Abstract
In light of the growing information on the pathophysiology and clinical aspects of unexpected perinatal loss and sudden infant death syndrome (SIDS), a novel approach to the inherent problems by pathologists has become necessary. Herein, we propose an up-to-date protocol for accurate examination of the central autonomic nervous system and of the cardiac conduction system, which can encompass morphological and/or functional abnormalities of reliable epicritical value in unexplained perinatal loss and SIDS, particularly in those cases (still quite numerous) lacking adequate clinical documentation. Anatomo-pathologic examination of the central autonomic nervous system includes an in-depth study on histological serial sections of the brainstem, cerebellum, and spinal cord, where the main structures participating in control of the vital functions are located. For the histological study of the cardiac conductions system, serial sections were obtained from two blocks, including the sino-atrial node and the atrio-ventricular system, respectively. This type of updated investigation is yielding important arguments for a broader discussion of the pathogenesis of unexpected stillbirth, early neonatal death, and SIDS, besides allowing a more complete forensic-medical documentation of individual cases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Unexpected perinatal loss, which includes stillbirth or fetal death after 22 completed weeks (154 days) and early neonatal death occurring during the first 7 days of life [30] and sudden infant death syndrome (SIDS), represent facets of a multifactorial problem that has not yet found a universally recognized approach on the clinical plane. The fundamental component is pathological anatomy.
These tragic events are still frequent despite the progress in the field of maternal–infant health care. SIDS strikes one infant every 700–1,000 live births, being the most frequent cause of death within the first year of life [5, 17]. Worldwide, there are over 6.3 million perinatal deaths a year, almost all of which occur in developing countries, where the risk of death around birth is six times greater than in developed countries. Stillbirths account for over half of all perinatal deaths, and two-thirds of stillbirths are unexplained [4, 30].
Recently, the International Stillbirth Alliance and the USA Academy of Pediatrics [4, 29] stressed the need to submit all victims of such deaths to neuropathologic studies. In fact, the possibility of preventing perinatal unexpected death and SIDS relies mainly on a better knowledge of the underlying alterations of the central autonomic nervous system (ANS) and of the cardiac conduction system (CCS), which functions under the control of the nervous system.
So far, neuropathological studies have been focused on the consequences of hypoxic cerebral damages (gliosis, etc.) and infections [3]. Instead, studies addressing the identification of peculiar lesions of the structures and/or the nuclei modulating the vital functions, are fundamental for pathogenic evaluations of the reflexogenic sudden death.
Therefore, the pathogenesis of stillbirth and SIDS must be taken in most cases to include some autonomic nervous dysfunctions in the control of the vital functions. A histopathological substrate should be searched for in a wide neuropathology field, including the brainstem nuclei, the cerebellum (cortex and deep nuclei), and the cardiac conduction system, subjected to strict autonomic control [2, 10, 11, 23, 26, 28].
The aim of this work is to propose a more accurate and up-to-date version of the previous guidelines [22], particularly with regard to the protocol for the examination of the nuclei and/or structures presiding over the vital activities (cardiorespiratory, arousal, upper digestive tract, etc.), which in all cases of unexpected perinatal loss and SIDS studied by our group, have shown congenital alterations.
Autopsy, neuropathological, and cardiac sampling and the method of examination of the ANS and the CCS
This protocol was applied in a very wide population of unexpected and unexplained perinatal and infant death victims. This was a selected set of cases, addressed to our Institute on the basis of a specific decree of the Lombardy Region [16]. This project, in fact, imposes that all the cases of unexplained perinatal death and/or of suspected SIDS must be sent to us, as Reference Center, for the in-depth anatomo-pathological examination. Therefore, cases with a defined cause of death (genetic abnormalities, infections, malformations, etc.) were excluded from this study.
In all cases of unexpected perinatal and infant death, a complete autopsy is performed according to the International Standardized Autopsy Protocol of the Global Strategy Task Force of SIDS International [6].
These autopsy guidelines include examination of the placental disk, umbilical cord, and membranes in fetuses and, in all cases, an in-depth histological examination of the cardiorespiratory autonomic nervous system [20, 22].
