Abstract
Neuroendocrine (NE) tumours of the lung include pure and mixed forms. In the former group, a continuum of lesions is recognised ranging from benign typical carcinoids to atypical carcinoids (having a low-grade behaviour, although often associated with regional and distant metastases), to the highly aggressive poorly differentiated carcinomas of the small and large cell types. In the mixed tumour group, the NE component is extensively represented in association with any of the non-small cell carcinoma subtypes (so-called combined carcinomas), or the NE component is restricted to a cell population scattered among adenocarcinoma cells (or more rarely within squamous or large cell carcinomas). The molecular profile of NE tumours has been widely investigated to identify features helpful for the diagnosis, prognosis and even therapy for this special lung tumour category. Specific chromosomal alterations, oncogene mutations and cell cycle molecule disregulation has been documented in NE tumours of the lung, as well as the expression of specific receptors or enzymes implicated in the response to biotherapies or to chemotherapeutic agents. The “molecular classification” of NE tumours should be integrated to morphology, for a better definition of the different histological types and a more appropriate selection of the therapeutic strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroendocrine tumours (NET) of the lung share most morphological and clinical features observed in neuroendocrine (NE; carcinoid) tumours of other organs, e.g. pancreas and intestine. Similarly, pulmonary NETs do not constitute a single, uniform entity but build up a spectrum of NE differentiated lesions associated to specific pathological features on the one side and to a variable clinical behaviour on the other. Typical carcinoids (TC) and atypical carcinoids (AC), as well as poorly differentiated NE carcinomas of the large and small cell types were identified.
Appropriate diagnostic and classification criteria, of high clinical and prognostic value, have been established for NETs of the lung, although a precise “functional” classification of these tumours, in terms of hormonal production, is still lacking. As a consequence, only “common” NE markers are of diagnostic significance in the definition and identification of pulmonary NETs. Among such markers, chromogranins/secretogranins are of major interest. Their detection by specific anti-bodies or by molecular procedures (to reveal the specific mRNA) is not only of diagnostic importance, but it allows drawing of information on cell metabolism and on the storage or release of neurosecretory granules as well. Chromogranin A (CgA), the most widely and intensely expressed member of the family, is stored in high amounts in carcinoids and to a variable extent in poorly differentiated NE carcinomas.
This review will briefly summarise the current diagnostic criteria of each single type of pulmonary NET (both pure and combined forms) with special reference to difficult issues for the differential diagnosis, and will finally consider the phenotypic and molecular data having a pathological and/or clinical relevance.
NE cells in normal lung and in non-neoplastic conditions
Normal NE cells (also called Kulchitsky cells) of the bronchial tree occur solitarily or in small aggregates, so-called neuroepithelial bodies, within the ciliated epithelium [17]. Lung NE cells produce a variety of peptides such as serotonin, bombesin/gastric releasing peptide, calcitonin and the recently identified ghrelin [60], while in hyperplastic or neoplastic conditions, adrenocorticotropic hormone (ACTH), vasoactive intestinal peptide or somatostatin productions have been reported [9].
A role of NE cells in lung development, growth and repair has been suggested based on their increased expression levels in infants with pulmonary dysplasia, cystic fibrosis or prolonged assisted ventilation [52].
NE cell hyperplasia is a microscopic finding only that displays three different patterns including: (1) increased number of scattered NE cells, (2) linear proliferations along the bronchial mucosa and (3) nodular hyperplasia in the mucosa. Most cases are idiopathic (“diffuse idiopathic pulmonary NE cell hyperplasia,” associated with obliterative bronchiolar fibrosis in the absence of known causes of interstitial or airway fibrosis or inflammation) [28], while others may be incidentally found in chronic bronchial inflammation [42], Langerhans cell hystiocytosis [2] or bronchopulmonary dysplasia [16].
At the extreme of this spectrum, the term “tumourlet” defines NE cell proliferations that extend beyond the basal membrane of bronchi and bronchioles, having a size less than 5 mm and a dense fibrous stroma surrounding cell clusters.
NE tumours
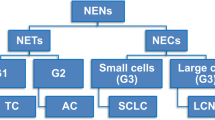
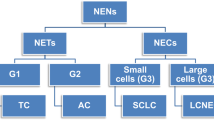
The WHO classification of NETs of the lung has combined architectural patterns (e.g. organoid growth vs small cell diffuse growth) with other parameters, with the mitotic index and the presence of necrosis as the two most relevant, for the purpose of recognising the four different categories proposed in the spectrum of pure NETs of the lung (Table 1).
Typical carcinoid
TC represents less than 1% of lung tumours. As opposed to other NE lung tumours, it is not related to smoking and develops in adults (mean age of 55 years) with an equal male/female distribution. The most common location is in the large airways, presenting as a polypoid mass, which protrudes in the bronchial lumen. Several histo-morphologic patterns can be recognised, most often in combination. Tumour cells are polygonal, with abundant, finely granular and eosinophilic cytoplasm. Nuclei show clumped open chromatin and small dark nucleoli. The growth pattern is most often trabecular with cords or ribbons of tumour cells dispersed in a delicate fibrovascular stroma. More rarely, spindle cell, acinar or glandular patterns, with occasional oncocytic and clear cell changes, can be recognised.
