standardised in our laboratory. Immunohistochemistry for KIT receptor CD117 protein was performed on a Bond™ Immunostainer (Bond Max, Vision BioSystems; Figs. 3 and 4). For KIT/ CD117 immunohistochemistry, sections were pretreated in the manufacturer’s ‘ Epitope Retrieval buffer 2’ (Tris–EDTA buffer at pH 8.8) for 20 min at 100°C, incubated with polyclonal antibody Anti CD117 primary antibody (DAKO, 1:1000), followed by Bond polymer refine detection kit according to the manufacturer’s protocol. A gastrointestinal tumour served as positive control. As it is known that KIT immunohistochemistry is prone to false positive staining results [20], the immunohistochemical reaction for KIT was carefully tested and set up in our laboratory with particular attention to negative controls so as to avoid false positive immunohistochemical staining results. For EGFR, immunohistochemistry was performed according to the EGFR pharmDx™, Code K1492 (Dako Cytomation) kit protocol. A cytospin of an EGFR-positive cell line contained in the kit served as positive control. A test array slide, containing a combination of normal and neoplastic human tissue cores, was included as negative control for each KIT and EGFR immunohistochemical reaction. Immunohistochemistry for PDGFRα was not performed because in our experience, PDGFRα immunohistochemistry is currently not reliable. Instead, all tumours were submitted to PDGFRα sequence analysis (see below).
KIT immunohistochemistry: a Membranous KIT expression in a blastemal nephroblastoma (KIT immunohistochemistry × 630). b Focal membranous KIT expression in a nephroblastoma with diffuse anaplasia (KIT immunohistochemistry × 630). c, d Focal membranous KIT expression in a nephroblastoma of mixed subtype (KIT immunohistochemistry × 630). c Represents a blastemal component of this tumour, whereas d depicts a focus of KIT expression in immature epithelium in addition to a group of blastemal cells (KIT immunohistochemistry × 630)
EGFR immunohistochemistry: a Membranous EGFR expression in a blastemal predominant nephroblastoma. At the top border of the figure is an EGFR-negative tubule (EGFR immunohistochemistry × 630). b Membranous EGFR expression in a focus of squamous epithelium. Surrounding stroma is negative for EGFR (EGFR immunohistochemistry × 630). c EGFR expression in a stromal predominant nephroblastoma. Foci of rhabdomyoblastic differentiation are negative for EGFR (EGFR immunohistochemistry × 630). d EGFR expression in blastemal foci and immature epithelium in a mixed type nephroblastoma (EGFR immunohistochemistry × 630)
Immunohistochemical staining for KIT/CD117 and EGFR was evaluated for each individual array punch tissue sample and recorded according to tumour tissue component and intensity of staining. Staining intensity was graded from + to +++, with + denoting faint cytoplasmatic staining, ++ intermediate cytoplasmatic staining and +++ exclusively only awarded to those tumours with distinct membranous in addition to cytoplasmic staining (Table 1). Immunohistochemical scoring was performed by SCW and EB independent of further tumour and patient information. Tumours were classified as KIT- or EGFR-positive, respectively, only in the presence of membranous KIT or EGFR expression and selected for subsequent DNA sequence analysis. Immunohistochemical scoring was amended to include the percentage of KIT- or EGFR-positive cells. In Table 1, the percentage of immunohistochemically positive tumour cells is provided.
KIT, PDGFRα and EGFR sequence analysis
For KIT, PDGFRα and EGFR sequence analysis, those tumours with strong immunohistochemical KIT and/or EGFR expression were selected. As in gastrointestinal stromal tumours the presence of either KIT or PDGFRα mutations is regarded as predictive of tumour response to receptor tyrosine kinase inhibition [2], we proceeded to submit all KIT expressing nephroblastomas not only to KIT but also to PDGFRα sequence analysis. KIT and PDGFRα sequence analysis was performed on 22 of 209 tumours with strong +++ membranous and cytoplasmic CD117 expression. EGFR sequencing was performed on 31 of 209 tumours with strong +++ membranous and cytoplasmic EGFR expression. Ten nephroblastomas revealed both EGFR and KIT expression and were submitted to KIT, PDGFRα as well as EGFR sequencing analysis. Consequently, KIT and EGFR DNA sequence analysis was performed on a total of 63 nephroblastomas. In addition, in the absence of an immunohistochemical PDGFRα screening test, all 177 CD117 negative nephroblastomas were submitted to PDGFRα sequence analysis, resulting in PDGFRα sequence analysis of all 209 nephroblastomas included in this study.
