Abstract
Caveolin-1, a principal component of caveolae, modulates growth signaling, endocytosis, and intracellular transport. We examined the expression of caveolin-1α and its relation to cell cycle and caveolin-interacting growth factor receptors in regenerating proximal tubules (PTs) after gentamicin-induced acute renal failure in rats. Caveolin-1α appeared in regenerating PTs as early as day 4 after last gentamicin, peaked at days 6 to 8, and showed cytoplasmic pattern after day 8. Immunoelectron microscopy revealed caveolin-1α-positive caveolae on the cell membrane and in cytoplasms in regenerating PTs at days 4 to 8 and caveolin-positivity confined to cytoplasms after day 10. The number of PT cells with proliferation markers peaked at day 6 and decreased afterwards as expression of cyclin-dependent kinase inhibitors increased. Platelet-derived growth factor receptor-β (PDGFR-β) and epidermal growth factor receptor (EGFR) were colocalized with caveolin-1α in proliferating PTs as early as day 4. Phosphorylated EGFR increased at day 8 and afterwards when caveolins dissociated from EGFR or decreased. In case of PDGFR-β, phosphorylation seemed to be associated with the increase and association of caveolins to the receptors. Our results suggest that transient expression of caveolin-1α in early regenerating PTs might contribute to the regenerating process of PTs through modulating growth factor receptors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Caveolae are described as 50- to 100-nm flask-shaped invaginations within the plasma membrane and are thought to play a role in important physiological functions such as cell surface signaling, endocytosis, and intracellular cholesterol transport [20]. Caveolin-1, caveolin-2, and caveolin-3, a family of ∼22 kD integral membrane proteins, are the main components of caveolae [20]. Caveolin-3 is expressed exclusively in myocytes, whereas caveolin-1 and caveolin-2 are often coexpressed and are found in many tissue types [20]. Caveolins interact with multiple caveolae-associated molecules [20], such as hormones, growth factor receptors including platelet-derived growth factor (PDGF) [8], and epidermal growth factor (EGF) [14], and signaling molecules, which are involved in cell growth and proliferation, suggesting that caveolae may function as unique cell surface signal transduction domains. It is also known that the signal transducing proteins are rapidly recruited into caveolae upon PDGF [8] and EGF [14] stimulations and that caveolin-1 and caveolin-3 but not caveolin-2 directly bind to PDGF receptor-β (PDGFR-β) and EGF receptor (EGFR), inhibiting their autophosphorylation in vitro as kinase inhibitors [2, 23].
Under normal condition, terminally differentiated, senescent cells such as adipocytes, endothelial cells, and fibroblasts [20] contain high caveolin-1 levels and therefore exhibit a marked insensitivity to growth factor-mediated stimulation. Renal proximal tubules (PTs) are highly differentiated but do not exhibit immunoreactive caveolin-1 [1]. Zager et al. [24] and Mahmoudi et al. [10] have reported marked increases in caveolin-1 in rat PT cells during the induction and maintenance phases of ischemia/reperfusion and myoglobinuric models of acute renal failure (ARF), suggesting that caveolin-1 expression may mediate PT cell death or survival of injured PT cells. However, it is not clear whether caveolins participate in regenerating PT cells after ARF. During cellular recovery after ARF, various growth factors/cytokines including EGF and PDGF [15, 16] have been reported to contribute to the tubular regeneration. As receptor tyrosine kinases, EGFR and PDGFR are caveolin-interacting molecules [2, 8, 14, 23]; caveolin might participate in the regenerating process of PT cells after ARF.
In the present study, we used a model of gentamicin-induced ARF in rats. This model is a well-established toxic ARF model known to induce PT damage followed by recovery mainly in the renal cortex [5]. We determined the expression and kinetics of caveolin-1α and defined the spatial and temporal correlations between caveolin-1α expression, state of cell cycle, and caveolin-interacting signaling molecules (PDGFR-β and EGFR) in regenerating PT cells after induction of ARF.
