Abstract
Sarcomatoid carcinomas (SC) of the lung are a heterogeneous group of nonsmall cell lung carcinomas (NSCLC) containing a sarcoma or sarcoma-like component. SC may represent an epithelial neoplasm undergoing divergent tissue differentiation originating from a single clone. Epithelial–mesenchymal transition (EMT) best describes the origin of the spindle and giant cells. We aimed to define chromosomal aberrations within the subgroups of SC and if EMT does play a role in SC. Twenty-two SC were investigated by chromosomal comparative genomic hybridization (CGH). Immunohistochemical staining was performed with antibodies for E-cadherin, Vimentin, c-Fos, c-Jun, Snail, TGFβ1, Notch1, β-catenin, Glycogen synthase kinase 3β (GSK3β), and Fascin. Gains occurred more frequently than losses (70.5 vs 29.5%). The shortest regions of overlap were gains on chromosomes 8q and 7 followed by 1q, 3q, and 19, supporting the common origin of the different subtypes of SC. The immunohistochemical staining suggests that the sarcomatoid components of SC might have undergone EMT, not triggered by the signaling pathways Notch1, Snail, and TGFβ1, but probably initiated by an upregulation of c-Jun and a consecutive overexpression of Vimentin and Fascin. The Wnt-pathway was not deregulated because combined membrane and cytoplasmic reactivity for β-catenin and GSK3β was observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcomatoid carcinomas (SC) of the lung are a group of rare malignancies, accounting for approximately 1% of all lung tumors. The average age of diagnosis is 60 years; the men to women ratio is almost 4:1. More than 90% of the patients are smokers or suffered from heavy tobacco abuse in the past [18]. In general, they have a worse prognosis than conventional nonsmall cell lung carcinomas (NSCLC) [15]. This type of malignant neoplasm consists of poorly differentiated NSCLC that contain a component of sarcoma or sarcoma-like differentiation, i.e., spindle and/or giant cells [15].
Five subgroups representing a pathological–morphologic continuum are currently recognized: pleomorphic carcinoma, pure spindle-cell carcinoma, pure giant cell carcinoma, carcinosarcoma, and pulmonary blastoma [18]. Histogenetically, SC may represent malignant epithelial neoplasm undergoing divergent tissue differentiation originating from a single clone [6, 8, 16].
In our present study, we tried to find concordant chromosomal aberrations within these five subgroups of SC to prove their similar histogenesis. It has been proven in the past that the different phenotypically appearing epithelial- and spindle-cell components within some subgroups of SC (urinary bladder and pharynx) might originate from the same clone [17]. Furthermore, epithelial–mesenchymal transition (EMT) could best describe the origin of the sarcoma-like appearing components of the SC [4]. Different signaling pathways that induce EMT are reported. The best known of these, the Notch, the Wnt, and the c-Jun [5, 9, 11, 21], were investigated in this study by immunohistochemistry (IHC) for key proteins.
The ancient cell-signaling system Notch could imply through Snail transduction the loss of cell polarity, a decrease in intercellular adhesion and an increase of motility, which leads to a mesenchymal phenotype [5]. Alternatively, abnormalities in the Wnt-signaling pathway could generate the mesenchymal appearing cells typical for SC [9]. Finally, we investigated c-Jun, which by synergizing with the activator protein Sp1 and binding to GC-box 1 can enhance Vimentin gene expression. Thus, Vimentin overexpression itself could also lead to EMT [21]. In addition, Fascin might contribute to a migratory phenotype of SC [1, 13].
Materials and methods
Study samples
Twenty-two cases of SC of the lung (including 16 cases of pleomorphic carcinomas, 2 of which have a predominant giant-cell and a minor large-cell component), 1 spindle-cell carcinoma, 2 cases of carcinosarcoma, 1 blastoma, and 2 cases of GC were collected as formalin-fixed, paraffin-embedded tissue blocks from the lung disease archive of the Institute of Pathology, Medical University of Graz, Austria.
DNA isolation
An experienced pathologist classified the tumor samples. Tumors were microdissected with needles, and tumor DNA was isolated from six consecutive, deparaffinized sections. Comparative genomic hybridization (CGH) [12, 20] was essentially done as previously described by Halbwedl et al. [7]. We did not separate the histologically different pleomorphic carcinoma components because in a previous report, no genetic differences between the epithelial- and spindle-cell component were found by CGH [17].
On the average, ten metaphase spreads were evaluated per case. Thresholds for chromosomal gains and losses were set to 1.2 and 0.8, respectively. Telomeres, centromeres, the Y-chromosomes, and gains on well-known CGH critical regions (1p36, 17p, 19p, and 22) were not evaluated. In two cases (one pleomorphic carcinoma and one blastoma), CGH could not be performed due to DNA degradation.
