Abstract
We examined ten cases of extrapulmonary lymphangioleiomyoma/lymphangioleiomyomatosis (LAM; all patients female; median age 46.5 years) for immunohistochemical labeling with a monoclonal antibody against podoplanin (D2-40), which is specific for lymphatic endothelial lining. We found positive staining in thin-wall branching vessels reflecting the lymphatic nature of tumor vessels in all cases tested. In contrast, perivascular (HMB-45 positive) myoid cells were not detected by D2-40. The D2-40 labeling confirms the current concept of lymphangiogenic origin of the tumor vessels in LAM. In addition, this study makes a further contribution to the immunohistochemical mapping of this antibody in vascular tumors. Finally, the use of this commercially available antibody provides an additional help in the differential diagnosis of LAM from other soft tissue tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lymphangio(leio)myoma is a rare disorder that nearly always affects women, mostly in the premenopausal period [5, 17]. This tumor may occur as a localized form or as a widespread multisystem disease, then called lymphangio(leio)myomatosis (LAM). Lymphangio(leio)myoma/LAM belongs to the tumor family of so-called perivascular epithelioid cell tumors (PEComas), i.e., they show perivascular epithelioid cell differentiation [6, 10]. Besides LAM, this group comprises angiomyolipoma as well as pulmonary and extrapulmonary clear cell “sugar” tumors. The concept of this tumor family is based on perivascular epithelioid cells with myomelanocytic origin [6, 10, 17]. Therefore, these cells show a characteristic positivity for smooth-muscle actin and variably desmin as well as for the (pre-)melanosomal marker HMB-45 and/or melan-A [6, 10].
LAM is different from the other PEComas in that it always contains large numbers of thin-walled branching vessels, sometimes surrounded by lymph follicles [6, 16, 17]. It was therefore proposed that these vessels are of lymphatic origin. This was confirmed by the fact that especially extrapulmonary LAMs are very often closely associated with lymph nodes or large lymphatic vessels such as the thoracic duct [16]. In addition, Kumasaka et al. [13] showed strong expression of vascular endothelial growth factor receptor-3 (VEGFR-3) in the vessels of LAM. This molecule has been demonstrated to be a marker for lymphatic endothelial cells in human tissues. Kumasaka et al. furthermore showed extensive lymphangiogenesis in association with the myoid cells [13].
Besides VEGFR-3, a new monoclonal antibody, D2-40, plays an increasing role in the routine diagnostics of vascular soft-tissue tumors. D2-40 is directed against an oncofetal antigen (M2A antigen) [15]. This protein was subsequently identified on the surface of rat glomerular podocytes, and because it was found to be involved in the flattening of foot processes in puromycin-induced nephrosis, it was named podoplanin [18]. Recently, D2-40 has been shown to react with a fixation-resistant epitope on lymphatic endothelium [12]. It is now considered to be the best commercially available antibody for the delineation of lymphatic endothelial cells, and has been tested for several vascular tumors [12]. Although positivity has been found for lymphangioma [8, 11], Kaposi’s sarcoma [8, 11], kaposiform hemangioendothelioma [4], hobnail hemangioma [7], and a subset of angiosarcomas [8, 11], labeling could not be demonstrated for hemangiomas, epitheloid hemangioendotheliomas, angiolipomas, and glomus tumors [8, 11]. Recently, sinonasal-type hemangiopericytoma could be further separated from the family of solitary fibrous tumors/hemangiopericytomas by the fact that the former commonly revealed strong labeling, whereas the latter did not at all [9]. However, until now, there are no reports concerning D2-40 labeling in LAM. We therefore analyzed LAM for D2-40 staining to contribute to immunohistochemical mapping of D2-40 in soft-tissue tumors and to confirm the lymphatic origin of this distinct tumor entity.