Necrosis is always absent and mitosis are less than 2 × 10 high power fields (HPF): both features are essential to differentiate TCs from other NE lung tumours of higher grade (see also Table 1).
Spindle cell variety of TC should be distinguished from nerve sheath proliferations and haemangiopericytomas, defined by S-100 protein and vascular markers immuno-reactivity, respectively, in contrast to the presence of NE markers in TCs. Carcinoids having glandular formation may be hardly distinguished from well-differentiated adenocarcinomas, especially when mucin secretion is observed. Clear cell changes may resemble clear cell (sugar) tumours, which are invariably positive for CD34 and HMB45 and lack NE markers.
Atypical carcinoid
AC represents nearly 10% of NE lung tumours, even if different series are difficult to compare because, in some of them, large cell NE carcinomas (LCNEC) are included in this same group. ACs are more often associated with cigarette smoking, they have a male pre-dominance and are more frequently peripherally located, compared to TCs. Conversely, they share the same morphologic, cytologic and architectural features as TCs (Fig. 1), and the correct diagnosis of the two forms may be difficult, especially in small biopsies. As already mentioned, the main differential criteria are the presence of necrosis and a higher mitotic count (2–10 × 10 HPF) in ACs. Necrosis has a peculiar focal, punctate, sharply demarcated appearance. Focal nuclear pleomorphism may also occur. It has to be considered that up to 20% of ACs may lack NE markers. Peripherally located tumours more frequently show spindle cell appearance and smaller cell size. These cases have to be separated from small cell carcinomas, which usually have a higher mitotic count, a dense and uniform nuclear chromatin, and extensive necrosis. The distinction between ACs and LCNECs is even more difficult and will be discussed in the next paragraph.
Spectrum of NETs of the lung: AC was separated from TC, based on high-grade morphological features [4]. AC had a more aggressive behaviour than benign carcinoid, but in this group, cases with indolent clinical course co-existed with highly aggressive neuroendocrine carcinomas
Large cell NE carcinoma
LCNEC was recognised in 1991 as a separate entity [54] based on the observation that a group of highly aggressive NE lung carcinomas exists, apart from the well-known small cell (oat cell) carcinoma. Because these tumours maintain at least in part the organoid and trabecular growth patterns (typical of carcinoid tumours) and are made of large cells, the term LCNEC was proposed. NE differentiated carcinomas with extensive necrosis, high mitotic index and marked propensity to invasion and distant spread were grouped in this novel category and found to have a clinical behaviour not different from that of small cell carcinoma [5, 54, 55] (Fig. 2). Unfortunately, in the last WHO classification of lung tumours [56], this tumour entity was included in the large cell carcinoma group, generally encompassing non-NE carcinoma subtypes and having nothing to do with LCNEC, with the possible exception of cell size. The main differential diagnostic problem is represented by ACs. LCNECs share the classical solid organoid and lobulated NE morphology but have an extensive, geographic-type necrosis, with a prominent infarct-like appearance and a mitotic count exceeding 10 mitoses per 10 HPF. Large tumour size, marked pleomorphism, lower nuclear to cytoplamic ratio and large nuclei with prominent nucleoli are additional features. The cell size distinguishes large cell NE from small cell carcinoma, but cases of co-existing small and large cell components are well documented. In such instances, an uncommittal report of poorly differentiated NE carcinoma with combined small land large cell features may be sufficient for the purpose of selecting the appropriate therapeutic strategy (chemotherapy; Figs. 3, 4). Two other highly malignant tumours enter into the differential diagnosis with LCNEC: basaloid carcinoma and large cell carcinomas. Minor morphological features (solid growth in large cell type, peripheral palisading in basaloid carcinoma) and, more importantly, expression of NE markers help to discriminate between these entities. The diagnostic immuno-histochemical profile of LCNEC includes more or less extensive (depending also on the use of antigen retrieval procedures) expression of cytoplasmic CgA and synaptophysin as well as neural cell adhesion molecule at the cell membrane level [25]. The simultaneous absence of high molecular weight cytokeratin (types 1, 5, 10, 14 of the Moll’s catalog) reactivity is an additional finding supporting a diagnosis of NET. These cytokeratins are in fact restricted to non-NE carcinomas of the lung [50, 59] and of other organs [36].
Spectrum of NETs of the lung: the intermediate group of NETs, i.e. the ACs, was further split into two entities, having a LCNEC subtype recognised [54]. The latter had a significantly different clinical behaviour from carcinoid tumors [55, 54], but no differences in outcome were seen between LCNEC and small cell carcinoma [5, 55]
The LCNEC subtype [54] (red circle) includes former anaplastic large cell carcinomas (LCC) with NE differentiation, former small cell cancers (SCLC) of the intermediate cell type and those former AC having >10 mitoses/10HPF
Small cell lung carcinoma
Small cell lung carcinoma (SCLC) usually arises as a rapidly growing tumour of the major airways. It is made up of small cells with scanty cytoplasm and condensed small nuclei, approximately three lymphocytes in size. Neoplastic cells grow in diffuse sheets loosely connected in a thin stroma. The distinction in three groups, according to the cell size, as oat cell, intermediate and combined cell type, has poor correspondence to clinical features and seems, at least for the first two forms, to be related more to tissue preservation, especially in biopsy specimens, than to true cell characteristics. In addition, several cases of intermediate cell variant are now re-classified as LCNEC. High mitotic rate and large infarct-like necrosis are additional main features.
