Abstract
Endometrial stromal sarcomas are rare malignant mesenchymal tumors that usually develop in the uterine corpus and occasionally arise at various extrauterine sites. This report describes the first case of primary extrauterine endometrial stromal sarcoma arising in the extraperitoneal portion of the round ligament presenting as a solitary inguinal mass in a 46-year-old woman. The patient presented gradually growing tumor in the right inguinal region. Local tumor resection was performed and no recurrence or metastasis was found at 15 months after the operation. Histological examination revealed that the tumor comprised uniform, spindle-shaped cells with blunt nuclear figure and scattered small arteries, and infiltrated into adjacent tissue. No endometriosis was morphologically identified in the lesion. Immunohistochemically, the tumor cells were positive for CD10, estrogen receptor, progesterone receptor, α-smooth muscle actin, and calponin. We confirmed JAZF1/JJAZ1 fusion by reverse transcription–polymerase chain reaction and the corresponding chromosomal translocation by interphase fluorescence in situ hybridization on paraffin sections. It is essential that the inguinal region should be recognized as a possible primary site of endometrial stromal sarcoma, and the detection of a JAZF1/JJAZ1 fusion can be useful when the diagnosis is not confirmed by microscopic observation or immunohistochemistry for the tumor arising in extrauterine sites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometrial stromal sarcoma (ESS) is a rare type of uterine tumor that accounts for less than 10% of uterine sarcomas [10]. The World Health Organization’s classification of tumors of the breast and female genital organs subclassified endometrial stromal tumors into three categories: endometrial stromal nodule, low-grade ESS, and undifferentiated endometrial sarcoma (UES) [3]. The tumors traditionally called high-grade ESS were recently reclassified as UES due to their lack of specific differentiation and histological resemblance to endometrial stroma [3]. Accordingly, ESS only means low-grade ESS. ESS was distinguished from the endometrial stromal nodule based on the presence of an infiltrative growing character or vascular invasion in ESS [4, 5, 7, 8].
Recently, a fusion of two zinc finger genes, JAZF1 and JJAZ1, caused by a reciprocal translocation t(7;17)(p15;q21) was detected in more than 80% of ESS cases [4, 5, 7, 8]. Furthermore, JAZF1/PHF1 or EPC1/PHF1 fusion also presented in a small number of ESS cases [9]. Diagnosis of ESS has been dependent on histological findings supported by immunohistochemical examination. However, it could be difficult to get to a definite diagnosis for monophasic spindle cell tumors, especially when an unusual location was involved. Molecular detection of specific fusion transcripts could overcome these problems and should become a potent diagnostic tool for ESS.
ESS’s usually develop as primary uterine tumor, but ESS’s of extrauterine origin, such as the ovary, fallopian tube, pelvic cavity, abdominal cavity, omentum, retroperitoneum, vagina, vulva, and sigmoid colon, have been reported [2, 6]. The round ligament passes through the inguinal canal and comprises mainly smooth muscle, fibrous tissue, blood vessels, and nerves [12]. Most round ligament tumors are leiomyomas or fibromas, and there is no previous report of ESS arising in the inguinal portion of the round ligament.
We described the first case of primary extrauterine ESS arising from the round ligament in the inguinal canal, unassociated with endometriosis, with a confirmed JAZF1/JJAZ1 fusion by reverse transcription–polymerase chain reaction (RT-PCR) and interphase fluorescence in situ hybridization (FISH) analysis.