Materials and methods
Induction of gentamicin-induced acute tubular injury
A total of 42 male Wistar rats (200–250 g, SLC, Shizuoka, Japan) were used in the present study. Thirty-five rats were injected subcutaneously with 400 mg/kg body weight/day of gentamicin sulfate solution (Sigma-Aldrich, St. Louis, MO, USA) for 2 days in divided doses (every 8 h). We previously reported [5] that subcutaneous injection of gentamicin at this dose in rats increased serum creatinine levels and induced proximal tubular damage only in the cortex, which reached peak levels at day 6 after the end of gentamicin (day 0 falls at 8 h after the last gentamicin). On the other hand, Ki67-positive proliferating PTs were found as early as day 4 and peaked in number at day 6. Regenerating PTs almost covered the denuded tubular basement membrane by day 10 [5]. Therefore, in the present study, rats were killed at days 4, 6, 8, 10, and 12 during the repair phase after PT injury. Rats at each time point and normal rats as controls were anesthetized with intraperitoneal pentobarbital sodium (30 mg/kg), then both kidneys were removed after flushing phosphate-buffered saline (PBS) for immunohistochemistry, immunofluorescence, conventional electron microscopy, immunoelectron microscopy, and Western blot analysis.
The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of Hamamatsu University School of Medicine.
Immunohistochemistry
The kidneys were bisected through the longitudinal axis, fixed with 4% paraformaldehyde, and embedded in paraffin. Three-micrometer-thick sections were deparaffinized, and endogenous peroxidase was blocked by treatment with 3% H2O2 for 30 min. The sections were then incubated with the primary antibody listed in Table 1 and reacted with the corresponding biotinylated secondary antibody (donkey polyclonal antibody against rabbit IgG, goat IgG, or mouse IgG, Chemicon International, Temecula, CA, USA) for 30 min at room temperature. Then, avidin-conjugated peroxidase (Nichirei, Tokyo, Japan) was added for 30 min. The reaction products were visualized by diaminobenzidine.
To examine the relationship between expression of caveolin-1α and state of cell cycle in PT cells, double-immunostaining only for caveolin-1α and cyclin D1 or caveolin-1α and Ki67 was performed using the method by Lan et al. [7]. After staining the sections with cyclin D1 or Ki67 as above, they were treated by microwave oven-heating for 2 × 5 min in 0.01 M sodium citrate, pH 6.0, at 800 W, then incubated with caveolin-1α antibody and with biotinylated secondary antibody. Then, Vectastain ABC-AP reagent (Vector Laboratories, Burlingame, CA, USA) was added, and the Vector Red alkaline Phosphatase Substrate Kit I (Vector Laboratories) was used as chromogen.
For control sections, the first antibodies were omitted or replaced by normal mouse or rabbit IgG. Signals in both control and experimental sections were negative or negligible.
Immunofluorescence studies
Portions of renal cortex were snap frozen in cold N-hexane and stored at −70°C for immunofluorescence studies. Immunofluorescence studies were performed on 4-μm-thick cryostat sections fixed in cold acetone. Sections were stained with rabbit polyclonal anti-caveolin-1α (Santa Cruz) followed by Alexa Fluor 488R donkey anti-rabbit IgG (Molecular Probes Eugene, OR, USA) for 30 min. After staining the sections for caveolin-1α, they were incubated with polyclonal goat anti-EGFR or goat anti-PDGFR and with Alexa Fluor 546R donkey anti-goat IgG (Molecular Probes) for 30 min at room temperature. All sections were observed under immunofluorescent microscopy.
Conventional electron microscopy
Small portions of renal tissues were fixed in 2% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) at 4°C for 2 h, postfixed in 1% OsO4 resolved in the same buffer for 1 h, and then embedded in Epon. Ultra-thin sections were counterstained with uranyl acetate and lead citrate, then examined under an electron microscope (JEM-1220, JEOL, Japan).
Immunoelectron microscopy
Renal tissues were immersed in 4% paraformaldehyde for 9 h, cryoprotected in graded sucrose solution, and then snap frozen in cooled N-hexane. Eight-micrometer cryostat sections were incubated with caveolin-1α antibody. After the sections had been fixed in 2% glutaraldehyde for 10 min, the reaction products were visualized by diaminobenzidine, then the sections were postfixed in 1% OsO4 for 2 h. Epon 812-embedded ultrathin sections were stained with lead citrate and examined with a JEM-1220 electron microscope.