Materials and methods
The records from the Soft Tissue Tumor Registry of the Institute of Pathology in Jena and the records of the Institute of Pathology in Mainz were searched for cases coded as LAM of the soft tissue from 1995 to the present. We found ten cases (listed in Table 1) with a median age of 46.5 years. All patients were female. Tumors were mostly localized in the retroperitoneum (n = 8), whereas one case was found in subcutaneous fat tissue of the neck, and one case was from the mediastinum. All tumors except one (patient no. 3) were found to diffusely involve soft tissues leading to the diagnosis of “lymphangioleiomyomatosis.”
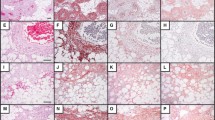
All specimens were prepared for routine histology using hematoxylin and eosin staining according to standard protocols. In brief, all tumors evaluated showed bundles of myoid cells, which were mostly arranged in a fascicular pattern. These bundles were surrounded by thin-walled and branching vessels (Fig. 1). Thus, these tumors showed the common and recently recapitulated morphological features of LAM [10].
Immunohistochemical staining was performed according to previous protocols [9] using the alkaline phosphatase-antialkaline phosphatase method. Besides D2-40, tissue specimens were investigated for expression of CD31, HMB-45, and α1-smooth-muscle actin (ASMA). Primary antibodies applied for immunohistochemical analysis are summarized in Table 2. D2-40 (Signet, Dedham, USA) was applied in a 1:400 dilution. Pretreatment by microwave (600 W, 3 × 5 min) was performed before the immunohistochemical procedure. Labeling was semiquantitatively analyzed and scored by one investigator (Hansen) as follows: no vessels/cells positive (=0), less than 20% of vessels/cells positive (=1), greater than 20% and less than 50% of vessels positive (=2), and greater than 50% of vessels positive (=3). Slides were evaluated and photographed by a Zeiss microscope (type Axiophot, Zeiss, Jena, Germany; digital camera Camedia, Olympus, Hamburg, Germany).
Results
In all cases, the typical morphological features of LAM were found as summarized above and shown in Fig. 1. Analysis of the immunohistochemical staining is summarized in Table 1. Strong expression of ASMA was demonstrated in the majority of myoid cells in nearly all patients (with more than 50% of cells positive in eight cases). In addition, this molecule was also strongly labeled in smooth muscle cells in the blood vessels (Fig. 2). By contrast, staining for HMB-45 was less intensive in the myoid cells. Most commonly, a diffuse staining pattern occurred in bundles of myoid cells, whereas vascular smooth muscle cells generally did not display HMB-45 positivity (Fig. 3). Moreover, CD31 was detected in the majority of thin-walled branching vessels between the myoid cells (see Table 1). Typically, linear staining pattern was seen. In addition, positivity for CD31 was also found in numerous blood vessels again exhibiting a linear staining (Fig. 4).
D2-40 labeled vessels occurred in all cases examined. In particular, the staining reaction was found exclusively in the thin-walled branching vasculature (Fig. 5). By contrast, neither fascicular bundles of myoid cells nor lymphocytes arranged in follicles in the vicinity of the bundles revealed any positivity. In the vessels, we commonly observed a linear staining of the thin cytoplasm of the endothelial cells, although nuclei were typically spared (Fig. 5). Some blood vessels were interspersed between myoid bundles and thin-walled vessels. These did not reveal positivity for D2-40 (Fig. 5). Semiquantitative analysis revealed five LAMs with more than 50% of vessels positive; four cases were found with more than 20% and less than 50% of vessels positive, whereas there was only one patient (no. 3) with less than 20% of the vessels positive. In the vicinity of the tumors, there were some residual thin-walled vessels without intraluminal erythrocytes, which were interpreted as lymphatic vessels, and which revealed D2-40 labeling as well (Fig. 6). However, residual blood vessels did not show any positivity for this marker.