For DNA extraction, one to three punch cylinders 0.6 mm in diameter were removed from identical matched tumour regions of the original paraffin tissue blocks of each selected nephroblastoma. DNA was extracted according to the SIOP 1 and the QIAamp® DNA mini kit DNA extraction protocols (Qiagen AG, Basel, Switzerland) [37]. The extracted DNA was submitted to a semi-nested or nested multiplex polymerase chain reaction (PCR) for KIT exons 9, 11, 13, PDGFRα exons 12, 14 and 18 and EGFR exons 18, 19, 20 and 21. DNA sequencing was performed on the PCR products on an ABI PRISM 310xl Genetic Analyzer (Applied BioSystems). Sequencing primers were identical to initial PCR primers. Primer sequences are given in Table 2.
Statistical analysis
For statistical analysis, associations between follow-up data, histological nephroblastoma subtype and immunohistochemical expression of KIT and EGFR were analysed, respectively. The association between survival, recurrence and metastasis with KIT and EGFR expression was evaluated using the chi-square test or Fisher’s exact test. P values < 0.05 were considered statistically significant. All analyses were carried out using SAS (version 9.0, The SAS Institute, NC, USA).
Results
In this study, we analyzed 209 nephroblastomas for immunohistochemical KIT and EGFR expression, a total of 63 tumours for mutations in KIT exons 9, 11, 13, EGFR exons 18, 19, 20 and 21, as well as PDGFRα exons 12, 14 and 18 in the total series of 209 nephroblastomas. Tumour localisation was equally distributed between both kidneys, with 108 tumours localised in the right and 101 in the left kidney.
The 209 tumours were classified according to the SIOP classification (Table 1, Fig. 1): two low-risk tumours (1%; CPDN: cystic partially differentiated nephroblastoma), 179 intermediate-risk tumours (86%), 27 high-risk tumours (12.5%) and one non-classifiable tumour (0.5%). Tumour group details are provided in Table 1. The 179 nephroblastomas of intermediate risk were: 91 mixed (52%), 42 regressive (23%), 29 stromal (16%), nine pre-chemotherapy blastemal (5%), six epithelial (3%) and two with focal anaplasia (1%). The 27 nephroblastomas of high-risk histology were: 21 of 27 (78%) post-chemotherapy blastemal and 6 of 27 diffuse anaplastic nephroblastomas (22.3%). The nephroblastomas classified as post-chemotherapy blastemal showed more than one third viable tissue of which more than two thirds of the viable tumour tissue was of blastemal type (i.e. blastema predominant).
Follow-up between 7 and 132 months (mean 66 months) was available for all 209 patients. Of 209 patients, nine (4%) died of tumour: five intermediate-risk tumours (56%; three regressive and two mixed), four high-risk tumours (44%; three post-chemotherapy blastemal, one diffuse anaplastic nephroblastoma; Fig. 1). Of the 27 patients with a high-risk histology tumour, four died (14.8%). Thus, our study confirms the association of high-risk nephroblastomas with poor outcome. Of the nine patients deceased, four had metastases, predominantly pulmonary: two patients with intermediate-risk tumours (regressive) and two high-risk tumours (one post-chemotherapy blastemal, one diffuse anaplastic nephroblastoma). A total of 27 (13%) tumours metastasised: 24 intermediate-risk tumours [89%; ten mixed (37%), two stromal (7%), ten regressive (37%; two patients died], one epithelial (4%), one pre-chemotherapy blastemal nephroblastoma (4%) and three high-risk tumours (11%; two post-chemotherapy blastemal (7%; one patient died), one with diffuse anaplasia (4%; this patient died).