Small cell carcinoma should be differentiated from several small cell proliferations, both of epithelial and mesenchymal origin. With regard to the latter, pulmonary lymphomas may resemble small cell carcinoma, and several nuclear and cytologic features may overlap, making immuno-histochemistry mandatory for a correct diagnosis. Other small cell tumours, which may be confused with small cell carcinoma include basaloid carcinoma, poorly differentiated squamous cell carcinoma and peripheral neuroectodermal tumours of the thoracic wall (so-called Askin tumours). The differential diagnosis with the former three is based on immuno-histochemical findings and on few morphologic features, such as loose connective tissue, nuclear condensation and scanty cytoplasm, which are more prominent in small cell carcinoma. The distinction from Askin tumour may be more difficult because of several shared features. In Askin tumour, however, the nuclei have a more dispersed chromatin, one or more small nucleoli, and specific immuno-histochemical markers, such as CD99, are constitutively expressed.
Although absent in TC and AC [50], TTF-1 is positive in the majority of both large and small cell NE carcinomas and is therefore useful to differentiate these lesions from poorly differentiated NE carcinomas of other sites. High molecular weight cytokeratins are not expressed in small cell lung cancer, in contrast with non-NE carcinomas [59].
Combined NE carcinomas
A small proportion of NE carcinomas are histologically heterogeneous. This occurs mostly with poorly differentiated carcinomas, both small and large cell types, which may grow in association with squamous cell carcinomas and adenocarcinomas, or, less frequently, with sarcomatous tumours. Combined carcinomas share many clinical, epidemiologic and prognostic features of the NE counterpart, so that, in the WHO classification of lung tumours, they are generally considered as variants of small and LCNECs (codes 8045/3 and 8013/3, respectively). The morphology of such tumours recapitulates the specific features of the individual components. The type of mixture of the two cell populations may either be represented by an intimate inter-mingling of cell types, with divergent differentiation along NE and “exocrine” lineages, or may represent a sort of “collision” tumour with a small/large cell carcinoma growing adjacent to a non-small cell lung carcinomas (NSCLC), with minimal interplay between the two components. Immuno-histochemistry is helpful for the correct identification of combined tumours and in our opinion a panel of markers, including CgA and CD56 (for NE cells) and high molecular weight cytokeratins (for NSCLC types), is sufficient to detect the differential reactivity of the two cell populations.
NE differentiation in NSCLC
More or less extensive areas of NE differentiation can be easily detected by CgA immuno-histochemistry in otherwise conventional NSCLC, as also observed in carcinomas of several other organs. This finding does not address to a diagnosis of mixed or combined NE-exocrine carcinoma, unless the extent of NE cells exceeds 30% of the whole tumour (according to the definition of mixed exocrine–endocrine carcinomas) and is generally restricted to a minor cell population. In fact, the recognition of a NE phenotype is possible with immuno-histochemistry only, in the absence of light microscopical features of the NE origin. NE features may be recognised in all lung carcinoma types, most frequently in adenocarcinomas but also in squamous and large cell carcinomas or in sarcomatoid carcinomas/blastomas [1, 6, 10, 47]. The percentage of NE cells may range from 3 to 25%, either as single cells scattered among non-NE glandular or squamous neoplastic cells, or more rarely as small clusters of neoplastic cells admixed within the non-NE component. Little is known about the significance of NE differentiation in NSCLC, and controversial data exist in the literature about the different behaviour and response to chemiotherapy for NE differentiated vs conventional NSCLCs. A recent study [19] indicates that NE differentiation has no impact on survival of a series of NSCLC, at variance with other studies [38], which found a worse prognosis in stage I NSCLC with NE differentiation. This is not surprising because NE differentiation is a well-known indicator of poor prognosis in prostate cancer [7] and in gastric adenocarcinoma [22].
Molecular profiling of lung NETs
The molecular profile of NE lung cancer has been extensively investigated with the goal of identifying features helpful for diagnosis, prognosis and even therapy for this special lung tumour category. The endpoint of genome, gene transcript, cell product, receptor and regulatory peptide expression analysis is ultimately to create a “molecular classification” of lung NETs that can serve as a complement or alternative to morphology to better define the different histological types and address the appropriate therapeutic strategy.
In the search for molecular markers of lung NETs, gene expression profile studies are increasing. Apart from common NE markers (such as chromogranins), insulinoma-associated (IA-1) gene and human achaete-scute homolog-1 (hASH1) gene overexpression were observed [8, 15, 51]. Among them, hASH1 is a recently described marker for NE differentiation in lung tumours [21] and was also suggested as a therapeutic target because of its cell growth modulation effect in lung cancer cell lines [33]. Other reports [3] found that carcinoids (typical and atypical) had genes that clustered with gliomas, while SCLC clustered with bronchial epithelium, suggesting a different histogenesis of these two tumour subtypes. Subsequent studies confirmed such distinct molecular subgroups [8, 23]. Moreover, by immuno-histochemistry, an expression profile of positive carboxipepditase E (CPE) and negative gamma-glutamil hydrolase (γGH) was preferentially detected in TC and AC thus significantly predicting good prognosis, whereas the opposite phenotype (negative CPE and positive γGH) was a feature of LCNEC and SCLC [18].