Discussion
In contrast to the other tumors comprising the group of the so-called “PEComas,” LAM shows large numbers of thin-walled branching vessels, which have been suggested to be of lymphatic origin [6, 16, 17]. D2-40 is a (relatively novel) monoclonal antibody directed against podoplanin, which has been shown to be specific for lymphatic endothelial lining [8–11]. In the present study, we investigated the D2-40 in LAMs to further contribute to the immunohistochemical mapping of this antibody for vascular tumors, and moreover, to confirm the lymphatic origin of this distinct tumor entity. We examined (extrapulmonary) cases of LAM of the soft tissue only, because previous examinations by Matsui et al. [16] showed distinct differences between pulmonary and extrapulmonary LAMs, principally regarding the histological features.
In nearly all LAM cases, D2-40 strongly labeled a large numbers of vessels both in the tumor areas as well as in surrounding vessels in the neighborhood of the tumor. Histological differences between negative and positive vessels were not found. It is known that D2-40 in some cases does not reveal a homogenous labeling of lymphatics. Furthermore, D2-40 does not detect lymph vessels only, but also reacts with several other cell types such as mesothelial cells, myofibroblasts, and some kinds of gonadal tumors (for review see Ordonez [18]). However, in comparison with the other commercially available vascular markers, D2-40 is generally accepted as an antibody that does react with lymphatic-derived vascular tumors with the highest sensitivity. In our study, CD31 showed strong expression with lymph vessels, but on the other hand, this molecule was also labeled in numerous blood vessels (which is a typical staining pattern as described previously [19]). Taken together, these firstly presented data of D2-40 in LAM contribute to the immunohistochemical mapping of this marker in vascular soft-tissue tumors. They are in line with the general view that lymphangiogenic origin of vascular tumors can be ascertained by this marker.
The LAM cases presented in our study show a similar local distribution as those in the study of Matsui et al. [16]. Thus, extrapulmonary LAMs can be observed in three major locations: the mediastinum, the upper retroperitoneum, and the pelvic cavity (a cervical localization, as presented in our study, has been described only in one prior case [1]). In general, the specific histological features of LAM lead to the correct diagnosis. Because of the presented restricted locations, a few distinct tumor entities have to be excluded: In the case of the localization in the upper retroperitoneum (especially in the neighborhood of the kidneys), one has to bear in mind that leiomyomas of the kidney, particularly of the renal capsule, can show reactivity for HMB-45 [2], and thus can be misinterpreted as a tumor of the PEComa family. In contrast to LAM, lymph vessels do not comprise major portions of renal leiomyomas, and therefore, D2-40 can aid in the differentiation between both diseases.
Because extrapulmonary LAMs are closely associated or even embedded in the regional (pelvic or iliacal) lymph nodes, metastasis of leiomyosarcoma is a further differential diagnosis. In fact, data concerning the incidence of lymph node metastasis in patients with uterine sarcomas are controversial, reaching from 45% (9/20 patients) of uterine stage I sarcomas in the study of Chen [3] to 0% (0/27 patients) of lymph node metastases of stage I/II uterine leiomyosarcomas in the study of Leitao et al. [14]. Nevertheless, for purposes of microscopical diagnosis, one should consider this possible pitfall, which can be solved by careful examination for signs of atypia (as a common finding for leiomyosarcoma) on the one hand, and numerous D2-40 positive branching lymphatic vessels arranged between myoid bundles without signs of atypia (as a common finding for LAM) on the other hand.
In summary, we examined ten cases of extrapulmonary LAM for labeling of D2-40 and contributed to the further immunohistochemical mapping of this marker. D2-40 positivity was revealed in all LAMs tested and confirms the current concept of lymphangiogenic origin of the tumor vessels in this neoplasm. Furthermore, the use of this commercially available antibody provides an additional help in the differential diagnosis of LAM from other soft-tissue tumors.