Immunohistochemistry for KIT and EGFR
Tumours with membranous immunohistochemical KIT or EGFR expression were graded +++ and classified as positive. Immunohistochemical results are provided in Table 1. In most tumours, KIT and EGFR expression was focal and observed in blastemal and immature epithelial tumour components (Figs. 3 and 4). For more precise analysis, the percentage of KIT- and EGFR-expressing tumour cells per punch was estimated and is indicated in Table 1. Assuming that each KIT- or EGFR-expressing tumour area might be of potential biological relevance, all tumours with membranous immunohistochemical KIT or EGFR expression were classified as positive irrespective of the percentage of positive tumour cells. Sixty-three (30.1%) nephroblastomas demonstrated expression of KIT, EGFR or both: 22 (10.6%) nephroblastomas were KIT-positive, 31 (14.8%) nephroblastomas were EGFR-positive, and 10 (4.8%) tumours expressed both KIT and EGFR. Consequently, a total of 32 of 209 nephroblastomas (15.3%) showed immunohistochemical KIT expression. Of these, 26 were intermediate-risk tumours (81.3%) and one was not classifiable (3.1%). KIT expression was observed in 12 (37.5%) mixed nephroblastomas, seven (21.9%) stromal, four (12.5%) regressive and three (9.4%) pre-chemotherapy blastemal nephroblastomas. Of the 27 high-risk histology nephroblastomas, six showed KIT expression (22.3%). Five of 21 post-chemotherapy blastemal nephroblastomas and one of six with diffuse anaplasia were KIT-positive. None of the six epithelial, focal anaplastic or cystic partially differentiated nephroblastomas showed KIT expression. EGFR expression was observed in 41 of 209 nephroblastomas (19.6%). Thirty-eight of them were intermediate-risk tumours (92.7%) and three high-risk tumours (7.3%). Similar to KIT, EGFR expression was observed predominantly in mixed nephroblastomas: 23 of 41 tumours (56.1%) were mixed, seven (17.1%) were stromal, seven (17.1%) regressive and one (2.4%) non-pretreated blastemal nephroblastomas. Only 3 of 27 high-risk nephroblastomas expressed EGFR, two (of 21) post-chemotherapy blastemal and one (of 6) nephroblastoma with diffuse anaplasia. None of the six epithelial, focal anaplastic or cystic partially differentiated nephroblastomas showed EGFR expression.
Immunohistochemical scoring was amended to include the percentage of KIT- or EGFR-positive cells in immunohistochemically positive tumour punches. In ESM Table 1, the percentage of immunohistochemically positive tumour cells per positive tumour punch is provided.
KIT exon 9, 11, 13, EGFR exon 18, 19, 20, 21, PDGFRα exon 12, 14 and 18 sequence analysis
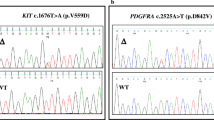
Sequence analysis for KIT and EGFR was performed on a total of 63 tumours and for PDGFRα on all 209 tumours from 209 patients. A total of 957 exons were sequenced. Twenty-two nephroblastomas with KIT expression were selected for KIT exon 9, 11, 13 and PDGFRα exon 12, 14 and 18 sequence analysis. All 22 tumours contained KIT exon 9, 11, 13 and PDGFRα exon 12, 14 and 18 wild-type sequences (Fig. 5). The 31 nephroblastomas with immunohistochemical staining for EGFR were selected for EGFR exon 18, 19, 20 and 21 sequence analysis. All 31 tumours contained EGFR exon 18, 19, 20 and 21 wild-type sequences (Fig. 5). The ten tumours with both KIT and EGFR expression were selected for KIT exon 9, 11, 13, PDGFRα exon 12, 14, 18 and for EGFR exon 18, 19, 20 and 21 sequence analysis. All ten tumours contained wild-type sequences of KIT exons 9, 11, 13, PDGFRα exons 12, 14, and 18 and for EGFR exons 18, 19, 20 and 21. In addition, all 177 immunohistochemically KIT-negative nephroblastomas were submitted to PDGFRα sequence analysis. Similar to the KIT immunohistochemically positive tumours, the immunohistochemically KIT-negative nephroblastomas contained PDGFRα exon 12, 14 and 18 wild-type sequences.
PDGFRα sequence analysis was performed in double independently in two separate laboratories at the Institute of Pathology at the University Hospital in Basel, Switzerland and at the Institute for Clinical Chemistry and Laboratory Medicine at the University of Regensburg, Regensburg, Germany.
All sequencing reactions were repeated twice. Repeat mutation analyses yielded identical results.