Aberrations of each case were listed in a Microsoft Access© database and displayed in a diagram to show overlapping genetic alterations.
Immunohistochemistry (IHC)
The same 22 paraffin-embedded tissue blocks as used for CGH were investigated. The immunohistochemical staining experiments were performed using primary antibodies and commercially available detection kits as recommended by the manufacturers (Table 1). Using not only tissues recommended by the manufacturer but also normal lung parenchyma, we tested reactivity and staining intensity. Internal positive and negative controls were identified within the tissue section. After having defined weak and strong stainings (1+ and 3+), the sections were evaluated for the percentage of positively stained tumor cells for each antibody at once (in increments of ten). The site of the reaction was also evaluated as nuclear, cytoplasmic, and membranous.
Statistical analysis of the IHC results was performed using Spearman’s rank-sum test for correlation used in Microsoft Excel© add-in program Winstat©.
All patient data were anonymized and thus they were in accordance with the rules of the ethical committee of our University.
Results
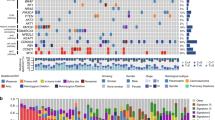
In our cases, we found more chromosomal gains than losses (70.5 vs 29.5%). Frequent chromosomal alterations were gains on chromosome 1 (seven cases, 31.8% of all cases), chromosome 8q (six cases, 27.3%), chromosome 3 (six cases, 27.3%), chromosome 7 (five cases, 22.7%), and chromosome 19q (five cases, 22.7%). In addition, we detected gains on chromosomes 11, 12p, 17q, and 20q. Deletions of genetic material were seen only in a few cases (most frequently on chromosomes 4q, 5q, 6q, and 13). Furthermore, two other cases (one pleomorphic carcinoma and one giant-cell carcinoma) did not exhibit any chromosomal aberrations as detected by CGH. In addition, two pleomorphic carcinomas characterized by predominant giant-cell components and minor large-cell carcinomas differed from the other SC by showing gains of 11p and 12p but not the common aberrations on chromosomes 1, 8q, 3, and 7. A detailed karyotype of all chromosomal alterations is shown in Fig. 1.
Overlapping karyograms of sarcomatoid carcinomas. Losses of genetic material are displayed to the right, while gains are pictured to the left of each chromosome. Pleomorphic carcinoma (green), carcinosarcoma (red), pleomorphic carcinoma with giant- and large-cell components (yellow), pure giant-cell carcinoma (blue)
In all control experiments, the 99% confidence interval was within the thresholds set to 1.2 and 0.8 except of the sex chromosomes. The deviation of the ratio at X-chromosomes was clearly detected in each control experiment hybridized in a sex-mismatch manner.
All immunohistochemical markers were evaluated semiquantitatively for their staining pattern and intensity. Antibodies for E-cadherin stained the epithelial components of SC more intensively, whereas Vimentin preferentially stained spindle and giant cells. Vimentin not only showed a positive immunoreactivity in the cytoplasm but in a few cases also stained the nuclei (Table 2, Fig. 2a). Immunohistochemical staining of the cell-cycle control proteins c-Jun and c-Fos was mostly confined to the mesenchymal parts of SC. C-Jun (on chromosome 1p32) showed a strongly positive nuclear reaction; however, in most cases also, a cytoplasmic positive immunostaining pattern could be observed (Fig. 2b). C-Fos (mean: 30%, 2+ intensity) was less intensively stained compared to c-Jun (mean: 70%, 3+ intensity). Weak overall immunoreactivity could be seen for proteins belonging to the Notch1/TGFβ1/Snail signaling pathway. Snail in most SC showed a low percentage of positive cells (mean: 5%); in Notch1, most cells were weakly stained (mean: 45%, 1+). In addition, with both markers only cytoplasmic and no nuclear reaction was seen (see Table 2). Reactivity for TGFβ was overall weak, and only a small percentage of the tumor cells were stained (mean: 5%, 1+).
a Immunohistochemistry for Vimentin. In most cases, there is positive reaction in the mesenchymal parts of SC, whereas the differentiated epithelial component is negative. Bar 20 μm. b Immunohistochemistry for c-Jun. Strong positive nuclear and a focal cytoplasmatic reaction is seen in this SC. There is no difference between the SC components. Bar 20 μm. c Immunohistochemistry for GSK3β. A positive staining is seen in the cytoplasm. Bar 50 μm. d Immunohistochemistry for β-catenin. Overall positive reaction at the cell membrane. Bar 20 μm. e Immunohistochemistry for Fascin. Strong overall reaction in the mesenchymal parts of SC. Most epithelial cells are negative. Bar 20 μm
In addition, diffuse cytoplasmic and membrane-confined staining was seen for Glycogen synthase kinase 3β (GSK3β) (Fig. 2c) and β-catenin (Fig. 2d), both representing the Wnt-signaling pathway. Fascin (fascin 1 and 3 on chromosome 7, fascin 2 on chromosome 17q), an actin-cross-linking protein, showed the strongest reaction of all antibodies used in this study and was remarkably only positive in the mesenchymal parts of SC (Fig. 2e). All results of the immunohistochemical study are given in Table 2.