References
Betlejewski S, Burduk D, Szukalski J (2004) Lymphangioma and lymphangiomyomatosis—the same lesion? Otolaryngol Pol 58:903–906
Bonsib S (1996) HMB-45 reactivity in renal leiomyomas and leiomyosarcomas. Mod Path 9:664–669
Chen SS (1989) Propensity of retroperitoneal lymph node metastasis in patients with stage I sarcoma of the uterus. Gynecol Oncol 32:215–217
Debelenko LV, Perez-Atayde AR, Mulliken JB, Liang MG, Archibald TH, Kozakevich HP (2005) D2-40 immunohistochemical analysis of pediatric vascular tumors reveals positivity in kaposiform hemangioendothelioma. Mod Path 18:1454–1460
Fiore MG, Sanguedolce F, Lolli I, Piscitelli D, Ricco R (2005) Abdominal lymphangioleiomyomatosis in a man with Klinefelter syndrome: the first reported case. Ann Diagn Pathol 9:96–100
Folpe AL (2002) Neoplasms with perivascular epithelioid cell differentiation (PEComas). In: Fletcher CDM, Unni KK, Mertens F (eds) WHO classification of tumours of soft tissue and bone. IARC, Lyon, pp 221–222
Franke FE, Steger K, Marks A, Kutzner H, Mentzel T (2004) Hobnail hemangiomas (targetoid hemosiderotic hemangiomas) are true lymphangiomas. J Cutan Pathol 31:362–367
Fukunaga M (2005) Expression of D2-40 in lymphatic endothelium of normal tissues and in vascular tumors. Histopathology 46:396–402
Hansen T, Katenkamp K, Katenkamp D (2006) D2-40 staining in sinonasal-type hemangiopericytoma—further evidence of distinction from conventional hemangiopericytoma and solitary fibrous tumor. Virchows Arch 448:459–462
Hornick JL, Fletcher CDM (2006) PEComa: what do we know so far? Histopathology 48:75–82
Kahn HJ, Bailey D, Marks A (2002) Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomas. Mod Path 15:434–440
Kaiserling E (2004) Immunohistochemical identification of lymph vessels with D2-40 in diagnostic pathology. Pathologe 25:362–374
Kumasaka T, Seyama K, Mitani K, Sato T, Souma S, Kondo T, Hayashi S, Minami M, Uekusa T, Fukuchi Y, Suda K (2004) Lymphangiogenesis in lymphangioleiomyomatosis. Its implication in the progression of lymphangioleiomyomatsosis. Am J Surg Pathol 28:1007–1016
Leitao MM, Sonoda Y, Brennan MF, Barakat RR, Chi DS (2003) Incidence of lymph node and ovarian metastases in leiomyosarcoma of the uterus. Gynecol Oncol 91:209–212
Marks A, Sutherland DR, Bailey D, Iglesias J, Law J, Lei M, Yeger H, Banerjee D, Baumal R (1999) Characterization and distribution of an oncofetal antigen (M2A antigen) expressed on testicular germ cell tumors. Br J Cancer 80:569–578
Matsui K, Tatsuguchi A, Valencia J, Yu ZX, Bechtle J, Beasley MB, Avila N, Travis WD, Moss J, Ferrans VJ (2000) Extrapulmonary lymphangioleiomyomatosis (LAM): clinicopathologic features in 22 cases. Human Pathol 31:1242–1248
Miettinen M (ed) (2003) Diagnostic soft tissue pathology. Churchill Livingstone, Philadelphia, pp 248–249
Ordonez NG (2006) Podoplanin: a novel diagnostic immunohistochemical marker. Adv Anat Pathol 13:83–88
Pusztaszeri MP, Seelentag W, Bosman FT (2006) Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and fli-1 in normal human tissues. J Histochem Cytochem 54:385–395
Acknowledgements
We thank Mrs. Bergholz, Mrs. Dietrich, and Mrs. Pelzer (Institute of Pathology, Jena) as well as Mrs. Roth (Institute of Pathology, Mainz) for the excellent technical assistance.
We are very grateful to all pathologists who sent case material and clinical details from patients included in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hansen, T., Katenkamp, K., Bittinger, F. et al. D2-40 labeling in lymphangiomyoma/lymphangiomyomatosis of the soft tissue: further evidence of lymphangiogenic tumor histogenesis. Virchows Arch 450, 449–453 (2007). https://doi.org/10.1007/s00428-007-0376-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-007-0376-9