Western blot analysis of caveolin-1α
Immunoblotting was repeated at least three times with samples from a minimum of three animals for each time point. Protein extracts were prepared from cold Triton X-100 insoluble extracts (detergent resistant membranes) of isolated renal cortical tissue using the method of Mendez et al. [13]. Equal amount of extracted protein (7.5 μg) were subjected to electrophoresis on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, then transferred onto polyvinylidene fluoride microporous membranes. The membranes were incubated overnight in 5% skim milk in Tris-buffered saline with Tween (TBS-T) buffer (20 mM Tris–HCl, pH 7.6; 150 mM NaCl; and 0.1% Tween-20) at room temperature for 1 h. Immunoblotting (caveolin-1α or β-actin) was then performed as described previously [25].
Semiquantitative analysis of immunohistochemically stained sections
The numbers of cyclin-D1-, Ki67-, p21-, p27- and p53-positive PT in the cortex in single immunostaining sections were counted at ×400 magnification.
Discrimination between proximal and distal tubules, even in conditions of regeneration, was performed by adapting the following criteria [18]: (a) PTs were characterized by the absence of clearly recognizable intercellular delineations and eventually by the presence of a brush border; (b) distal tubules were identified on the basis of clearly recognizable intercellular delineations and by the presence of stripes, originating from the basal cell surface and extending towards the cell apex. The number of immunoreactive cells in each rat represented the average number of 40 fields examined and the mean numbers before and at days 4, 6, 8, 10, and 12 after gentamicin injection were displayed in histograms.
Statistical analysis
Data are expressed as mean ± SD. Differences between data sets were examined for statistical significance using one-way analysis of variance followed by Fisher’s t test. A p value of <0.05 was accepted as statistically significant.
Results
Cell-cycle-related molecules in regenerating PTs
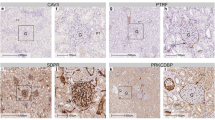
Cyclin-D1 (G1 phase marker)-positive and Ki67 (S phase marker)-positive proliferating PTs were found as early as day 4, and both peaked in number at day 6, then decreased (Fig. 1a–d,k,l). Cyclin-dependent kinase inhibitors p21 and p27 were found in PTs, peaked in number at days 10 and 8–10, respectively (Fig. 1e–h,m,n). p53 was also increased in PTs and peaked in number at day 8 (Fig. 1i,j,o).
a–j Immunohistochemistry of cell-cycle-related molecules (cyclin D1, Ki67, p21, p27, and p53) in PT cells before (left column) and at days 6 (b, d), 8 (h, j), and 10 (f) after (middle column) induction of acute tubular injury. Asterisks PTs; DT distal tubules; brown nuclei indicate positive immunoreaction. k–o The number of cyclin D1+, Ki67+, p21+, p27+, or p53+ PT cells per field before (n) and days after induction of acute tubular injury (right column). Data are expressed as mean ± SD of five rats. *p < 0.05, **p < 0.01 vs normal control. NS Not significant
Redifferentiation of regenerating PTs
Megalin, as a differentiated phenotype of PT, was found in the brush-border of PT in the normal kidney and reappeared in the reappeared brush-border of some regenerating PTs after day 8 and in almost all PTs by day 12 (Fig. 2 and Table 2).
Immunohistochemistry of caveolin-1α and caveolin-2 and relation between caveolin-1α and cell cycle in regenerating PTs
As reported by other investigators [1, 24], immunoreactive caveolin-1 was detected in glomerular/vascular or distal tubule structures but not in PT cells in the cortex of normal rat kidneys (Fig. 3a,f). Peritubular capillary endothelial cells showed a faint staining of caveolin-1α in normal rat kidneys, and such staining did not change after ARF (Fig. 3f,g). Caveolin-1α was found in injured PTs and exfoliated PTs after induction of ARF in the cortex (Fig. 3b,c,g). The regenerating PTs showed a weak expression of caveolin-1α as early as day 4 (Fig. 3b) and strong expression at days 6 to 8 (Fig. 3c,d,g) as membrane/cytoplasmic pattern (Table 2). Caveolin-1α staining began to be confined in the cytoplasm exhibiting a coarse granular pattern and with reduced intensity after day 8 (Fig. 3d,e). Double immunohistochemical staining for caveolin-1α and cyclin D1 revealed that cyclin-D1-positive (G1 phase marker) PT cells were also positively stained for caveolin-1α (Fig. 3h), indicating that early regenerating PT cells expressed caveolin-1. Double immunohistochemistry of caveolin-1α and Ki67 (S phase marker) revealed that caveolin-1α showed a tendency for weak intensity and granular staining in the cytoplasm of Ki67-negative PT cells after day 8 compared to that in Ki67-positive PT cells (Fig. 3i).