Comparative genomic hybridisation identifies genome alterations of chromosome copy number, gains and losses, as well as gene amplifications, although the precise localisation of the altered loci cannot be obtained with this methodology. The most common genetic feature in both TCs and ACs is the allelic deletion of the long arm of chromosome 11. Different loci in 11q are altered in these tumours, being 11q13 (linked to the MEN1 gene locus) lost in nearly one third of sporadic carcinoids, both typical and atypical. It was recently demonstrated that the main genetic defect of MEN1 consist in loss of heterozygosity (LOH) or micro-satellite instability (MSI), which lead to the consequent absence of the gene product menin in TC and AC [58]. However, the development of lung carcinoids in MEN1 syndrome is a rare occurrence. MEN1 gene alterations are also virtually absent in poorly differentiated NE carcinomas, and MEN1 kindreds do not present these types of tumours as inherited carcinomas. Interestingly, familial lung carcinoid syndromes different from MEN1 have been described, and still need to be better defined [31].
With regard to SCLC, the vast majority shows deletions of the short arm of chromosome 3, being the putative tumours suppressor genes still to be completely identified. The von Hippel Lindau (3p25) and FHIT (3p14.2) genes seem to be the most likely candidates, and LOH of both has been detected in a high percentage of SCLC. However, although the frequency of LOH at 3p was higher in the group of poorly differentiated NE carcinomas, large and small cell types had different LOH patterns at 3p and also at 22q), and a significantly different genetic background was observed in these two forms [53]. Finally, DNA gain in 17q24–25 was demonstrated as markers of brain metastases in SCLC [41].
Among alternative mechanisms of gene inactivation, hypermethylation contributes to the silencing of genes acting as tumour suppressors (DNA repair, cell cycle regulation, angiogenesis and invasion modulators, etc). As compared to other lung cancer types, this mechanism is rarely acting in lung NETs. In fact, DNA methylation rarely involves TC, whereas RASSF1A and Caspase 8 are more frequently altered in AC and SCLC [48].
Among genetic alterations involving cell cycle regulators, p53 gene point mutations are frequently present in LCNEC and SCLC, while absent in all TCs and most ACs [32]. In this context, immuno-histochemical detection of p53 protein has been proposed in the differential diagnosis of NE lung tumours. Mutations in RB1 gene with loss of function of the retinoblastoma gene product have also been detected in most SCLC [13, 24]. Similarly, mutations of the PTEN gene were also mainly restricted to SCLC [61].
Concerning key pathways that regulate the cell cycle, altered expression patterns of proteins acting in several steps have been described, with diagnostic or prognostic implications. Rb/p16/cyclin D1 pathway aberration is one of the most common in NETs, with a variety of molecules and different mechanisms involved, probably reflecting variable genetic divergency among individual tumours [20]. Signalling pathway of the E2F family of transcriptional factors (E2F1, E2F3) was studied in lung NETs [11]. Recently, Salon et al. [46] provided evidence of a direct and functional interconnection between the E2F-1, Skp2 and cyclin E oncoproteins in poorly differentiated lung NE carcinomas, but not in TC and AC, suggesting their co-operation in the development of these tumours.
Telomerase activity was widely studied in NE lung tumours. Telomerases are the enzymes that synthesise telomeric DNA strands, thereby compensating the DNA losses during each cell division. A high intra-cellular telomerase activity can be considered the compensation to telomere length shortening resulting from uncontrolled proliferation. SCLCs have high telomerase activity in nearly 90–100% of the cases, confirming the model of a high proliferation with extensive genetic instability, as demonstrated by their numerous allelic losses [26]. Moreover, a significantly higher telomerase activity in LCNEC than in TC was recently described [30, 62]. In lung cancer, a significant correlation between telomere length and diagnostic/prognostic factors has only occasionally been documented. Telomerase inhibitors are being investigated in the clinical setting, in combination with cytotoxic chemotherapy for advanced lung cancers, although anti-telomerase therapies need a better comprehension of the telomere elongation machinery and of the response to telomere shortening induced-DNA damage [27].
Finally, limited data have been generated on molecules regulating invasive growth in pulmonary NETs. Salon et al. [45] described a correlation between progression and levels of E-cadherin and β-catenin. In particular impaired expression of E-cadherin and β-catenin correlate with lymph node metastasis and advanced stage disease in carcinoids and LCNEC [39].
Tumour profiling for therapeutic purposes
Novel therapeutic strategies are arising from a better understanding of cancer biology. Innovative therapies targeted to peptide receptors, signalling molecules or catalytic enzymes proved to be helpful in patients with several tumour types, including NETs. An accurate expression profile of the various peptide receptors in lung NETs can be helpful for therapeutic purposes, including the choice of radioligands or non-radioactive peptide analogues [40, 44]. Among them, somatostatin receptors (SSTRs) have been demonstated in NE and non-NE lung tumour [37]. The tissue localisation of SSTRs in lung tumours was widely analised by means of auto-radiography [43], in situ hybridisation, immuno-histochemistry and RT-PCR [35, 37]. Recently, the SSTR type 2 immuno-histochemical expression was investigated in 58 NETs of the lung (12 TC, 20 AC, 19 LCNEC and 7 SCLC) and correlated with follow-up [40]. A strong membrane reactivity was detected in 91% of TC, 65% of AC, 57% of LCNEC and 43% of SCLC.