Protocol for examination of the central ANS
Anatomo-pathologic study of the central autonomic nervous system includes an in-depth study of the brainstem, cerebellum, and spinal cord, where the main structures participating in control of the vital functions are located (cardiorespiratory, arousal, upper digestive tract, etc.).
Brainstem (medulla oblongata, pons, and midbrain)
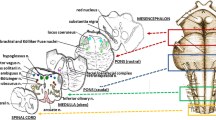
Examination of the brainstem includes sampling of three specimens, as shown in Fig. 1. The first specimen, ponto-mesencephalic, includes the upper third of the pons and the adjacent portion of midbrain. The second extends from the upper third of the medulla oblongata to the portion adjacent to the pons. The third specimen takes as reference point the obex and extends 2–3 mm above it and below it.
Transverse serial sections are made at intervals of 30 µm. For each level, twelve 5-µm sections are obtained, two of which are routinely stained for histological examination alternately using hematoxylin–eosin and Klüver–Barrera stains, and three additional sections at each level are subjected to immunohistochemistry to study the functional activities. The remaining sections are saved and stained as deemed necessary for further investigations.
The routine histological evaluation of the brainstem is focused on the locus coeruleus, the parafacial nucleus, the parabrachial/Kölliker–Fuse complex in the pons/mesencephalon, and on the hypoglossus, the dorsal motor vagal, the tractus solitarius, the ambiguus, the pre-Bötzinger, the inferior olivary, and the arcuate nuclei in the medulla oblongata.
Figure 2 shows the histological sections corresponding to the three brainstem specimens of Fig. 1 indicating the main nuclei and structures to be examined.
Cerebellum
It is essential to make an accurate examination of the cerebellum, too, in view of its role in controlling the respiratory muscles and of the observation of developmental anomalies of the cortex and deep nuclei in cases of perinatal unexpected death and SIDS. To examine both the cerebellar cortex and the nuclei (dentate, etc.), a specimen of hemisphere extending all along the major diameter should be sampled.
Spinal cord (cervico-thoracic tract)
The removal of the first five thoracic levels of the spinal cord enables examination of the orthosympathetic intermediolateral nucleus.
Protocol for examination of the functional activities of the central ANS
To analyze the immunoexpression of neurotransmitters, particularly somatostatin, substance P and tyrosine-hydroxylase, sections are subjected to immunohistochemical methods with specific primary antibodies after application of the avidin–biotin–peroxidase technique, in conformity with conventional immunohistochemical procedures. Immunohistochemical visualization of apoptotic cells was obtained by the TUNEL method (TdT-mediated dUTP-biotin nick end labeling).
A detailed description of the immunohistochemical methods, including the immunopositivity evaluation, applied in this study is available in our previous works [9, 12–14].
Protocol for morphometric analysis of the central ANS
The morphologic examination of the main nuclei and structures of the brainstem and cerebellum is usually supplemented by morphometric analysis, performed with an image analyzer. The following parameters are evaluated and indicated as mean values and standard deviation: area (expressed in mm2), neuronal density (expressed as number of neurons per mm2), and neuronal size (cell body area, expressed in μm2). An in-depth description of the methodology can be found in our previous works [7, 8, 13, 15, 19, 21].
Protocol for examination of the CCS
The neonatal and infant hearts are regularly examined for pathologic changes in the atria, septa, ventricles, pericardium, endocardium, and coronary arteries. Samples of the myocardium are stained with hematoxylin–eosin and trichromic Heidenhain (azan).
Histological observations of the heart are focused on the cardiac conduction system. The conducting tissue, although fairly constant in layout and structure, is subject to noteworthy individual variations; hence, histological examination on serial sections is expected to provide the necessary data on both topography and pathology of the specialized tissue. The CCS is removed in two blocks for paraffin embedding.
Block 1
This contains the sinu-atrial (SA) node, its atrial approaches, and the Crista Terminalis and the SA node gangliar plexus. The main visual reference for removal is centered upon the Sulcus–Crista Terminalis. Two longitudinal cuts are driven, parallel to the Sulcus–Crista line, through the atrial wall, with a medial prolongation on the right side to encompass the anterior aspect of the inlet of the superior vena cava; on the left side, the cava-cava bridge has to be sectioned very medially, prolonging the cut on the superior vena cava wall. Of the two transverse cuts, the superior one is oriented to remove as much as possible of the cava funnel in between the longitudinal sections. The inferior cut removes, more or less distally (according to the atrial volume), the fan of pectinate muscles that radiate from the Crista Terminalis (Fig. 3).