a–g Immunohistochemistry of caveolin-1α in PT cells in normal (a, f) and at days 4 (b), 6 (c, g), 8 (d), and 10 (e) after last gentamicin injection. Brown color indicates positive immunoreaction. Asterisks PT cells; DT distal tubular cells; g glomerulus; a small artery; c peritubular capillary. Double arrows indicate injured PT cells with positive caveolin-1α. h, i Double immunohistochemistry of caveolin-1α (pink color) and cyclin D1 (brown nuclei) at day 4 (h), or caveolin-1α (pink color) and Ki67 (brown nuclei) at day 8 (i). Arrows indicate positive immunoreactive nuclei and arrowheads indicate negative immunoreactive nuclei. j–n Immunohistochemistry of caveolin-2 in PT cells in normal (j) and at days 4 (k), 6 (l), 8 (m), and 10 (n) after induction of acute tubular injury. Brown color indicates positive immunoreaction. Asterisks PT cells
Immunoreactive caveolin-2 showed a distribution pattern similar to that of caveolin-1α in the normal kidneys (Fig. 3a,j). After induction of acute tubular injury, the regenerating PTs showed moderate caveolin-2 expression at day 4 (Fig. 3k) and strong expression at day 6 (Fig. 3l; Table 2). Caveolin-2 staining rapidly decreased on day 8 (Fig. 3m) and became almost negative without coarse granular pattern in the cytoplasm (Fig. 3n; Table 2).
Ultrastructural findings of caveolae and caveolin-1α−positive structures in regenerating PTs
Conventional electron microscopy revealed almost no caveolae formation in normal PT cells (not shown). However, flask-like structures of about 100 nm diameter could be focally found, some of which were open to the apical or basal fronts of the regenerating PT cells at days 4 to 6, suggesting caveolae formation (Fig. 4a). Possible caveolae-derived vesicles including interconnected structures could also be found in cytoplasm in the regenerating PT cells at days 4 to 8 (Fig. 4b), but apparent caveolae-derived structures could not be identified after day 10. Immunoelectron microscopy supported the conventional electron microscopic findings. There was almost no caveolin-1-positive structures in the normal PT (Fig. 4c); however, caveolin-1α-positive flask-like structures with or without opening to the free or basal fronts of cells or apparently free in the cytoplasm could be found at days 4 to 6 (Fig. 4d,e). The majority of the caveolin-1α-positive structures were part of large clusters of interconnected structures, grape-like structures, or with unusual shapes, accumulated at the cell periphery and in the cytoplasm (Fig. 4d,e). With time, the caveolin-1α-positive structures at the cell periphery were decreased in density and were not found in the regenerated brush-border at day 8 (Fig. 4f). After day 10, most caveolin-1 positivity was limited to certain intracellular compartments including mitochondria (Fig. 4g).
Conventional electron micrographs of PT cells days 4 (a) and 8 (b) after induction of acute tubular injury. Arrowheads Caveolae; arrows possible caveolae-derived vesicles. Immunoelectron microscopic findings of immunoreactive caveolin-1α (black color) in PT cells in normal (c) and days 4 (d), 6 (e), 8 (f), and 10 (g) after induction of acute tubular injury. Arrowheads Caveolin-1α-positive caveolae; double arrows caveolin-1α-positive vesicles or interior compartments in cytoplasm; arrows caveolin-1α-positive caveolae or clusters of interconnected structures, grape-like structures or with unusual shapes; asterisks brush-border; inset caveolin-1α-positive interior compartment. N Nucleus; m mitochondria; Bm tubular basement membranes; En endothelial cells. a, b, d Bars = 0.5 μm; c, e–g Bars = 5.0 μm
Western blot analysis of caveolin-1α
Western blot analysis of Triton X-100 insoluble fraction showed marked increase in caveolin-1α levels in the renal cortex after induction of ARF and peaked at day 6 (Fig. 5).