Statistical analysis
Correlation of tyrosine kinase receptor expression and outcome
Metastases occurred in 30 patients (14.1%), tumour recurrences were observed in 17 patients (8%), and nine patients died (4.2%). Although we observed a trend for KIT expression in high-risk histology tumours with adverse events, statistically, KIT expression was not significantly associated with outcome, neither in the whole group of nephroblastomas (p = 0.18) nor in the group of low- or intermediate-risk histology tumours nor in high-risk histology tumours (p = 0.062). KIT expression was independent of immunohistochemical EGFR expression. Similar to KIT, EGFR expression did not correlate with adverse events for the total group of nephroblastomas analysed (p = 0.224).
Comparison of treated and untreated nephroblastomas
Preoperative chemotherapy had been administered to 181 patients according to the current SIOP protocol, and 28 patients underwent primary tumour resection without preoperative chemotherapy (see Table 3). In the tumour group with preoperative chemotherapy, there were 27 high-risk histology nephroblastomas, 153 intermediate risk histology nephroblastomas and one unclassified nephroblastoma. In the tumour group without preoperative chemotherapy, there were two low-risk histology tumours and 26 intermediate-risk histology tumours, but no high-risk tumour. None of the high-risk histology tumours were treated with primary resection; all of these had received preoperative chemotherapy.
Comparing the immunohistochemical results of the total group of 209 tumours with those tumours treated preoperatively, there is no statistical difference between the total group and the group treated with preoperative chemotherapy.
Comparison of immunohistochemical expression among the preoperatively treated versus the preoperatively untreated group yields a tendency towards a higher percentage of KIT-expressing tumours in the preoperatively untreated group (p = 0.069). Seven of 26 preoperatively untreated nephroblastomas of intermediate-risk histology showed KIT expression, corresponding to 27%, as opposed to 19 of 153 (12%) of intermediate-risk histology nephroblastomas with preoperative chemotherapy. As for EGFR expression, the trend is similar but to a lesser degree (p = 0.443): Seven of 26 (27%) preoperatively untreated nephroblastomas of intermediate-risk histology showed EGFR expression versus 31 of 153 (21%) preoperatively treated intermediate-risk histology nephroblastomas.
However, the numbers of individual intermediate-risk histology subgroups without preoperative chemotherapy are small, and analysis of larger numbers is required to confirm such a trend. Similarly, the tumour spectrum within the preoperatively treated versus preoperatively untreated tumour group is different and is therefore likely to introduce a bias into the comparison of these two nephroblastoma groups.
As for outcome analysis among the preoperatively untreated tumours and comparison with the preoperatively treated tumours, a similar bias is likely.
None of the adverse events occurred in these 28 preoperatively untreated tumours except one surviving patient with metastases of a KIT- and EGFR-negative tumour of the intermediate-risk histology group. No patient in the preoperatively untreated group died. All other adverse events occurred in those patients with preoperative chemotherapy. All patients who died were in the group with preoperative chemotherapy.
Discussion
Enhanced understanding of genetic alterations in nephroblastoma and identification of key molecular markers is expected to provide the foundation for novel targeted therapies to address currently poor prognostic nephroblastoma subgroups. Transmembrane receptor tyrosine kinases play a crucial role in growth factor signalling cascades. The role of tyrosine kinases for tumour growth and angiogenesis in nephroblastomas has been described in previous studies [1, 12, 14, 41]. Constitutional protein kinase activation by somatic mutation is a frequent mechanism of tumourigenesis [33]. Targeted small molecule drugs have emerged as elegant and efficient anti-cancer strategies, and selective receptor tyrosine kinase inhibition evolves as focus of research for novel therapies. As receptor tyrosine kinases, KIT and EGFR play a significant role in renal organogenesis and expression has previously been described in a small series of nephroblastomas, we set out to determine the prevalence of KIT and EGFR expression in a large series of 209 nephroblastomas. So far, as known from the experience in gastrointestinal stromal tumours, leukemias and non-small-cell lung cancer, susceptibility to kinase inhibition appears to be linked to constitutionally activating gene mutations, which has held true for KIT as well as for EGFR. Neither KIT nor EGFR mutations have been described in nephroblastomas so far. In a separate small set of 12 KIT-positive nephroblastomas, we detected mutations of KIT exon 11 in two tumours (Bruder et al., manuscript submitted). Therefore, we also determined KIT and EGFR gene sequences of most commonly involved exons in 63 nephroblastomas and PDGFRα of all 209 nephroblastomas to investigate a rationale for potential targeted tyrosine kinase inhibitor therapy in nephroblastoma.