The procedure for removing the two blocks for the study of the cardiac conduction system. Block 1 contains the sino-atrial node (SAN), its atrial approaches, the Crista Terminalis, and the sinu-atrial node gangliar plexus. The main visual reference for the removal is centered upon the Sulcus-Crista Terminalis. Block 2 contains the atrio-ventricular node (AVN), the His bundle (HB), and bundle branches. The reference points for the excision are, on the right side, the outlet of the coronary sinus and the pars membranacea septi
Block 2
This contains the atrium–ventricular (AV) system with its atrial approaches: the reference points of the excision are, on the right side, the outlet of the coronary sinus and the pars membranacea septi. By holding the already opened heart so as to expose the interventricular septum against a fairly intense light source, the transparent area of the pars membranacea can be clearly spotted and pinned between thumb and index; thereupon, the procedure continues with excision of the interventricular septum together with the central fibrous body, the lowermost part of the atrial septum, and the adjacent segments of the AV fibrous annuli. The cuts will be driven as follows: (a) an inferior, longitudinal incision through the posterior part of the septum, across the AV annulus fibrous and up to the superior margin of the coronary sinus ostium; (b) an anterior longitudinal incision parallel to the former, through the superior part of the septum, extending to the aortic valvular ring; (c) and (d) two cuts perpendicular to (a) and (b), to remove the tissue block, with its upper margin about 1.5 cm above the AV ring and its lower margin encompassing the base of the medial tricuspid papillary muscle and possibly the moderator band.
Both blocks 1 and 2 are routinely fixed in 10% buffered formalin that, besides simplicity, has the advantage of allowing further silver impregnations of the nervous system, whenever needed. On paraffin embedding, it is advisable to place the medial margin of block 1 and the posterior–inferior margin of block 2 at the bottom. The resulting section plane of block 1 is perpendicular to the atrial endocardial surface and parallel to the major axis of the Crista Terminalis. The block 2 section is also perpendicular to both endocardial surfaces, along the major septal axis. Besides the generic differences from these oversimplified models, individual variations must be expected, due to the remarkable variability of the conduction system and to the slight discrepancies occurring in the excision of the heart fragments from case to case.
Regarding fetal hearts, the small hearts from fetuses aged 22–35 gestational weeks are processed entire and serially cut. The section plane of this paraffin block is perpendicular to the interatrial and interventricular septa and parallel to the anterior heart surface. For near to term and term fetuses aged 36–42 gestational weeks, the hearts are sampled according to the above-described method for infant and neonatal hearts with the exception of fetuses that are small for gestational age (Fig. 4).
Histological slide, trichromic Heidenhain (azan) stained, of a heart from a female fetus aged 23+4 gestational weeks. The heart was processed entire and serially cut. The section plane of the heart’s paraffin block was perpendicular to the interatrial and interventricular septa and parallel to the anterior heart surface
For the histological examination of the conduction tissue, the one or two paraffin blocks are cut serially at intervals of 20–40 μm (levels). For each level, three sections are retained and mounted, and one of the mounted sections is stained alternately with hematoxylin–eosin, azan, and terminal uridine deoxynucleotidyl transferase dUTP nick end labeling for apoptosis. All intervening sections are kept and stained as deemed necessary. For each heart, in our experience, the average number of histological sections stained and examined was about 100. A 3-D reconstruction of the conduction system can be obtained from the series of 2-D slides [18, 24, 25, 27].
The morphological investigations of both ANS and CCS should be completed by the application of molecular biology methods with the purpose of disclosing genetic abnormalities (particularly mutations and genetic polymorphisms) and the presence of any microorganisms, including viruses.
Results of the application of the guidelines in perinatal and infant unexpected death
Our morphological investigations in 71 cases of sudden perinatal death (57 late fetal and 14 neonatal deaths) and 138 cases of SIDS have disclosed a variety of congenital lesions, as reported in our previous studies, of the autonomic nervous system (particularly of the brainstem and cerebellum) [7–11, 12–15, 19, 21] and of the cardiac conduction system [18, 24, 25, 27].