Expression of PDGFR-β and EGFR and their phosphorylated forms in regenerating PTs
Immunoreactive PDGFR-β was negative in normal PTs but was found in the regenerating PTs as early as day 4 and peaked at days 6 to 8, then decreased rapidly (Fig. 6a–e and Table 2). p-PDGFR-β was found as early as day 4, peaked and concentrated at the apical membrane at day 6, then almost disappeared by day 10 (Fig. 6f–j and Table 2).
Immunoreactive EGFR was constitutively positive in the brush-border in normal PTs (Fig. 6k). It was also positive in the regenerating PTs as early as day 4, and peaked at days 6 to 8 (Fig. 6l-n). It increasingly reappeared in the brush-border after day 8 (Fig. 6n,o). Two different antibodies against EGFR used in this study provided similar results. p-EGFR was constitutively positive in the brush-border in normal PTs (Fig. 6p). p-EGFR was almost negative at day 4 but was found weakly at day 6 and increasingly reappeared in the brush-border after day 8 (Fig. 6q–t and Table 2).
Relationship between caveolin-1α and PDGFR-β or EGFR in regenerating PTs
Caveolin-1α colocalized with PDGFR-β partially on the cell membrane at days 4 to 8 and in the cytoplasm of the regenerating PTs at day 8 (Fig. 7a–c). Caveolin-1α was also colocalized with EGFR partially on the cell membrane and in the cytoplasm of regenerating PTs at days 4 to 8 (Fig. 7d–f). After day 10, most EGFR was found on the apical surface of PT (corresponding to the brush-border) without caveolin-1α colocalization (Fig. 7g–i). EGFR transiently localized in the nuclei at days 4 to 6 (Fig. 7h).
Double fluorescence staining for caveolin-1α and PDGFR-β at 6 days (a–c) and 8 days (d–f) after last gentamicin injection, and for caveolin-1 and EGFR at 6 days (g–i) and 10 days (j–l) after last gentamicin injection. Caveolin-1α expression (green) (a, d, g, j) and PDGFR-β expression (red) (b, e) or EGFR (red) (h, k) were observed in regenerating PT cells. Superimposition of caveolin-1α and PDGFR-β images and caveolin-1α and EGFR produced by immunofluorescence staining showed yellow fluorescence on the membrane (c, i) and in the cytoplasm (c, f, i), indicating colocalization of caveolin-1α and PDGFR-β (c, f) or EGFR (i). Asterisks Tubular lumen; N nuclei
Discussion
In the present study, induction of acute tubular injury by gentamicin resulted in appearance of caveolin-1α expression in cyclin-D1-positive PT cells at day 4. As we previously found almost no proliferating PT cells at day 2 after ARF in this model [5], cyclin-D1-positive PT cells at day 4 were early regenerating PT cells in this study. Caveolin-1α expression peaked at day 6, as shown by immunohistochemistry, at the time of peak PT proliferation, judged by the number of Ki67-positive PT cells. Western blotting also showed increased caveolin-1α levels in the cortex as early as day 4, peaking at day 6. However, the strong increasing of caveolin-1α at day 4 displayed by Western blot did not seem to correlate well with the results of corresponding immunostains in PT cells. As there were many caveolin-1α-positive injured PT cells within the tubular lumen at day 4 (Fig. 3b), it is possible that the results of the Western blot may reflect caveolin-1α protein not simply in regenerating PT cells but also in injured PT cells and other cell types. Coarse granular cytoplasmic staining of caveolin-1α was found in the late regenerating PT cells after day 8 and tended to become weaker in Ki67-negative PT cells. Some PT cells were redifferentiated after day 8, judged by megalin-positivity. Conventional electron microscopy and immunoelectron microscopy revealed the presence of caveolin-1α-positive flask-like structures, representing caveolae [20], on the plasma cell membranes of early regenerating PT cells and caveolin-1α-positive structures were increasingly internalized with time in the cytoplasm as grape-like vesicles. Caveolin-1α-positive vesicles were almost confined in various intracellular compartments in the late regenerating PT cells after day 10. The findings showed that the early proliferating, regenerating PT cells transiently expressed caveolae/caveolin-1α on the plasma membrane, and the subsequent internalization of the caveolin-1α was suggested in the regenerating process of PT cells.