Thymidylate synthetase (TS) is an enzyme playing an important role in cellular proliferation and growth [29], catalysing the methylation of fluorodeoxyuridine monophosphate to deoxythimidine monophosphate, an essential precursor for DNA synthesis [14]. Five-fluorouracil (5-FU) is an anti-cancer agent largely used in various human neoplasms, that inhibits TS and blocks DNA synthesis [49]. The predictive role of TS quantification in tumours treated with anti-folate drugs such as 5-FU has been extensively described in a variety of human tumours. NETs represent potential targets of anti-folate agents, and we have recently conducted a study on the quantification of TS mRNA and protein levels in these tumours, showing a differential expression of TS in the spectrum of pulmonary NETs, being higher in poorly differentiated NE carcinomas, and indicated TS as a predictive marker of clinical response in a group of patients treated with 5-FU (Ceppi et al., submitted).
The signalling pathway involving mTOR (the mammalian target of rapamycin) is one of the main regulators of cell growth and proliferation and is located at the crossroad of several major signal transduction molecules (including, PTEN/Pi3-kinase, AMKP, Ras/Raf). Its functions in mammalian cells include the control of mRNA translation and metabolism. All mTOR functions are blocked by rapamycin as well as by other mTOR inhibitors under development, such as everolimus and temsirolimus [12, 63]. The only available literature data on mTOR in NE lung tumours are represented by experimental models in SCLC cells. In fact, it has been shown that blocking PI3k/Akt/mTOR pathway with rapamycin is sufficient to overcome chemoresistance and promote apoptosis in SCLC cells [57]. A recent preliminary report on phase II clinical trial, in which the rapamycin derivative temsirolimus was evaluated in SCLC patients after chemotherapy, failed to show any beneficial effect for the patients [34]. No data exist on the distribution of mTor protein (with special reference to its Serin 2448-phosphorylated active form) and its downstream targets (S6K and 4EBP1) in the spectrum of pulmonary NETs. The immuno-histochemical expression of these molecules was analysed in the same series of 58 lung NETs. The preliminary data indicate that well-differentiated tumours had a higher expression level of mTor (75% of TC vs 43% of SCLC), but no correlation with the expression of the target proteins S6K and 4EBP1 (implicated in protein synthesis and cell proliferation) was observed. With regard to the expression of hypoxia-related factors, HIF1alpha (which is targeted by mTor via the initiation factor eIF4E), but not HIF2, was strongly expressed in the nuclei of mTor positive tumours only, although no differential reactivity was observed between well-differentiated tumours and high-grade NETs (unpublished data).
Conclusive remarks
A spectrum of NETs of the lung exists including pure forms and mixed forms. In the former group, a continuum of lesions is recognised ranging from benign TCs to ACs (having a low-grade behaviour, although often associated with regional and distant metastases), to the highly aggressive poorly differentiated carcinomas of the small and large cell types, which share the same poor prognosis. In the mixed tumour group, again several tumour types are recognised, being the NE component extensively represented in association with any of the NSCLC subtypes (so-called combined carcinomas), or being the NE component restricted to a cell population scattered among conventional adenocarcinoma cells (or more rarely within squamous or large cell carcinomas).
The correct morphological identification of all these tumour types together with the definition of a molecular profile may allow to better characterise individual NETs, with the ultimate goal of providing a combined morphological and molecular classification of lung NETs, useful for the choice of the most appropriate therapeutic strategy.