Among the 209 nephroblastomas investigated in this study, we found KIT and/or EGFR expression in 63 tumours. Interestingly, KIT expression was more frequent among tumours of high-risk histology (6/27; 22.3%) compared with those of low- and intermediate-risk (26/181; 14.4%). In contrast, EGFR expression was less frequent in high-risk nephroblastomas (3/27; 11%) than in intermediate- and low-risk tumours (38/181; 20%). Did KIT or EGFR expression confer a different prognosis in our set of nephroblastomas? If nephroblastomas with adverse events defined as local recurrence, metastasis or death from disease were regarded separately, adverse events occurred in 37 (17.7%) of the total series of 209 tumours and in five (12.2%) of 41 tumours with EGFR expression. Conversely, adverse events occurred in 32 (19%) of 168 tumours without EGFR expression. Furthermore, adverse events were recorded in three (9.4%) of 32 KIT-expressing tumours, as opposed to adverse events in 34 (19.2%) of 177 KIT-negative tumours. In contrast, in 200 surviving patients, KIT and EGFR were positive in 15% and 19.5%, respectively. It therefore appears that KIT or EGFR expression alone may be associated with slightly better tumour outcome if regarded independently from tumour histology. However, in nine patients who died of their tumour, two (22.3%) tumours showed strong KIT expression and another 2 (22.3%) showed strong EGFR expression, while KIT and EGFR were positive in slightly lower proportions in the 200 surviving patients, 15% and 19.5%, respectively. Moreover, if evaluated in the context of tumour histology, four of the nine patients who died of disease had tumours of high-risk histology, and among these four tumours, two strongly expressed KIT, and one was EGFR-positive (p = 0.371). Although these findings suggest a tendency that KIT expression, and to a minor degree also EGFR expression, may potentially be associated with poor outcome in the context of unfavourable histology, we were unable to statistically prove such a correlation. This is also true for tumours of low- and intermediate-risk histology associated with adverse events in relation to KIT and EGFR expression: Of 182 tumours with low- and intermediate-risk histology, only one (3.8%) of 26 KIT-expressing tumours and four (10.5%) of 38 EGFR expressing tumours (p = 0.206) were associated with adverse events, whereas, conversely, 31 of 156 (19.9%) KIT-negative and 28 of 144 (19.4%) EGFR-negative tumours were associated with adverse events. Even if these results might suggest a tendency that low- and intermediate-risk histology nephroblastomas with KIT and EGFR expression appear to be associated with a better prognosis, this correlation is statistically not significant. As numbers of subgroups are small, this issue requires to be addressed in a still larger study. Nevertheless, these results suggest that KIT and EGFR expression should be evaluated in the context of tumour histology and outcome. The potential correlation of KIT and EGFR expression with better prognosis in low-/intermediate-risk histology nephroblastomas in this series is an interesting and important observation.
Could a subset of nephroblastomas merit a trial of selective tyrosine receptor kinase inhibition and be susceptible to this therapeutic approach? As the presence of either KIT or PDGFRα mutations is predictive of tumour response to selective receptor tyrosine kinase inhibition with imatinib in gastrointestinal stromal tumours [2, 18, 20], though to a lesser degree for PDGFRα mutations and no KIT mutations were detected in the subset of nephroblastomas investigated in this study, we therefore proceeded to determine also PDGFRα sequences in those nephroblastomas with KIT expression, and in the absence of an immunohistochemical screening test for PDGFRα, also in those tumours without KIT expression. Moreover, we investigated EGFR-expressing nephroblastomas for EGFR sequence alterations. None of the 63 nephroblastomas investigated showed any sequence alteration in any receptor tyrosine kinase analysed. Our present study of mutation analysis of 63 and 209 nephroblastomas, respectively, suggests that neither KIT, EGFR nor PDGFRα mutations appear to play a significant role in tumour pathogenesis. KIT and EGFR expression therefore do not appear to result from constitutionally activating mutations in the investigated exons in these nephroblastomas. Why do these tumours express KIT and/or EGFR in the absence of respective gene mutations? Importantly, KIT and PDGFRα receptor tyrosine kinases may be activated by mechanisms other than gene mutation, such as gene fusion and amplification, autocrine and paracrine stimulation of the receptor by its ligand, loss of phosphatase activity, cross-activation by other kinases and epigenetic promoter activation via methylation status alteration. Therefore, absence of KIT or PDGFRα mutations does not exclude selective tyrosine kinase inhibition with imatinib as targeted therapeutic strategy in the KIT-expressing nephroblastomas [35]. Importantly, imatinib has been shown to also effectively inhibit wild-type KIT and PDGFR receptors [2]. Similarly, in a previous study correlating KIT immuno-phenotype with response to tyrosine kinase inhibitor, imatinib even showed a response in gastrointestinal stromal tumours with only weak immunohistochemical staining [4]. Of note, in non-small-cell lung cancer, EGFR receptor tyrosine kinase inhibition with erlotinib/Tarceva® has recently been found to be effective in non-small-cell lung cancer with wild-type EGFR [5]. Therefore, as numbers in this current study are small, further investigations are required to evaluate a potential benefit, and future clinical therapeutic studies might be considered for the limited poor prognostic subgroup of nephroblastomas with high-risk histology also in the absence of KIT, PDGFRα or EGFR mutations. Nevertheless, at present, the question remains open whether the small group of poor prognostic nephroblastomas will benefit from additional receptor tyrosine kinase inhibition.