Caveolin-1 expression was demonstrated to arrest NIH 3T3 cells in the G0/G1 phase of the cell cycle through a p53/p21-dependent pathway [4]. During the process of reduction in PT proliferation in this model, p53 and p21 expression levels were significantly increased, and the p53- and p21-expressing cells reached peak numbers among regenerating PT cells at days 8 and 10, respectively. Furthermore, the expression level of p27 increased, and the p27-positive cells reached peak numbers from days 8 to 10. The number of p27-positive PT cells was higher than that of p21-positive PT cells. These results suggest that it is unlikely that caveolin-1α expression in the regenerating PT cells mainly contributed to the induction of PT cells to exit the S-phase of the cell cycle through a p53/p21-dependent pathway in this model.
It was reported that formation of caveolae is polarized in basolateral distribution in wild-type MDCK II and caveolin-1 transfected Caco-2 cells [21]. Moreover, in nontransformed NIH 3T3 cells, as cells become confluent, caveolin-1 was found to be concentrated at the areas of cell–cell contact, suggesting the ability of caveolin-1 to regulate contact inhibition and growth arrest in nontransformed cells [22]. However, in our model, caveolae were distributed focally both on the basal and free (apical) plasma membranes of early regenerating PT cells but disappeared from the plasma membrane, suggesting that caveolin-1α does not seem to play a role in regulation of contact inhibition in our model.
In the process of caveolin-1 recycling, caveolin-1 normally moves with caveolae-derived vesicles to various intracellular compartments, such as endoplasmic reticulum and Golgi apparatus, and then recycles back to the cell surface [9]. In addition, it is suggested that caveolin-1 can enter the cytoplasm as a soluble protein embedded in lipid particles. Soluble caveolin-1 may translocate to the endoplasmic reticulum, remain in the cytosol, or to mitochondria [9]. Thus, it is possible that caveolin-1α-positive vesicles could be found in endomembranes and mitochondria in the present model. However, loss of caveolae on the plasma membrane of the redifferentiated PT cells may rather suggest that caveolin-1α-positive vesicles formed in Golgi apparatus were not sorted into the plasma membrane in the process of reduction in caveolin-1α synthesis, although the messenger RNA level of caveolin-1α was not examined.
Caveolin-1 and caveolin-2 are often coexpressed and are found in many tissue types because caveolin-2 seems to interact with caveolin-1, forming hetero-oligomers [20] that determine the localization of these proteins within the cell [19]. In the present study, caveolin-1α and caveolin-2 were coexpressed in proliferating PT cells; however, unlike caveolin-1α, caveolin-2 was not found in significant amounts in the cytoplasm of redifferentiating PT cells. This might reflect the dependence of caveolin-2 on caveolin-1 for plasma membrane localization [19].
During cellular recovery after acute tubular injury, various growth factors/cytokines have been reported to contribute to tubular regeneration [6, 16]. Given the roles of caveolins in modulating/integrating signal transduction in diverse cell types [20], we speculated that transient expression of caveolins on the plasma membrane might play roles on regenerating process of the PT cells by regulating signal transduction of growth factors. PDGF-b and EGF are known as renotropic growth factors [15, 16], and their receptors, receptor tyrosine kinases (PDGFR [8, 23] and EGFR [2, 14]) are caveolae-localized and caveolin-interacting molecules. In the present study, both EGFR and PDGFR-β were found in the early regenerating PT cells (Table 2). Phosphorylated EGFR increased at day 8 and afterwards (Table 2) when caveolins dissociated from EGFR (Fig. 7l) or decreased (Table 2). This consists with the traditional hypothesis that caveolins inhibit activation of various cell surface receptors as seen in the mesangial cells [3]. In case of PDGFR-β, phosphorylation seemed to be associated with the increase and association of caveolins to the receptors in the regenerating PT cells from the very early phase of proliferation (Table 2). Caveolin-1 was demonstrated to inhibit the autophosphorylation of PDGFR and EGFR in a dose-dependent manner in vitro [2, 23]. It was reported that only in the presence of ligands, EGFR and PDGFR transiently associate with caveolae and that ligand-induced stimulation of EGFR or PDGFR can cause heterologous desensitization of other receptors by sequestration in caveolin-containing membranes or caveolae [3, 12]. Thus, it might be possible that EGFR and PDGFR are differentially phosphorylated in this model because concentration and/or kinetics of ligands for EGFR and PDGFR should be differently regulated in vivo. However, only immunohistochemical methods in this study limit drawing any functional roles of caveolins.
