Abstract
Malignant fibrous histiocytoma (MFH) and inflammatory myofibroblastic tumor (IMT) are uncommon primary non-epithelial cell tumors of the pancreas. In addition, there are inflammatory pseudotumors (IPT) that may arise in the course of autoimmune pancreatitis (AIP). In the English language literature, only 24 cases of IMT and nine cases of MFH in the pancreas have been reported to date. We investigated three individual spindle cell tumors of the pancreas that were identified as MFH, IMT, and IPT, respectively, using immunohistochemical and molecular analysis. Both the MFH and the IMT, but not the IPT, showed nuclear p53 expression and mutations of the p53 gene. The MFH and the IMT also had higher mitotic and Ki-67 (MIB-1) indexes than the IPT. The IPT was found to be a tumor-like case of AIP. Many IgG4-positive plasma cells, which are considered to be a feature of AIP, were found in all three tumors. It is concluded that in this series of spindle cell tumors of the pancreas, apart from immunohistochemical features, the demonstration of p53 mutations may be helpful in distinguishing true neoplastic tumors from pseudotumors such as IPTs arising in the context of AIP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant tumors of the pancreas are mostly of epithelial origin, predominantly ductal adenocarcinomas. Non-epithelial malignant tumors of mesenchymal origin are extremely rare and account for only 0.6% of the cases [4, 25]. The most common among the latter are leiomyosarcomas, liposarcomas, neurogenic sarcomas (schwannomas or peripheral neuroectodermal tumors), and malignant lymphomas [25, 43]. These tumors may cause difficulties in the differential diagnosis, even after extensive immunohistochemical analysis [25, 43]. In particular, in tumors with a spindle cell pattern, it may be difficult to distinguish malignant fibrous histocytomas (MFH) (now often called myxofibrosarcomas [11]) from inflammatory myofibroblastic tumors (IMT) or inflammatory pseudotumors (IPT), but this distinction is crucial for predicting the prognosis and selecting the proper treatment [7, 11, 43].
In the pancreas, so far, only nine cases of MFH [1, 3, 12, 14, 23, 26, 27, 33, 46] and 24 cases of IMT [2, 8, 10, 17, 20, 24, 28, 31, 32, 34–36, 39, 41, 42, 44, 47, 48, 50, 52] have been reported in the English language literature. Furthermore, IPT and IMT have often been used synonymously [10, 48, 50], though they can differ in their pathogenesis [29, 30]. We recently encountered representative cases of primary MFH, IMT, and IPT. On these cases, we conducted immunohistochemical and gene mutational analyses which, we believe, provide valuable clues for the characterization of these tumors and important information for the differential diagnosis. We will also discuss the relation of IPT to autoimmune pancreatitis (AIP).
Patients’ histories
Case 1
A 44-year-old woman was admitted to the hospital in May 2003 with a mass in the left upper abdomen and epigastralgia. She had a history of uterine cervical cancer (early stage) that had been successfully operated on 4 years earlier. Blood tests revealed mild anemia and slightly elevated levels of the tumor marker immune-suppressor acid protein (IAP) (521 μg/ml, normal <500 μg/ml). C-reactive protein (1.1, <0.3 mg/dl) was elevated, but immunoglobulins were not examined. Liver function tests and pancreatic enzymes were normal and there was no elevation in markers of cholestasis (alkaline phosphatase, bilirubin, etc). Ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI) revealed a tumor mass in the dorsal area of the stomach and enlarged peripancreatic and para-aortic lymph nodes. The latter lymph nodes appeared to obstruct the left ureter, causing hydronephrosis. Angiography results revealed that the tumor originated from the pancreas. A creatinine clearance test for 24 h showed dysfunction of the kidney. Administration of Ga-diethylenetriamine pentaacetic acid (DTPA) did not result in enhancement of the mass. A malignant pancreatic tumor with lymph node metastasis was diagnosed and the tumor and a lymph node were resected. However, as the tumor adhered firmly, radical resection was not achieved. After the operation, the patient received extensive chemotherapy. During a follow-up period of 20 months, there was no recurrence.
Case 2
A 64-year-old male was admitted to the hospital in October 1995 for left lower thoracic pain at inspiration and an abdominal mass in the left upper abdomen. His past history was not informative. A blood test revealed mild anemia and elevated serum amylase (269, 50∼228 IU/l), elastase (470, 100–400 ng/dl), and IAP (739, <500 mg/ml). Liver function tests were normal, and there was no sign of bile duct stenosis. Immunoglobulin was not examined. US, CT, and MRI results revealed a well-circumscribed homogeneous mass originating in the pancreas body. After administration of Ga-DTPA, the area surrounding the mass was enhanced. Endoscopic retrograde pancreatography showed an irregular stricture of the pancreatic duct in the body. A pancreatic carcinoma was suspected and a resection of the tail and body of the pancreas was performed. No metastasis was found during operation. Five years after the operation, the patient is disease-free.
Case 3
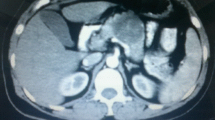
A 40-year-old male was admitted to the hospital with epigastric pain and fever. He had been free of disease until this event. He had noticed thirst, polydipsia, and weight loss for the last 6 months and was found to have diabetes and acute hepatitis. Two months later, he developed jaundice, general fatigue, epigastric pain, and restlessness. He was referred to our hospital and diagnosed as having a pancreatic mass and acute obstructive cholangitis. Laboratory data revealed marked anemia, leukocytosis, elevated levels of bilirubin, bile tract markers, CA19-9 (41.3, <37 IU/ml), and serum ferritin (941, 25–280 ng/ml). Immunoglobulin was not examined. Abdominal ultrasound and CT results disclosed a large low-density mass in the head of the pancreas with diffuse swelling of the body. A diagnosis of pancreatic carcinoma was made clinically and a Whipple procedure was performed.