Addressing the question of preoperative chemotherapy and evaluation of the patient outcome in relation to expression of KIT and EGFR, comparing with patients without preoperative chemotherapy, such a comparison is likely to be biased for the following reasons. According to the current SIOP protocol, most nephroblastoma patients are treated by preoperative chemotherapy [15]. Only a minority of very young patients are judged not to require chemotherapy because of a high likelihood of congenital mesoblastic nephroma. Therefore, the criteria of patient inclusion will render the preoperatively treated versus the preoperatively untreated nephroblastoma groups incongruent.
It has been argued that preoperative chemotherapy may unpredictably alter tumour protein expression and genetics; therefore, only analysis of untreated tumour tissue should be regarded as reliable. However, it is now being recognised that surviving blastemal tumour tissue after chemotherapy constitutes an indicator towards poor chemotherapy response and prognosis, and therefore, nephroblastomas of post-chemotherapy blastemal predominant histology are currently classified among the high-risk histology group accordingly, in contrast to those blastemal predominant nephroblastomas without preoperative chemotherapy. Analysis of a study population of nephroblastomas with available pre-chemotherapy pre-surgical biopsy as well as post-chemotherapy specimens of identical tumours should be undertaken in the future to further contribute to resolve this issue.
In summary, a minor proportion of nephroblastomas express KIT and EGFR. However, KIT, PDGFRα and EGFR mutations do not appear to significantly contribute to nephroblastoma pathogenesis. Hence, unlike in gastrointestinal stromal tumours, leukemia and non-small-cell lung cancer, there appears to be no comparable molecular basis for potential selective tyrosine kinase inhibition in nephroblastoma. In nephroblastomas, immunohistochemical KIT and EGFR expression does not appear to bear a direct association with outcome. Nevertheless, as numbers of high-risk histology nephroblastomas are small, further clinical evaluation studies might be considered to elucidate a potential therapeutic benefit for this poor prognostic subgroup of patients.
References
Alpers CE, Hudkins KL, Ferguson M, Johnson RJ, Rutledge JC (1995) Platelet-derived growth factor A—chain expression in developing and mature human kidneys and in Wilms’ tumor. Kidney Int 48(1):146–154
Buchdunger E, Cioffi CL, Law N, Stover D, Ohno-Jones S, Druker BJ, Lydon NB (2000) Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther 295:139–145
Charles AK, Brown KW, Berry PJ (1998) Microdissecting the genetics events in nephrogenic rests and Wilms tumor development. Am J Pathol 153:991–1000
Chirieac RL, Trent JC, Steinert DM, Choi H, Yang Y, Zhang J, Patel SR, Benjamin RS, Raymond AK (2006) Correlation of immunophenotype with progression-free survival in patients with gastrointestinal stromal tumors treated with imatinib mesylate. Cancer 107:2237–2244
Cho BC, Im CK, Park MS, Kim SK, Chang J, Park JP, Choi HJ, Kim YJ, Shin SJ, Sohn JH, Kim H, Kim JH (2007) Phase II study of erlotinib in advanced non-small-cell lung cancer after failure of geftinib. J Clin Oncol 25:2528–2533
Chow LQM, Eckhardt SG (2007) Sunitinib: from rational design to clinical efficacy. J Clin Oncol 25:884–896
Corless CL, Schroeder A, Griffith D, Town A, Mc Greevey L, Harrell P, Shiraga S, Bainbridge T, Morich J, Heinrich MC (2005) PDGFRA mutations in gastrointestinal stromal tumors: frequency, spectrum and in vitro sensitivity to imatinib. J Clin Pathol 23:5357–5365
Dei Tos AP (2003) The reappraisal of gastrointestinal stromal tumors: from Stout to the KIT revolution. Virchows Arch 442:421–428