Several reports indicate that clathrin-independent uptake of various endogenous plasma molecules, including signaling receptors to a distinct class of caveolin-1-containing endosome, termed a caveosome, allows different types of endocytic mechanisms to have different functional consequences for the cell [17]. Together with the immunoelectron microscopic findings that caveolin-1α was translocated from the plasma cell surface to intracellular compartments of regenerating PT cells, the immunohistochemistry of caveolin-1α, EGFR, and PDGFR indicates the possibility that caveolins may play a role in endocytosis of these cell surface receptors, or that caveolins may play as an intracellular transporter of cell surface receptors in this model. We also found that EGFR localized in the nuclei. Although transient nuclear localization of EGFR has been reported in regenerating rat liver [11], it cannot be excluded that it is just an artifact.
In conclusion, caveolin-1α expression and caveolae formation appeared on the plasma cell membrane exclusively in the early proliferating phase of regenerating PT cells in association with caveolin-interacting receptors for renotropic growth factors, then caveolin-1α might associate with endocytosis or intracellular transport of these cell surface receptors, suggesting that caveolin-1α may play a role in the regenerating process of PT cells after ARF. As caveolin has multifunctional roles, further studies such as inhibition of caveolin-1α expression are necessary to elucidate the physiological significance of transient caveolin-1α expression in regenerating PT cells in this model.
References
Breton S, Lisanti MP, Tyszkowski R, McLaughlin M, Brown D (1998) Basolateral distribution of caveolin-1 in the kidney. Absence from H+-atpase-coated endocytic vesicles in intercalated cells. J Histochem Cytochem 46:205–214
Couet J, Sargiacomo M, Lisanti MP (1997) Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J Biol Chem 272:30429–30438
Fujita Y, Maruyama S, Kogo H, Matsuo S, Fujimoto T (2004) Caveolin-1 in mesangial cells suppresses MAP kinase activation and cell proliferation induced by bFGF and PDGF. Kidney Int 66:1794–1804
Galbiati F, Volonte D, Liu J, Capozza F, Frank PG, Zhu L, Pestell RG, Lisanti MP (2001) Caveolin-1 expression negatively regulates cell cycle progression by inducing G0/G1 arrest via p53/p21WAF1/Cip1-dependent mechanism. Mol Biol Cell 12:2229–2244
Goto T, Fujigaki Y, Sun DF, Yamamoto T, Hishida A (2004) Plasma protein extravasation and vascular endothelial growth factor expression with endothelial nitric oxide synthase induction in gentamicin-induced acute renal failure in rats. Virchows Arch 444:362–374
Harris RC (1997) Growth factors and cytokines in acute renal failure. Adv Renal Replace Ther 4:43–53
Lan HY, Mu W, Nikolic-Paterson DJ, Atkins RC (1995) A novel, simple, reliable, and sensitive method for multiple immunoenzyme staining: use of microwave oven heating to block antibody crossreactivity and retrieve antigens. J Histochem Cytochem 43:97–102
Liu P, Ying Y, Anderson RG (1997) Platelet-derived growth factor activates mitogen-activated protein kinase in isolated caveolae. Proc Natl Acad Sci USA 94:13666–13670
Liu P, Rudick M, Anderson RG (2002) Multiple functions of caveolin-1. J Biol Chem 277:41295–41298
Mahmoudi M, Willgoss D, Cuttle L, Yang T, Pat B, Winterford C, Endre Z, Johnson DW, Gobe GC (2003) In vivo and in vitro models demonstrate a role for caveolin-1 in the pathogenesis of ischaemic acute renal failure. J Pathol 200:396–405