References
Abbona G, Papotti M, Viberti L, Macri L, Stella A, Bussolati G (1998) Chromogranin A gene expression in non-small cell lung carcinomas. J Pathol 186:151–156
Aguayo SM, King TE Jr, Waldron JA Jr, Sherritt KM, Kane MA, Miller YE (1990) Increased pulmonary neuroendocrine cells with bombesin-like immunoreactivity in adult patients with eosinophilic granuloma. J Clin Invest 86:838–844
Anbazhagan R, Tihan T, Bornman DM, Johnston JC, Saltz JH, Weigering A, Piantadosi S, Gabrielson E (1999) Classification of small cell lung cancer and pulmonary carcinoid by gene expression profiles. Cancer Res 59:5119–5122
Arrigoni MG, Woolner LB, Bernatz PE (1972) Atypical carcinoid tumors of the lung. J Thorac Cardiovasc Surg 64:413–421
Asamura H, Kameya T, Matsuno Y, Noguchi M, Tada H, Ishikawa Y, Yokose T, Jiang SX, Inoue T, Nakagawa K, Tajima K, Nagai K (2006) Neuroendocrine neoplasms of the lung: a prognostic spectrum. J Clin Oncol 24:70–76
Berendsen HH, de Leij L, Poppema S, Postmus PE, Boes A, Sluiter HJ, The H (1989) Clinical characterization of non-small-cell lung cancer tumors showing neuroendocrine differentiation features. J Clin Oncol 7:1614–1620
Berruti A, Mosca A, Tucci M, Terrone C, Torta M, Tarabuzzi R, Russo L, Cracco C, Bollito E, Scarpa RM, Angeli A, Dogliotti L (2005) Independent prognostic role of circulating chromogranin A in prostate cancer patients with hormone-refractory disease. Endocr Relat Cancer 12:109–117
Bhattacharjee A, Richards WG, Staunton J, Li C, Monti S, Vasa P, Ladd C, Beheshti J, Bueno R, Gillette M, Loda M, Weber G, Mark EJ, Lander ES, Wong W, Johnson BE, Golub TR, Sugarbaker DJ, Meyerson M (2001) Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc Natl Acad Sci USA 98:13790–13795
Brambilla C, Brambilla E (eds) (1999) Lung tumors. Fundamental biology and clinical managment. Marcel Dekker, New York
Chejfec G, Cosnow I, Gould NS, Husain AN, Gould VE (1990) Pulmonary blastoma with neuroendocrine differentiation in cell morules resembling neuroepithelial bodies. Histopathology 17:353–358
Cooper CS, Nicholson AG, Foster C, Dodson A, Edwards S, Fletcher A, Roe T, Clark J, Joshi A, Norman A, Feber A, Lin D, Gao Y, Shipley J, Cheng SJ (2006) Nuclear overexpression of the E2F3 transcription factor in human lung cancer. Lung Cancer 54:155–162
Dancey JE (2005) Inhibitors of the mammalian target of rapamycin. Expert Opin Investig Drugs 14:313–328
Eymin B, Gazzeri S, Brambilla C, Brambilla E (2001) Distinct pattern of E2F1 expression in human lung tumors: E2F1 is up regulated in small cell lung carcinoma. Oncogene 20:1678–1687
Friedkin M, Crawford EJ, Donovan E, Pastore EJ (1962) The enzymatic synthesis of thymidylate. III. The further purification of thymidylate synthetase and its separation from natural fluorescent inhibitors. J Biol Chem 237:3811–3814
Garber ME, Troyanskaya OG, Schluens K, Petersen S, Thaesler Z, Pacyna-Gengelbach M, van de Rijn M, Rosen GD, Perou CM, Whyte RI, Altman RB, Brown PO, Botstein D, Petersen I (2001) Diversity of gene expression in adenocarcinoma of the lung. Proc Natl Acad Sci USA 98:13784–13789
Gillan JE, Cutz E (1993) Abnormal pulmonary bombesin immunoreactive cells in Wilson-Mikity syndrome (pulmonary dysmaturity) and bronchopulmonary dysplasia. Pediatr Pathol 13:165–180
Gould VE, Linnoila RI, Memoli VA, Warren WH (1983) Neuroendocrine components of the bronchopulmonary tract: hyperplasias, dysplasias, and neoplasms. Lab Invest 49:519–537
He P, Varticovski L, Bowman ED, Fukuoka J, Welsh JA, Miura K, Jen J, Gabrielson E, Brambilla E, Travis WD, Harris CC (2004) Identification of carboxypeptidase E and gamma-glutamyl hydrolase as biomarkers for pulmonary neuroendocrine tumors by cDNA microarray. Hum Pathol 35:1196–1209
Howe MC, Chapman A, Kerr K, Dougal M, Anderson H, Hasleton PS (2005) Neuroendocrine differentiation in non-small cell lung cancer and its relation to prognosis and therapy. Histopathology 46:195–201
Igarashi T, Jiang SX, Kameya T, Asamura H, Sato Y, Nagai K, Okayasu I (2004) Divergent cyclin B1 expression and Rb/p16/cyclin D1 pathway aberrations among pulmonary neuroendocrine tumors. Mod Pathol 17:1259–1267
Jiang SX, Kameya T, Asamura H, Umezawa A, Sato Y, Shinada J, Kawakubo Y, Igarashi T, Nagai K, Okayasu I (2004) hASH1 expression is closely correlated with endocrine phenotype and differentiation extent in pulmonary neuroendocrine tumors. Mod Pathol 17:222–229
Jiang SX, Mikami T, Umezawa A, Saegusa M, Kameya T, Okayasu I (2006) Gastric large cell neuroendocrine carcinomas: a distinct clinicopathologic entity. Am J Surg Pathol 30:945–953
Jones MH, Virtanen C, Honjoh D, Miyoshi T, Satoh Y, Okumura S, Nakagawa K, Nomura H, Ishikawa Y (2004) Two prognostically significant subtypes of high-grade lung neuroendocrine tumors independent of small-cell and large-cell neuroendocrine carcinomas identified by gene expression profiles. Lancet 363:775–781