Clinical history
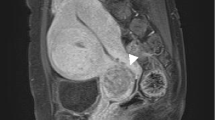
A 46-year-old woman, para 3 and gravida 3, presented with an indolent mass in the right inguinal region. Her menstrual cycle was regular, and her gynecologic history was unremarkable. Physical examination suggested an inguinal hernia. The MRI showed a demarcated 3.0-cm solid mass in the right inguinal canal (Fig. 1a), and no significant lesion in the uterus. Tumor resection was performed. At the operation, the tumor was not covered with a hernia sac nor adhered to the peritoneum but attached to the extraperitoneal portion of the round ligament. There was no evidence of recurrence or metastasis 15 months after surgery.
a MRI showing a mass (arrow head) in the right inguinal canal. b Macroscopic appearance of the cut surface of the tumor showing a yellow-white tumor. c Panoramic view of the tumor demonstrating invasion to adjacent tissue (arrow). H & E staining; scale bar, 5 mm. d The tumor cells infiltratively growth into the adjacent smooth muscle tissue of the round ligament. H & E staining (original magnification ×40). e The tumor is densely cellular, and whorls around small arteries are observed. H & E staining (original magnification ×100). f The tumor cells are uniform, short, and spindle shaped. H & E staining (original magnification ×400)
Materials and methods
Surgically resected specimen was fixed in 10% neutral-buffered formalin, cut into 6 tissue blocks of 5 mm in thickness, and embedded in paraffin. Sections, 4-μm thick, were used for hematoxylin and eosin staining, immunohistochemical studies, molecular analysis, and interphase FISH.
Immunohistochemistry
Immunohistochemical examinations were performed on deparaffinized thin sections using the standard avidin–biotin–peroxidase complex method with an automated immunostainer (Ventana Medical System, Tucson, AZ, USA). Primary antibodies against following antigens were used: low molecular weight cytokeratin (CAM5.2, prediluted, Becton–Dickinson, San Jose, CA, USA), high molecular weight cytokeratin (34βE12, 1:75, Dako, Glostrup, Denmark), vimentin (V9, 1:100, Dako), α-smooth muscle actin (1A4, 1:200, Dako), desmin (D33, 1:100, Dako), calponin (CALP, 1:75, Dako), high-molecular-weight caldesmon (h-caldesmon; h-CD, 1:100, Dako), bcl-2 (124, 1:50, Dako), E-cadherin (NCH-38, 1:100, Dako), epithelial membrane antigen (EMA; E29, 1:100, Dako), CD10 (56C6, 1:20, Novocastra, Newcastle upon Tyne, UK), CD34 (NU-4A1, 1:100, Nichirei, Tokyo, Japan), CD117 (c-kit; polyclonal, 1:80, Dako), estrogen receptor (1D5, 1:50, Dako), progesterone receptor (PgR636, 1:800, Dako), calretinin (polyclonal, prediluted, Invitrogen, Carlsbad, CA, USA), inhibin-α (R1, 1:50, Dako), S-100 protein (2A10, Immuno-Biological Laboratories, Gunma, Japan), and Ki-67 (MIB-1, 1:50, Dako). Antigen retrieval was carried out with appropriate methods for each antibody.
Molecular analysis
Three sections cut from paraffin-embedded tissue were prepared for RT-PCR. The RNA extraction and RT-PCR to identify the JAZF1/JJAZ1 fusion was carried out as previously described using the following primers: JAZF1-369-FW (5′-CCACCCATCACCCCCTCCT-3′) and JJAZ1-400-RV (5′-TGCTATGAGATTCCGAGTTC-3′) [5]. To confirm the JAZF1/JJAZ1 fusion, PCR products extracted from a corresponding band in agarose gel were analyzed by direct sequencing. The RT-PCR for detection of SYT/SSX fusion transcripts, frequently observed in synovial sarcoma, were performed as reported elsewhere [11, 13].