Marti U, Hug M (1995) Acinar and cellular distribution and mRNA expression of the epidermal growth factor receptor are changed during liver regeneration. J Hepatol 23:318–327
Matveev SV, Smart EJ (2002) Heterologous desensitization of EGF receptors and PDGF receptors by sequestration in caveolae. Am J Physiol Cell Physiol 282:C935–C946
Mendez AJ, Lin G, wade DP, Lawn RM, Oram JF (2001) Membrane lipid domains distinct from cholesterol/sphingomyelin rich rafts are involved in the ABCA-1-mediated lipid secretory pathway. J Biol Chem 276:3158–3166
Mineo C, James GL, Smart EJ, Anderson RG (1996) Localization of epidermal growth factor-stimulated Ras/Raf-1 interaction to caveolae membrane. J Biol Chem 271:11930–11935
Morin NJ, Laurent G, Nonclercq D, Toubeau G, Heuson-Stiennon JA, Bergeron MG, Beauchamp D (1992) Epidermal growth factor accelerates renal tissue repair in a model of gentamicin nephrotoxicity in rats. Am J Physiol 263:F806–F811
Nakagawa T, Sasahara M, Haneda M, Kataoka H, Nakagawa H, Yagi M, Kikkawa R, Hazama F (1999) Role of PDGF B-chain and PDGF receptors in rat tubular regeneration after acute injury. Am J Pathol 155:1689–1699
Nichols B (2003) Caveosomes and endocytosis of lipid rafts. J Cell Sci 116:4707–4714
Nouwen EJ, Verstrepen WA, Buyssens N, Zhu MQ, De Broe ME (1994) Hyperplasia, hypertrophy, and phenotypic alterations in the distal nephron after acute proximal tubular injury in the rat. Lab Invest 70:479–493
Parolini I, Sargiacomo M, Galbiati F, Rizzo G, Grignani F, Engelman JA, Okamoto T, Ikezu T, Scherer PE, Mora R, Rodriguez-Boulan E, Peschle C, Lisanti MP. (1999) Expression of caveolin-1 is required for the transport of caveolin-2 to the plasma membrane. Retention of caveolin-2 at the level of the Golgi complex. J Biol Chem 274:25718–25725
Razani B, Woodman SE, Lisanti MP (2002) Caveolae: from cell biology to animal physiology. Pharmacol Rev 54:431–467
Vogel U, Sandvig K, van Deurs B (1998) Expression of caveolin-1 and polarized formation of invaginated caveolae in Caco-2 and MDCK II cells. J Cell Sci 111:825–832
Volonte D, Galbiati F, Lisanti MP (1999) Visualization of caveolin-1, a caveolar marker protein, in living cells using green fluorescent protein (GFP) chimeras. The subcellular distribution of caveolin-1 is modulated by cell-cell contact. FEBS Lett 445:431–439
Yamamoto M, Toya Y, Jensen RA, Ishikawa Y (1999) Caveolin is an inhibitor of platelet-derived growth factor receptor signaling. Exp Cell Res 247:380–388
Zager RA, Johnson A, Hanson S, dela Rosa V (2002) Altered cholesterol localization and caveolin expression during the evolution of acute renal failure. Kidney Int 61:1674–1683
Zhou H, Kato A, Yasuda H, Miyaji T, Fujigaki Y, Yamamoto T, Yonemura K, Hishida A (2004) The induction of cell cycle regulatory and DNA repair proteins in cisplatin-induced acute renal failure. Toxicol Appl Pharmacol 200:111–120
Acknowledgement
We thank Ms. Etsuko Hakamata for the excellent technical assistance. This work was presented in part at the XLIIth Congress of the ERA-EDTA in Istanbul, Turkey, 2005.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fujigaki, Y., Sakakima, M., Sun, Y. et al. Immunohistochemical study on caveolin-1α in regenerating process of tubular cells in gentamicin-induced acute tubular injury in rats. Virchows Arch 450, 671–681 (2007). https://doi.org/10.1007/s00428-007-0417-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-007-0417-4