The statistical analysis (n=22) revealed a strong positive correlation for Fascin and c-Jun, correlation factor r=0.48 (p=0.011), Fascin/Vimentin, r=0.52 (p=0.006), and c-Jun/Vimentin, r=0.37 (p=0.046). In contrast, we did not evaluate any significant positive correlation between Notch/TGFβ1/Snail and Vimentin or Fascin. Because β-catenin did not show a nuclear staining pattern and therefore might not function as a transcription factor, we did not include it in our statistical analysis.
Discussions
CGH results for SC revealed some overlaps of chromosomal aberrations indicating a possible common histogenetic origin of the different subtypes of SC.
Pleomorphic and spindle-cell carcinomas all showed gains on either 8q, 7, 5, 3q, 1 or 19, and 5p. In addition to pleomorphic carcinoma, carcinosarcoma also showed gains on 3q, 5p, and 19. This might be interpreted that carcinosarcoma and pleomorphic and spindle-cell carcinoma are closely related according to their genetic origin, which is also supported by the studies of Rossi et al., Pelosi et al., and Fishback et al. [3, 13–15]. Gains of genetic material on chromosomes 2q and 14q separate carcinosarcoma from the other subtypes of SC. This, however, should be interpreted with caution due to the small numbers of cases investigated.
The chromosomal aberrations of giant-cell carcinomas also showed gains on chromosomes 1q, 3q, and 7, indicating a genetic relation to pleomorphic carcinoma and carcinosarcoma. Nevertheless, chromosomal losses on 13p and 15p were only found in giant-cell carcinoma, probably pointing to the differences in the phenotypic appearance as well as their much more aggressive behavior [3].
The two cases of pleomorphic carcinomas with giant- and large-cell components did clearly show chromosomal alterations, which are not found in the other subgroups of SC. Gains on chromosomes 11p, 12p, 9q, and 17q and losses on 13q were exclusively detected at that type of carcinomas. There was no similarity with pure giant-cell carcinoma; however, the number of cases is probably too small to draw a final conclusion.
Some of the chromosomal aberrations found in SC are not commonly seen in adenocarcinomas (7+ and 12p+) and gains of 1q, 7+, and 8+ not in squamous cell carcinomas [10, 19]. This clearly separates SC from adenocarcinomas and squamous cell carcinomas, although both are the most frequent found components in SC. In one study, the various parts of SC were investigated separately by CGH, and no influence on the chromosomal aberrations was seen [17].
With respect to EMT [2, 4, 5], it seems that the well-known signaling-pathway Notch and its downstream ligands such as TGF-β do not play a crucial role in our cases. Moreover, the combined membranous and cytoplasmic staining for β-catenin and the cytoplasmic staining for GSK3β could implicate a normally regulated Wnt-pathway [9, 11]. The weak staining for E-cadherin might be interpreted as a result of mutation, which would cause β–catenin to dissociate from its membranous partners and shed into the cytoplasm. High expression of GSK3β in the cytoplasm could be an indicator of immediate ubiquitinylation of β-catenin; however, GSK3β needs to be phosphorylated. This was not proven in our experiments.
It is interesting to note that we could show that high nuclear activity of the transcription factor c-Jun might be responsible for the expression of Vimentin in SC, as previously investigated by Wu et al. [21] in cell lines, and probably also Fascin, which is also substantiated by our statistical analysis [13]. Vimentin might therefore be responsible for the EMT and Fascin for an increase in cell motility.
In conclusion, our CGH data point to a close relationship of all subgroups in SC, except the pleomorphic, giant-, and large-cell carcinomas. We provide some evidences that the newly described signaling pathway of c-Jun/ Vimentin acts as probable promoter of EMT in SC. Thus, the members of the c-Jun pathway might be the targets for a new therapeutic treatment concept in these highly aggressive carcinomas.