Interphase fluorescence in situ hybridization
The interphase FISH was performed on the paraffin-embedded tissue sections. The probes used were derived from bacterial artificial chromosomes (BAC). BAC clones were commercially purchased from Invitrogen. To detect chromosomal translocations, JAZF1/JJAZ1 fusion, the probes were selected as follows: RP11-350G16 encompassing the entire JAZF1 gene mapping to 7p15 and CTD-3205H10 fully covering the JJAZ1 gene mapping to 17q21 according to the mapping data at the NCBI map viewer website (http://www.ncbi.nlm.nih.gov/mapview/static/MVstart.html). RP11-350G16 and CTD-3205H10 were labeled with digoxigenin and biotin, respectively, with Nick translation mix (Roche Diagnostics, Basel, Switzerland). The slides were deparaffinized in xylene and rehydrated in ethanol. The slides were pretreated by microwaving in 10-mmol citrate buffer (pH 6.0) at 90°C for 15 min. The extracellular matrix in the tissue section was digested using 0.2% pepsin (in 0.01 NHCl) for 10 min at 37°C. The DNA in the tissue sections and the probes were codenatured for 10 min at 90°C, and incubated in a humidified box at 37°C for 48 h. Digoxigenin-labeled probes were detected with rhodamine-conjugated antidigoxigenin antibody, and biotinylated probes were detected with conjugated avidin (Alexa Fluor 488, Molecular Probes, Eugene, OR, USA). The nuclei were counterstained with mounting medium containing 4′,6-diamidino-2-phenylindole (Vector, Burlingame, CA, USA). The probe signals were detected using a fluorescence microscope with a digital camera, with rhodamine as red and Alexa Fluor 488 as green label. For each slide, more than 100 nuclei of tumor cells were analyzed. The composed images were reconstructed with Adobe Photoshop 6.0 software (Adobe, San Jose, CA, USA).
Results
Macroscopically, the tumor was demarcated, adhered to fibromuscular tissue of the round ligament, and had a maximum diameter of 3 cm. The cutting surface of the tumor displayed a yellow-white color and had an elastic hard consistency (Fig. 1b). No necrosis or hemorrhage was apparent.
Microscopically, the tumor cells infiltrated the adjacent smooth muscle tissue of the round ligament at the periphery, 10 mm away from the main mass (Fig. 1c and d). The tumor was highly cellular, and whorls of the tumor cells around small arteries were observed (Fig. 1e). The tumor cells were uniform and spindle-shaped, and had bland nuclei and indistinct cytoplasmic margins (Fig. 1f). Mitotic figures were rare. There was no evidence of vascular invasion or endometriosis throughout the entire specimen.
Immunohistochemically, the tumor cells were positive for CD10, estrogen receptor, progesterone receptor, bcl-2, vimentin, α-smooth muscle actin, and calponin but negative for low and high molecular weight cytokeratin, desmin, h-caldesmon, E-cadherin, EMA, CD34, CD117, calretinin, inhibin-α, and S-100 protein. Less than 1% of tumor cells were positive for Ki-67.
The RT-PCR for the detection of the JAZF1/JJAZ1 fusion transcript showed a fusion product at the expected level of 93 bp as previously reported (Fig. 2a). The fusion of JAZF1 and JJAZ1 genes was confirmed by direct sequencing (Fig. 2b). No SYT–SSX1 or SYT–SSX2 fusions were detected.
Detection of the JAZF1/JJAZ1 fusion transcript. a Fusion transcript is amplified and identified in the expected range of 93 bp. b Partial chromatogram showing the junction of the JAZF1/JJAZ1 fusion gene (arrows). M 100-bp DNA ladder, PGK phosphoglycerate kinase (247 bp, internal control), PBGD porphobilinogen deaminase (127 bp, internal control), Case RNA extracted from the present tumor
The interphase FISH revealed a JAZF1/JJAZ1 fusion as a result of a reciprocal translocation between 7p15 and 17q21 (Fig. 3).
Discussion
ESS’s usually arise in the uterine corpus and are composed of uniform ovoid to slightly spindle-shaped cells. However, these tumors occasionally present elongated spindle-shaped cells that are hard to distinguish from various spindle cell tumors and may be confused with other tumors. In this case, histological diagnosis was difficult because the tumor developed in the extrauterine site, the inguinal region, and comprised uniform, elongated spindle-shaped cells.