From the overall analysis of the neuropathological results, the following prominent data emerged:
-
1.
In all cases of sudden perinatal and infant death, one or more congenital morphological and/or functional abnormalities of the brainstem and cerebellum were observed.
-
2.
In the SIDS victims, the main alteration was hypoplasia of the arcuate nucleus, sometimes associated to hypoplasia of the reticular formation and of the hypoglossus nucleus. This hypoplasia was present with the same incidence in fetal and infant unexpected deaths.
-
3.
Hypoplasia of the facial/parafacial complex was a very frequent and specific anomaly of sudden fetal deaths never observed after birth.
-
4.
Hypoplasia of the Kölliker–Fuse nucleus was frequently observed in perinatal deaths (stillbirths and early neonatal deaths).
-
5.
The cerebellar cortex showed in many cases delayed maturation particularly in SIDS victims.
-
6.
Among the functional alterations, altered expression of neurotransmitters in the brainstem was frequently diagnosed, particularly of catecholamine in the locus coeruleus and of somatostatin in the hypoglossus nucleus, besides defective apoptotic programs in cerebellum (precisely in the Purkinje and internal granular layers of the cerebellar cortex and in the dentate nucleus).
From the overall analysis of the cardiac conduction system results, the following prominent data emerged:
-
1.
Accessory atrioventricular pathways, mostly Mahaim fibers, in 27% of stillbirth and in 23% of SIDS victims.
-
2.
Cartilaginous hypermetaplasia in 20% of stillbirth and in 12% of SIDS.
All of these cardiac conduction findings may be isolated or associated with central nervous system alterations. These associations were observed in 30% of cases of our studies.
Discussion
In light of the growing information on the pathophysiology and clinical aspects of unexpected perinatal loss and SIDS, a novel approach to the inherent problems by pathologists (particularly by those entrusted with forensic medical authority) has become necessary.
This type of updated investigation, extending from the cardiac conduction system up to the brainstem and cerebellar vital centers, has heretofore been neglected even in the recent standardization of diagnostic criteria of SIDS proposed by a very wide group of researchers in this field [1].
Herein, we have reported a new and more updated version of the standard technical protocol for performing accurate examination of the central autonomic nervous system and of the cardiac conduction system in cases of unexplained perinatal loss and SIDS.
Our studies, performed according to this protocol, have revealed frequent structural and/or functional alterations of the autonomic nervous system and of the cardiac conduction system in the victims of perinatal unexpected death and SIDS [7–9, 12–15, 19, 21]. These alterations, which are frequently associated, are mainly of a congenital nature and therefore represent a common morphological substrate in both perinatal and infant unexplained death.
A particularly common finding in these pathologies was hypoplasia of the arcuate nucleus, a chemoreceptorial component of the ventral surface, which was present in over 50% of the victims, albeit with different degrees of extension and severity. In perinatal deaths, in addition to hypoplasia of the arcuate nucleus, we observed hypodevelopment of the reticular formation, consisting of the presence of very sparse branching of the dendrites into the neuropil, prevalently in the ambiguous and pre-Bötzinger nuclei areas, besides hypoplasia of the parabrachial/Kölliker–Fuse complex. Moreover, exclusively in unexplained fetal death victims, apart from hypoplasia of the arcuate nucleus, we frequently observed hypoplasia of the parafacial nucleus. In unexplained death victims, we also observed functional alterations of different nuclei and/or structures of the brainstem, mainly consisting of severe disturbances of the neuroreceptoral activities (particularly in the locus coeruleus and in the hypoglossus nucleus) [9, 13].
Among the abnormalities of the cardiac conduction system, sometimes associated with abnormalities of the autonomic nervous system, the most frequent were accessory atrioventricular pathways, mostly Mahaim fibers [18, 24, 25, 27].
Subtle isolated or associated lesions involving these structures are likely responsible for the disruption of the neuronal pathways. In particular, neuropathological involvement of the vagal-glossopharyngeal neuronal circuit may explain some lethal reflexogenic mechanisms of sudden perinatal death (in both the near term fetus and the newborn) and early infant death, including SIDS.