Kaye FJ (2002) RB and cyclin dependent kinase pathways: defining a distinction between RB and p16 loss in lung cancer. Oncogene 21:6908–6914
Lantuejoul S, Moro D, Michalides RJ, Brambilla C, Brambilla E (1998) Neural cell adhesion molecules (NCAM) and NCAM-PSA expression in neuroendocrine lung tumors. Am J Surg Pathol 22:1267–1276
Lantuejoul S, Soria JC, Moro-Sibilot D, Morat L, Veyrenc S, Lorimier P, Brichon PY, Sabatier L, Brambilla C, Brambilla E (2004) Differential expression of telomerase reverse transcriptase (hTERT) in lung tumors. Br J Cancer 90:1222–1229
Lantuejoul S, Salon C, Soria JC, Brambilla E (2007) Telomerase expression in lung preneoplasia and neoplasia. Int J Cancer 120:1835–1841
Miller RR, Muller NL (1995) Neuroendocrine cell hyperplasia and obliterative bronchiolitis in patients with peripheral carcinoid tumors. Am J Surg Pathol 19:653–658
Navalgund LG, Rossana C, Muench AJ, Johnson LF (1980) Cell cycle regulation of thymidylate synthetase gene expression in cultured mouse fibroblasts. J Biol Chem 255:7386–7390
Nishio Y, Nakanishi K, Ozeki Y, Jiang SX, Kameya T, Hebisawa A, Mukai M, Travis WD, Franks TJ, Kawai T (2007) Telomere length, telomerase activity, and expressions of human telomerase mRNA component (hTERC) and human telomerase reverse transcriptase (hTERT) mRNA in pulmonary neuroendocrine tumors. Jpn J Clin Oncol 37:16–22
Oliveira AM, Tazelaar HD, Wentzlaff KA, Kosugi NS, Hai N, Benson A, Miller DL, Yang P (2001) Familial pulmonary carcinoid tumors. Cancer 91:2104–2109
Onuki N, Wistuba II, Travis WD, Virmani AK, Yashima K, Brambilla E, Hasleton P, Gazdar AF (1999) Genetic changes in the spectrum of neuroendocrine lung tumors. Cancer 85:600–607
Osada H, Tatematsu Y, Yatabe Y, Horio Y, Takahashi T (2005) ASH1 gene is a specific therapeutic target for lung cancers with neuroendocrine features. Cancer Res 65:10680–10685
Pandya K, Levy D, Hidalgo M (2005) A randomized, phase II ECOG trial of two dose levels of temsirolimus (CCI-779) in patients with extensive stage small cell lung cancer in remission after induction chemotherapy. A preliminary report. ASCO annual meeting proceedings, Abstract 7005, pp 622s
Papotti M, Croce S, Macri L, Funaro A, Pecchioni C, Schindler M, Bussolati G (2000) Correlative immunohistochemical and reverse transcriptase polymerase chain reaction analysis of somatostatin receptor type 2 in neuroendocrine tumors of the lung. Diagn Mol Pathol 9:47–57
Papotti M, Sapino A, Righi L, Chiappone S, Bussolati G (2001) 34betaE12 cytokeratin immunodetection in the differential diagnosis of neuroendocrine carcinomas of the breast. Appl Immunohistochem Mol Morphol 9:229–233
Papotti M, Croce S, Bello M, Bongiovanni M, Allia E, Schindler M, Bussolati G (2001) Expression of somatostatin receptor types 2, 3 and 5 in biopsies and surgical specimens of human lung tumors. Correlation with preoperative octreotide scintigraphy. Virchows Arch 439:787–797
Pelosi G, Pasini F, Sonzogni A, Maffini F, Maisonneuve P, Iannucci A, Terzi A, De Manzoni G, Bresaola E, Viale G (2003) Prognostic implications of neuroendocrine differentiation and hormone production in patients with Stage I nonsmall cell lung carcinoma. Cancer 97:2487–2497
Pelosi G, Scarpa A, Puppa G, Veronesi G, Spaggiari L, Pasini F, Maisonneuve P, Iannucci A, Arrigoni G, Viale G (2005) Alteration of the E-cadherin/beta-catenin cell adhesion system is common in pulmonary neuroendocrine tumors and is an independent predictor of lymph node metastasis in atypical carcinoids. Cancer 103:1154–1164
Pelosi G, Volante M, Papotti M, Sonzogni A, Masullo M, Viale G (2006) Peptide receptors in neuroendocrine tumors of the lung as potential tools for radionuclide diagnosis and therapy. Q J Nucl Med Mol Imaging 50:272–287
Petersen I, Hidalgo A, Petersen S, Schluns K, Schewe C, Pacyna-Gengelbach M, Goeze A, Krebber B, Knosel T, Kaufmann O, Szymas J, von Deimling A (2000) Chromosomal imbalances in brain metastases of solid tumors. Brain Pathol 10:395–401
Pilmane M, Luts A, Sundler F (1995) Changes in neuroendocrine elements in bronchial mucosa in chronic lung disease in adults. Thorax 50:551–554
Reubi JC, Kappeler A, Waser B, Laissue J, Hipkin RW, Schonbrunn A (1998) Immunohistochemical localization of somatostatin receptors sst2A in human tumors. Am J Pathol 153:233–245
Reubi JC (2003) Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev 24:389–427
Salon C, Moro D, Lantuejoul S, Brichon Py P, Drabkin H, Brambilla C, Brambilla E (2004) E-cadherin, beta-catenin adhesion complex in neuroendocrine tumors of the lung: a suggested role upon local invasion and metastasis. Hum Pathol 35:1148–1155