Immunohistochemical findings supported the differential diagnosis for spindle cell tumor. The present tumor was positive for CD10, estrogen receptor, progesterone receptor, α-smooth muscle actin, calponin but negative for desmin and h-caldesmon. These findings supported the diagnosis of ESS. Monophasic fibrous synovial sarcoma (MFSS) is a representative spindle-shaped soft tissue tumor that resembles ESS histologically, demonstrating SYT/SSX1 or SYT/SSX2 chimera gene in more than 90% of the cases [13]. In this case, although these chimera genes were not revealed by RT-PCR using RNA extracted from paraffin-embedded tissues, the diagnosis of MFSS could not completely be excluded from these negative results only.
Previous studies examining the genetic aspects of ESS’s revealed frequent chromosomal abnormalities of 6p, 7p, and 17q [8]. In recent years, a specific chromosomal rearrangement t(7;17)(p15;q21) resulting in a JAZF1/JJAZ1 fusion transcript was reported in ESS [4, 5, 7, 8]. The JAZF1/JJAZ1 fusion in ESS was described in 5 of 5 cases by Koontz et al. [7], 1 of 3 cases by Micci et al. [8], 3 of 13 cases including variant types by Huang et al. [4], and 16 of 20 cases by Hrzenjak et al. [5]. Micci et al. [9] identified ESS cases with two other genetic alterations, t(6;7)(p21;p15) leading to a JAZF1/PHF1 fusion gene and t(6;10;10)(p21;q22;p11) causing a EPC1/PHF1 fusion gene. The JAZF1, JJAZ1, and PHF1 genes all encode zinc finger domains often found in DNA-binding proteins, and fusion genes previously reported in ESS, JAZF1/JJAZ1, JAZF1/PHF1, and EPC1/PHF1 could express unusual proteins with zinc finger motifs. Wild-type JAZF1 is expressed in normal endometrium, and loss of expression for normal JAZF1 was demonstrated in some ESS cases [7]. The function of the JAZF1 gene has not been elucidated, but it has been suggested that JAZF1 might have a role in tumor suppression, and abnormal fusion proteins with DNA-binding activities could deregulate the transcriptional process of other genes [7, 9]. The recognition of the JAZF1/JJAZ1 fusion gene was crucial and unique in diagnosing ESS. The detection of these chimera genes should become a specific diagnostic tool, especially when the ESS arises at an extrauterine site. In this case, JAZF1/JJAZ1 fusion was confirmed by RT-PCR and interphase FISH.
Primary ESS’s have been reported in various extrauterine locations [2, 6]. Irvin et al. [6] reported a case of ESS that occurred in the endometriosis of the vulva in association with the extrapelvic portion of the round ligament; however, an ESS arising from the round ligament in the inguinal canal has not been described. Some cases of extrauterine ESS included endometriosis, but many of the extrauterine ESS’s described in the literature have been unassociated with endometriosis [2]. There are many reports of inguinal endometriosis often presenting as an inguinal hernia, and approximately 90% of these cases have right-side lesion [1]. The present case showed a right groin mass, but there was no endometriosis within the lesion as determined by histological examination.
In conclusion, it is important to recognize that ESS can develop in extrauterine sites such as the inguinal region, and the detection of JAZF1/JJAZ1 fusion can be useful when the diagnosis is not definitive by other studies including light microscopy and immunohistochemistry.