Already in 2002 at the International Congress on SIDS [20], we indicated the need to analyze all these structures in the context of an in-depth neuropathological examination of the autonomic nervous system and proposed the new definition of SIDS as the “sudden death of an infant under one year of age which remains unexplained after a thorough case investigation, including performance of a complete autopsy and in-depth histology of the cardiorespiratory autonomic nervous system, examination of the death scene, and a review of the clinical history.”
All in all, the above-illustrated autopsy examination procedures enable analysis of the morpho-functional substrates of perinatal loss and of SIDS, namely, the reflexogenic histopathology focused upon the vital (namely cardiorespiratory) neuronal circuitry. In conclusion, neuropathology is an integral component and a consistent substrate of both SIDS and unexpected perinatal loss; an updated appraisal of fundamental aspects of the histopathology of the autonomic nervous system and the cardiac conduction system could help to foster a better understanding of the problem at issue.
It is hoped that the neuropathological protocol we propose will prove useful to the pathologist faced with victims of unexpected perinatal loss and SIDS, in the autopsy room, to explain their deaths and hopefully, in the future, to save other lives.
References
Bajanowski T, Vege A, Byard RW, Krous HF, Arnestad M, Bachs L, Banner J, Blair PS, Borthne A, Dettmeyer R, Fleming P, Gaustad P, Gregersen M, Grøgaard J, Holter E, Isaksen CV, Jorgensen JV, de Lange C, Madea B, Moore I, Morland J, Opdal SH, Råsten-Almqvist P, Schlaud M, Sidebotham P, Skullerud K, Stoltenburg-Didinger G, Stray-Pedersen A, Sveum L, Rognum TO (2007) Sudden infant death syndrome (SIDS)—standardised investigations and classification: recommendations. Forensic Sci Int 165:129–143
Blanco CE, Dawes GS, Hanson MA, McCooke HB (1984) The response to hypoxia of arterial chemoreceptors in fetal sheep and new-born lambs. J Physiol 351:25–37
Grafe MR, Kinney H (2002) Neuropathology associated with stillbirth. Sem Neuropathol 26:83–88
International Stillbirth Alliance (2007) http://www.stillbirthalliance.org/. Cited June 9, 2007
Kochanek KD, Murphy SL, Anderson RN, Scott C (2004) Deaths: final data for 2002. Natl Vital Stat Rep 53:1–115
Krous HF (1996) Instruction and reference manual for the International Standardise Autopsy Protocol for sudden unexpected infant death. J SIDS Infant Mortal 1:203–246
Lavezzi AM, Ottaviani G, Mauri M, Matturri L (2003) Hypoplasia of the arcuate nucleus and maternal smoking during pregnancy, in perinatal and infant sudden unexpected death. Neuropathology 24:284–289
Lavezzi AM, Ottaviani G, Ballabio GM, Rossi L, Matturri L (2004) Preliminary study on the cytoarchitecture of the human parabrachial/Kölliker–Fuse complex with reference to sudden infant death syndrome and sudden intrauterine unexplained death. Pediatr Dev Pathol 7:171–179
Lavezzi AM, Ottaviani G, Matturri L (2004) Role of somatostatin and apoptosis in breathing control in sudden perinatal and infant unexplained death. Clin Neuropathol 23:304–310
Lavezzi AM, Ottaviani G, Rossi L, Matturri L (2004) Cytoarchitectural organization of the parabrachial/Kölliker–Fuse complex in man. Brain Dev 26:316–320
Lavezzi AM, Ottaviani G, Rossi L, Matturri L (2004) Hypoplasia of the Parabrachial/Kölliker–Fuse complex in perinatal death. Biol Neonate 86:92–97
Lavezzi AM, Ottaviani G, Matturri L (2005) Adverse effects of prenatal tabacco smoke exposure on biological parameters of the developing brainstem. Neurobiol Dis 20:601–607
Lavezzi AM, Ottaviani G, Mingrone R, Matturri L (2005) Analysis of the human locus coeruleus in perinatal and infant sudden unexplained death. Possible role of the cigarette smoking in the development of this nucleus. Dev Brain Res 154:71–80