References
Adams JC (2004) Roles of fascin in cell adhesion and motility. Curr Opin Cell Biol 16:590–596
Akhurst RJ, Derynck R (2001) TGF-beta signaling in cancer—a double-edged sword. Trends Cell Biol 11:S44–S51
Fishback NF, Travis WD, Moran CA, Guinee DG Jr, McCarthy WF, Koss MN (1994) Pleomorphic (spindle/giant cell) carcinoma of the lung. A clinicopathologic correlation of 78 cases. Cancer 73:2936–2945
Gotzmann J, Mikula M, Eger A, Schulte-Hermann R, Foisner R, Beug H, Mikulits W (2004) Molecular aspects of epithelial cell plasticity: implications for local tumor invasion and metastasis. Mutat Res 566:9–20
Grego-Bessa J, Diez J, Timmerman L, de la Pompa JL (2004) Notch and epithelial-mesenchyme transition in development and tumor progression: another turn of the screw. Cell Cycle 3:718–721
Guarino M, Micheli P, Pallotti F, Giordano F (1999) Pathological relevance of epithelial and mesenchymal phenotype plasticity. Pathol Res Pract 6(195):379–389
Halbwedl I, Ullmann R, Kremser ML, Man YG, Isadi-Moud N, Lax S, Denk H, Popper HH, Tavassoli FA, Moinfar F (2005) Chromosomal alterations in low-grade endometrial stromal sarcoma and undifferentiated endometrial sarcoma as detected by comparative genomic hybridization. Gynecol Oncol 2:582–587
Holst VA, Finkelstein S, Colby TV, Myers JL, Yousem SA (1997) p53 and K-ras mutational genotyping in pulmonary carcinosarcoma, spindle cell carcinoma, and pulmonary blastoma: implications for histogenesis. Am J Surg Pathol 21:801–811
Ilyas M (2005) Wnt signalling and the mechanistic basis of tumour development. J Pathol 205:130–144
Jiang F, Yin Z, Caraway NP, Li R, Katz RL (2004) Genomic profiles in stage I primary non small cell lung cancer using comparative genomic hybridization analysis of cDNA microarrays. Neoplasia 6:623–635
Kallionemi A, Kallionemi OP, Sudar D, Rutovitz D, Gray JW, Waldman F, Pinkel D (1992) Comparative genomic hybridization for molecular cytogenetic analysis of solid tumors. Science 258:818–821
Klymkowsky MW (2005) Editorial. β-catenin and its regulatory network. Human Pathol 36:225–227
Pelosi G, Fragetta F, Nappi O, Pastorino U, Maisonneuve P, Pasini F, Iannucci A, Solli P, Musavinasab HS, de Manzoni G, Terzi A, Viale G (2003) Pleomorphic carcinomas of the lung show a selective distribution of gene products involved in cell differentiation, cell cycle control, tumor growth, and tumor cell motility: a clinicopathologic and immunohistochemical study of 31 cases. Am J Surg Pathol 27:1203–1215
Pelosi G, Scarpa A, Manzotti M, Veronesi G, Spaggiari L, Fragetta F, Nappi O, Benino E, Pasini F, Antonello D, Iannucci A, Maisonneuve P, Viale G (2004) K-ras gene mutational analysis supports a monoclonal origin of biphasic pleomorphic carcinoma of the lung. Mod Path 17:538–546
Rossi G, Cavezza A, Sturm N, Migali M, Facciolongo N, Longo L, Maiorana A, Brambilla E (2003) Pulmonary carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements: a clinicopathologic and immunohistochemical study of 75 cases. Am J Surg Pathol 27:311–324
Thompson L, Chang B, Barsky SH (1996) Monoclonal origins of malignant mixed tumors (carcinosarcomas). Evidence for a divergent histogenesis. Am J Surg Pathol 20:277–285
Torenbeek R, Hermsen MA, Meijer GA, Baak JPA, Meijer CJLM (1999) Analysis by comparative genomic hybridization of epithelial and spindle cell components in sarcomatoid carcinoma and carcinosarcoma: histogenetic aspects. J Pathol 189:338–343
Travis W, Brambilla E (2004) Pathology and genetics of tumours of the lung, pleura, thymus and heart. WHO, Lyon
Sy SM, Wong N, Lee TW, Tse G, Mok TS, Fan B, Pang E, Johnson PJ, Yim A (2004) Distinct patterns of genetic alterations in adenocarcinoma and squamous cell carcinoma of the lung. Eur J Cancer 40:1082–1094
Weiss MM, Hermsen MA, Meijer GA, van Grieken NC, Baak JP, Kuipers EJ, van Diest PJ (1999) Comparative genomic hybridisation. Mol Pathol 52:243–251
Wu Y, Zhang X, Zehner ZE (2003) c-Jun and the dominant-negative mutant, TAM67, induce vimentin gene expression by interacting with the activator Sp1. Oncogene 22:8891–8901
Acknowledgement
This study was supported by the Medical University of Graz.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blaukovitsch, M., Halbwedl, I., Kothmaier, H. et al. Sarcomatoid carcinomas of the lung—are these histogenetically heterogeneous tumors?. Virchows Arch 449, 455–461 (2006). https://doi.org/10.1007/s00428-006-0256-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-006-0256-8