References
Candiani GB, Vercellini P, Fedele L, Vendola N, Carinelli S, Scaglione V (1991) Inguinal endometriosis: pathogenetic and clinical implications. Obstet Gynecol 78:191–194
Chang KL, Crabtree GS, Lim-Tan SK, Kempson RL, Hendrickson MR (1993) Primary extrauterine endometrial stromal neoplasms: a clinicopathologic study of 20 cases and a review of the literature. Int J Gynecol Pathol 12:282–296
Hendrickson MR, Tavassoli FA, Kempson RL, McCluggage WG, Haller U, Kubik-Huch RA (2003) Mesenchymal tumours and related lesions. In: Tavassoli FA, Devilee P (eds) World Health Organization classification of tumours. Pathology and genetics of tumours of the breast and female genital organs. IARC, Lyon, pp 233–244
Huang HY, Ladanyi M, Soslow RA (2004) Molecular detection of JAZF1–JJAZ1 gene fusion in endometrial stromal neoplasms with classic and variant histology: evidence for genetic heterogeneity. Am J Surg Pathol 28:224–232
Hrzenjak A, Moinfar F, Tavassoli FA, Strohmeier B, Kremser ML, Zatloukal K, Denk H (2005) JAZF1/JJAZ1 gene fusion in endometrial stromal sarcomas: molecular analysis by reverse transcriptase–polymerase chain reaction optimized for paraffin-embedded tissue. J Mol Diagn 7:388–395
Irvin W, Pelkey T, Rice L, Andersen W (1998) Endometrial stromal sarcoma of the vulva arising in extraovarian endometriosis: a case report and literature review. Gynecol Oncol 71:313–316
Koontz JI, Soreng AL, Nucci M, Kuo FC, Pauwels P, van Den Berghe H, Cin PD, Fletcher JA, Sklar J (2001) Frequent fusion of the JAZF1 and JJAZ1 genes in endometrial stromal tumors. Proc Natl Acad Sci USA 98:6348–6353
Micci F, Walter CU, Teixeira MR, Panagopoulos I, Bjerkehagen B, Saeter G, Heim S (2003) Cytogenetic and molecular genetic analyses of endometrial stromal sarcoma: nonrandom involvement of chromosome arms 6p and 7p and confirmation of JAZF1/JJAZ1 gene fusion in t(7;17). Cancer Genet Cytogenet 144:119–124
Micci F, Panagopoulos I, Bjerkehagen B, Heim S (2006) Consistent rearrangement of chromosomal band 6p21 with generation of fusion genes JAZF1/PHF1 and EPC1/PHF1 in endometrial stromal sarcoma. Cancer Res 66:107–112
Moinfar F, Gogg-Kamerer M, Sommersacher A, Regitnig P, Man YG, Zatloukal K, Denk H, Tavassoli FA (2005) Endometrial stromal sarcomas frequently express epidermal growth factor receptor (EGFR, HER-1): potential basis for a new therapeutic approach. Am J Surg Pathol 29:485–489
Okamoto S, Hisaoka M, Daa T, Hatakeyama K, Iwamasa T, Hashimoto H (2004) Primary pulmonary synovial sarcoma: a clinicopathologic, immunohistochemical, and molecular study of 11 cases. Hum Pathol 35:850–856
Smith P, Heimer G, Norgren A, Ulmsten U (1993) The round ligament: a target organ for steroid hormones. Gynecol Endocrinol 7:97–100
Tsuji S, Hisaoka M, Morimitsu Y, Hashimoto H, Shimajiri S, Komiya S, Ushijima M, Nakamura T (1998) Detection of SYT-SSX fusion transcripts in synovial sarcoma by reverse transcription–polymerase chain reaction using archival paraffin-embedded tissues. Am J Pathol 153:1807–1812
Acknowledgment
The authors thank Hideaki Ninomiya and Yoshitame Yanaida (Department of Pathology, Kanazawa Medical University Hospital, Ishikawa, Japan) for the immunohistochemistry. This work was supported by a grant for promoted research from Kanazawa Medical University (S2006-3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sato, K., Ueda, Y., Sugaya, J. et al. Extrauterine endometrial stromal sarcoma with JAZF1/JJAZ1 fusion confirmed by RT-PCR and interphase FISH presenting as an inguinal tumor. Virchows Arch 450, 349–353 (2007). https://doi.org/10.1007/s00428-006-0345-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-006-0345-8