Lavezzi AM, Ottaviani G, Mauri M, Matturri L (2006) Alterations of biological features of the cerebellum in sudden perinatal and infant death. Curr Mol Med 6:429–435
Lavezzi AM, Matturri L (2007) Functional neuroanatomy of the human pre-Bötzinger complex with particular reference to sudden unexplained perinatal and infant death. Neuropathology (in press)
Lombardy Regional Decree n. 11693 of 06-20-02 (2007) “Legislative measures on adoption of anatomo-pathological and forensic-pathological intervention targeted on the prevention, knowledge, and identification of the sudden infant death cases.” http://users.unimi.it/~pathol/pdf/decreto.pdf. Cited August 21, 2007
Matthews T, McDonnell M, McGarvey C, Loftus G, O’Regan M (2004) A multivariate time based analysis of SIDS risk factors. Arch Dis Child 89:267–271
Matturri L, Ottaviani G, Ramos SG, Rossi L (2000) Sudden infant death syndrome (SIDS): a study of cardiac conduction system. Cardiovasc Pathol 9:137–145
Matturri L, Biondo B, Suarez-Mier MP, Rossi L (2002) Brain stem lesions in the sudden infant death syndrome: variability in the hypoplasia of the arcuate nucleus. Acta Neuropathol (Berl) 104:12–20
Matturri L, Lavezzi AM, Rossi L (2002) Proposal to modify the definition of SIDS, with regard to the post mortem exam In: Proceed. 7th SIDS International Conference. Florence, Italy; August 31-September 4, 2002, p. 103
Matturri L, Minoli I, Lavezzi AM, Cappellini A, Ramos S, Rossi L (2002) Hypoplasia of medullary arcuate nucleus in unexpected late fetal death (stillborn infants): a pathologic study. Pediatrics 109:1–5
Matturri L, Ottaviani G, Lavezzi AM (2005) Techniques and criteria in pathologic and forensic-medical diagnostics of sudden unexpected infant and perinatal death. Am J Clin Pathol 124:259–268
McKay LC, Janczewski WA, Feldman JL (2005) Sleep-disordered breathing after targeted ablation of preBötzinger complex neurons. Nat Neurosci 8:1142–1144
Ottaviani G, Matturri L, Rossi L, James TN (2003) Crib death: further support for the concept of fatal cardiac electrical instability as the final common pathway. Int J Cardiol 92:17–26
Ottaviani G, Matturri L (2007) Histopathology of the cardiac conduction system in sudden intrauterine unexplained death (SIUD). Cardiovasc Pathol (in press)
Rekling JC, Feldman JL (1998) PreBötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu Rev Physiol 60:385–405
Rossi L, Matturri L (1990) Clinicopathological approach to cardiac arrhythmias. Centro Scient Torinese, Turin. Distributed by Futura, Mount Kisco, NY
Teitel DF (1996) Fetal chemoreception: a developing story. Reprod Fertil Dev 8:471–482
USA Academy of Pediatrics (2005) http://www.msnbc.msn.com/id/9649335/. Cited June 9, 2007
World Health Organization (WHO) (2006) Neonatal and perinatal mortality: country, regional and global estimates. WHO, Geneva, pp 1–75
Acknowledgments
This study was supported by the Italian Lombardy Region target project n. 49210/2000 (Program of research and intervention for the reduction of the risk of SIDS and unexpected fetal death), by the agreement Lombardy Region-Pfizer Italia S.r.l. n. 814/2006, and by Ministry of Foreign Affairs (joined projects of particular relevance “Anatomopathologic and genetic study of the unexplained perinatal death and SIDS”) n. 269/P/0085087/2004 and n. 0083227/20006.
Author information
Authors and Affiliations
Corresponding author
Additional information
“Lino Rossi” Research Center for the study and prevention of unexpected perinatal death and SIDS, Institute of Pathology, University of Milan, Italy.
Rights and permissions
About this article
Cite this article
Matturri, L., Ottaviani, G. & Lavezzi, A.M. Guidelines for neuropathologic diagnostics of perinatal unexpected loss and sudden infant death syndrome (SIDS)—a technical protocol. Virchows Arch 452, 19–25 (2008). https://doi.org/10.1007/s00428-007-0527-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-007-0527-z