Demetri GD (2001) Targeting c-kit mutations in solid tumors: scientific rationale and novel therapeutic options. Semin Oncol 28(5 Suppl 17):19–26
Dome JS, Coppes MJ (2002) Recent advances in Wilms tumor genetics. Curr Opin Pediatr 14:5–11
Eble JN, Sauter G, Epstein JI, Sesterhenn IA (2004) Pathology and genetics, tumours of the urinary system and male genital organs. World health organization classification of tumours. IATC, Lyon
Fraizer GE, Bowen-Pope DF, Vogel AM (1987) Production of platelet-derived growth factor by cultured Wilm’s tumor cells and fetal kidney cells. J Cell Physiol 133:169–174
Frost MJ, Ferrao PT, Hughes TP, Asman LK (2002) Juxtamembrane mutant V560Gkit is more sensitive to Imatinib (STI571) compared with wild-type c-kit whereas the kinase domain mutant D816Vkit is resistant. Mol Cancer Ther 1:1115–1124
Ghanem MA, Van Der Kwast TH, Den Hollander JC, Sudaryo MK, Mathoera RB, Van den Heuvel MM, Noordzji MA, Nijman RJM, van Steenbrugge GJ (2001) Expression and prognostic value of epidermal growth factor receptor, transforming growth factor-a, and c-erb B-2 in nephroblastoma. Cancer 92:3120–3129
Graf N, Semler O, Reinhard H (2004) Prognosis of Wilm’s tumor in the course of the SIOP trials and studies. Urologe A 43:421–428
Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ, Kiese B, Eisenberg B, Roberts PJ, Singer S, Fletcher CD, Silbermen S, Dimitrijevic S, Fletcher JA (2003) Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 21:4342–4349
Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y (1998) Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279:577–580
Hirota S, Ohashi A, Nishida T, Isozaki K, Kinoshity K, Shinomura Y, Kitamura Y (2003) Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumors. Gastroenterology 125:660–667
Hirsch FR, Varella-Garcia M, Bunn PA Jr, Franklin WA, Dziadziuszko R, Thather N, Chang A, Parikh P, Pereira JR, Ciuleanu T, von Pawel J, Watkins C, Flannery A, Ellison G, Donald E, Knight L, Parums D, Botwood N, Holloway B (2006) Molecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non-small-cell lung cancer. J Clin Oncol 24:5034–5042
Hornick JL, Fletcher CDM (2007) The role of KIT in the management of patients with gastrointestinal stromal tumors. Human Pathology 38:679–687
Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Teervahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitrijevic S, Druker B, Demetri GD (2001) Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med 344:1052–1056
Kato N, Honma K, Hojo H, Sasou S, Matsuzaki O, Motoyama T (2005) KIT expression in normal and neoplastic renal tissues: immunohistochemical and molecular genetic analysis. Pathol Int 55:479–483
Kitadai Y, Sasaki T, Kuwai T, Nakamura T, Bucana CD, Hamilton SR, Fidler IJ (2006) Expression of activated platelet-derived growth factor receptor in stromal cells of human colon carcinomas is associated with metastatic potential. Int J Cancer 119:2567–2574
Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP (1998) Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 4:844–847
Lasota J, Wozniak A, Sarlomo-Rikala M, Rys J, Kordek R, Nassar A, Sobin LH, Miettinen M (2000) Mutations in exons 9 and 13 of KIT gene are rare events in gastrointestinal stromal tumors. A study of 200 cases. Am J Pathol 157:1091–1095
Medinger M, Drevs J (2005) Receptor tyrosine kinases and anticancer therapy. Curr Pharm Des 11:1139–1149
Miliaras D, Karasavvidou F, Papanikolaou A, Sioutopoulou D (2004) KIT expression in fetal, normal adult, and neoplastic renal tissues. J Clin Pathol 57:463–466