Salon C, Merdzhanova G, Brambilla C, Brambilla E, Gazzeri S, Eymin B (2007) E2F-1, Skp2 and cyclin E oncoproteins are upregulated and directly correlated in high-grade neuroendocrine lung tumors. Oncogene
Schleusener JT, Tazelaar HD, Jung SH, Cha SS, Cera PJ, Myers JL, Creagan ET, Goldberg RM, Marschke RF Jr (1996) Neuroendocrine differentiation is an independent prognostic factor in chemotherapy-treated nonsmall cell lung carcinoma. Cancer 77:1284–1291
Shivapurkar N, Toyooka S, Eby MT, Huang CX, Sathyanarayana UG, Cunningham HT, Reddy JL, Brambilla E, Takahashi T, Minna JD, Chaudhary PM, Gazdar AF (2002) Differential inactivation of caspase-8 in lung cancers. Cancer Biother 1:65–69
Spears CP, Gustavsson BG, Mitchell MS, Spicer D, Berne M, Bernstein L, Danenberg PV (1984) Thymidylate synthetase inhibition in malignant tumors and normal liver of patients given intravenous 5-fluorouracil. Cancer Res 44:4144–4150
Sturm N, Rossi G, Lantuejoul S, Papotti M, Frachon S, Claraz C, Brichon PY, Brambilla C, Brambilla E (2002) Expression of thyroid transcription factor-1 in the spectrum of neuroendocrine cell lung proliferations with special interest in carcinoids. Hum Pathol 33:175–182
Sugita M, Geraci M, Gao B, Powell RL, Hirsch FR, Johnson G, Lapadat R, Gabrielson E, Bremnes R, Bunn PA, Franklin WA (2002) Combined use of oligonucleotide and tissue microarrays identifies cancer/testis antigens as biomarkers in lung carcinoma. Cancer Res 62:3971–3979
Sunday ME, Kaplan LM, Motoyama E, Chin WW, Spindel ER (1988) Gastrin-releasing peptide (mammalian bombesin) gene expression in health and disease. Lab Invest 59:5–24
Takeuchi T, Minami Y, Iijima T, Kameya T, Asamura H, Noguchi M (2006) Characteristics of loss of heterozygosity in large cell neuroendocrine carcinomas of the lung and small cell lung carcinomas. Pathol Int 56:434–439
Travis WD, Linnoila RI, Tsokos MG, Hitchcock CL, Cutler GB Jr, Nieman L, Chrousos G, Pass H, Doppman J (1991) Neuroendocrine tumors of the lung with proposed criteria for large-cell neuroendocrine carcinoma. An ultrastructural, immunohistochemical, and flow cytometric study of 35 cases. Am J Surg Pathol 15:529–553
Travis WD, Gal AA, Colby TV, Klimstra DS, Falk R, Koss MN (1998) Reproducibility of neuroendocrine lung tumor classification. Hum Pathol 29:272–279
Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC (eds) (2004) World Health Organization classification of tumors. Pathology and genetics of tumors of the lung, pleura, thymus and heart. IARC, Lyon, France
Tsurutani J, West KA, Sayyah J, Gills JJ, Dennis PA (2005) Inhibition of the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin pathway but not the MEK/ERK pathway attenuates laminin-mediated small cell lung cancer cellular survival and resistance to imatinib mesylate or chemotherapy. Cancer Res 65:8423–8432
Vageli D, Daniil Z, Dahabreh J, Karagianni E, Liloglou T, Koukoulis G, Gourgoulianis K (2006) Microsatellite instability and loss of heterozygosity at the MEN1 locus in lung carcinoid tumors: a novel approach using real-time PCR with melting curve analysis in histopathologic material. Oncol Rep 15:557–564
Viberti L, Bongiovanni M, Croce S, Bussolati G (2000) 34betaE12 Cytokeratin immunodetection in the differential diagnosis of small cell tumors of lung. Int J Surg Pathol 8:317–322
Volante M, Fulcheri E, Allia E, Cerrato M, Pucci A, Papotti M (2002) Ghrelin expression in fetal, infant, and adult human lung. J Histochem Cytochem 50:1013–1021
Yokomizo A, Tindall DJ, Drabkin H, Gemmill R, Franklin W, Yang P, Sugio K, Smith DI, Liu W (1998) PTEN/MMAC1 mutations identified in small cell, but not in non-small cell lung cancers. Oncogene 17:475–479
Zaffaroni N, Villa R, Pastorino U, Cirincione R, Incarbone M, Alloisio M, Curto M, Pilotti S, Daidone MG (2005) Lack of telomerase activity in lung carcinoids is dependent on human telomerase reverse transcriptase transcription and alternative splicing and is associated with long telomeres. Clin Cancer Res 11:2832–2839
Zitzmann K, De Toni EN, Brand S, Goke B, Meinecke J, Spottl G, Meyer HH, Auernhammer CJ (2007) The novel mTOR inhibitor RAD001 (everolimus) induces antiproliferative effects in human pancreatic neuroendocrine tumor cells. Neuroendocrinology 85:54–60
Acknowledgements
Work partially supported by the Italian Ministry of Research (MIUR, Rome; grant ex-60% to MP).
Conflict of interest statement
We declare that we have no conflict of Interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Righi, L., Volante, M., Rapa, I. et al. Neuro-endocrine tumours of the lung. A review of relevant pathological and molecular data. Virchows Arch 451 (Suppl 1), 51–59 (2007). https://doi.org/10.1007/s00428-007-0445-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-007-0445-0