Nocito A, Bubendorf L, Tinner EM, Süess K, Wagner U, Forster T, Kononen J, Fijan A, Bruderer J, Schmid U, Ackermann D, Maurer R, Alund G, Knönagel H, Rist M, Anabitarte M, Hering F, Hardmeier T, Schoenenberger AJ, Flury R, Jäger P, Fehr JL, Schraml P, Moch H, Mihatsch MJ, Gasser T, Sauter G (2001) Microarrays of bladder cancer tissue are highly representative of proliferation index and histological grade. J Pathol 194:349–357
Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fuji Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304:1497–1500
Pan CC, Chih-Hsueh C, Chiang H (2004) Overexpression of KIT (CD117) in chromophobe renal cell carcinoma and renal oncocytoma. Am J Clin Pathol 121:878–883
Peres EM, Savasan S, Cushing B, Abella S, Mohamed A (2004) Chromosome analyses of 16 cases of Wilms tumor: different pattern in unfavorable histology. Cancer Genet Cytogenet 148:66–70
Pritchard-Jones K (2002) Controversies and advances in the management of Wilms’ tumour. Arch Dis Child 26:486–493
Ranson M (2004) Epidermal growth factor receptor tyrosine kinase inhibitors. Br J Cancer 90:2250–2255
Schnadig ID, Blanke CD (2006) Gastrointestinal stroma tumors: imatinib and beyond. Curr Treat Options Oncol 7:427–437
Sihto H, Sarlomo-Rikala M, Tynninen O, Tanner M, Andersson LC, Franssila K, Nupponem NN, Joensuu H (2005) KIT and platelet-derived growth factor receptor alpha tyrosine kinase gene mutations and KIT amplifications in human solid tumors. J Clin Oncol 23:49–58
Smithey BE, Pappo AS, Hill DA (2002) C-kit expression in pediatric solid tumors: a comparative immunohistochemical study. Am J Surg Pathol 26:486–492
Tornillo L, Duchini G, Carafa V, Lugli A, Dirnhofer S, Di Vizio D, Boscaiano A, Russo R, Tapia C, Schneider-Stock R, Sauter G, Insabato L, Terracciano LM (2005) Patterns of gene amplification in gastrointestinal stromal tumors (GIST). Lab Invest 85:921–931
Tornillo L, Terracciano LM (2006) An update on molecular genetics of gastrointestinal stromal tumours. J Clin Pathol 59:557–563
Vujanic GM, Sandstedt B, Harms D, Kelsey A, Leuschner I, De Kraker J (2002) Revised International Society of Paediatric Oncology (SIOP) working classification of renal tumors of childhood. Med Pediatr Oncol 38:79–82
Yarden Y, Kuang WJ, Yang-Feng T, Coussens L, Munemitsu S, Dull TJ, Chen E, Schlessinger J, Francke U, Ullrich A (1987) Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J 6:3341–3351
Yokoi A, Mc Cruddden KW, Huang J, Kim ES, Soffer SZ, Frischer JS, Serur A, New T, Yuan J, Mansukhani M, O’toole K, Yamashiro DJ, Kandel JJ (2003) Human epidermal growth factor receptor signaling contributes to tumor growth via angiogenensis in her2/neu-expressing experimental Wilms’ tumor. J Pediatr Surg 38:1569–1573
Acknowledgments
We are grateful to Barbara Stalder, Molecular Pathology Section, Institute for Pathology, University Hospital Basel, Switzerland for excellent performance of immunohistochemistry and to Melanie Schlangstedt, Institute for Clinical Chemistry and Laboratory Medicine at the University of Regensburg, Regensburg, Germany for PDGFRα sequence analysis. The study experiments comply with the current laws in the country in which they were performed.
This study was funded by a grant of the Basel Cancer League to EB.
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM
Loc recur local recurrence, Meta metastasis, DOD death of disease, KIT % percent of tumour cells with immunohistochemical KIT expression per positive punch cylinder, EGFR % percent of tumour cells with immunohistochemical EGFR expression per positive punch cylinder, Pre-op Chemo preoperative chemotherapy, Follow-up follow-up period indicated in months (XLS 56.5 kb)
Rights and permissions
About this article
Cite this article
Wetli, S.C., Leuschner, I., Harms, D. et al. KIT, PDGFRα and EGFR analysis in nephroblastoma. Virchows Arch 452, 637–650 (2008). https://doi.org/10.1007/s00428-008-0605-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-008-0605-x