After the operation, the patient suffered from diabetes, which was controlled by insulin injection. However, 8 months after the operation, his general condition worsened and he died. An autopsy was not performed.
Materials and methods
Tissues
Specimens from the pancreatic tumors were fixed in 10% formalin and processed to obtain 4-μm paraffin sections that were stained with hematoxylin and eosin, Azan, silver, elastica van Gieson, periodic acid–Schiff , and alcian blue.
Immunohistochemistry
For immunohistochemistry, the standard streptavidin–biotin technique was applied. The list of antibodies used and their sources as well as staining conditions are summarized in Table 1. Negative control stains were performed by omitting the primary antibodies or substituting nonimmune rabbit or swine sera. The number of cells showing nuclear staining for p53 and Ki-67 (MIB-1) was determined in areas with high cellularity and recorded as the number of positive cells per 100 tumor cells.
Genetic analysis
For the detection of p53 and K-ras mutations, genomic DNA was extracted from sections cut from paraffin-embedded tissue blocks. Briefly, the sections were deparaffinized with xylene, washed with 100% ethanol, and subsequently dried. Tumor tissue was scratched off the slides with a fine needle. DNA was extracted and purified with DNAeasy extraction kits (QIAGEN, Valencia, CA, USA). Polymerase chain reaction (PCR) was performed as previously described [37]. PCR Amplicon was ligated to the vector with a TOPO cloning kit (Invitrogen, Carlsbad, CA, USA). After blue–white selection, purified vector was digested with EcoRI. An about 100-bp insert clone was loaded onto a 3% agarose gel. The positive clone was amplified using a VIC dye sequence kit (Applied Biosystems, Foster City, CA, USA) and a purified Qiagen kit. Direct sequencing was carried out with a Perkin Elmer ABI Prism 310 sequence analyzer (Applied Biosystems).
Results
Pathological findings
Case 1
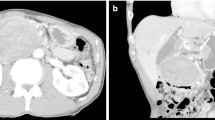
The left-sided pancreatectomy specimen contained a well-encapsulated, light yellowish firm mass measuring 8.3×5.6×6.0 cm (Fig. 1a). Microscopic spindle- to oval-shaped tumor cells mixed with variable numbers of polymorphic or multinucleated giant cells were arranged in a storiform pattern (Fig. 2a). There were some areas with high cellularity, and atypical mitoses were also seen. Lymphocytes, plasma cells, and eosinophils infiltrated within the tumor area (Fig. 2b). There were areas of stromal myxoid changes, but no necrosis or hemorrhage was found. The resected lymph node revealed a metastasis of the pancreatic tumor.
Light microscopic appearances of pancreatic MFH (case 1), IMT (case 2), and IPT (case 3). Case 1: the low power view shows a diffuse eosinophilic fibrous area mixed with sporadic lymphoid aggregates (a). The high power view shows haphazardly oriented large pleomorphic cells with a typical storiform-like growth pattern (b). Case 2: the low power view shows conspicuous inflammatory changes occupying a large area (c). Some atrophic exocrine tissues are visible. The high power view reveals spindle cells admixed with eosinophilic and lymphoid cells (d). Case 3: the low power view shows a large inflammatory area with marked proliferation of spindle cells adjacent to atrophic exocrine tissues (e). In the high power view, spindle cells show nuclear enlargement and pleomorphism growing with a typical storiform pattern mixed with inflammatory cells (f)
Case 2
The left-sided pancreatectomy specimen contained a light yellowish firm mass in the pancreatic body measuring 10.0×9.0×8.5 cm (Fig. 1b). The tumor consisted of mainly microscopic spindle- to oval-shaped cells arranged in a storiform pattern. There were scattered polymorphic or multinucleated cells (Fig. 2c,d), but nuclear polymorphism was less than in case 1. Mitoses were not found. The stroma was rich in collagen, and there was extensive lymphoplasmacytic infiltration.
Case 3
The Whipple resection specimen contained a mass 7.5×3.6×2.0 cm in size in the head of the pancreas. The tumor had a hard consistency and its cut surface was solid, lobulated, and relatively well circumscribed. The normal pancreatic tissue was replaced by a meshwork of microscopic spindle-shaped fibroblastic cells arranged in a storiform pattern and some foamy histiocytes (Fig. 2e). The spindle cells showed nuclear enlargement and hyperchromatism (Fig. 2f). Mitoses were not found. Detailed examinations of the tumor and its surrounding pancreatic tissue revealed features of autoimmune pancreatitis, such as periductal lymphoplasmacytic infiltration or venulitis. The lymphoplasmacytic infiltration extended into the pancreatic parenchyma, replacing most of the acinar cells and encasing medium-sized ducts. Mild inflammatory changes were also seen in the bile duct wall, the gallbladder, and the ampulla of Vater.
All clinicopathological features are summarized in Table 2.
Immunohistochemistry
Table 1 summarizes the immunohistochemical results. In case 1, spindle cells and pleomorphic cells showing a storiform pattern were positive for vimentin but negative for epithelial, muscle, neural, adipocytic, or lymphoma markers as well as anaplastic lymphoma kinase (ALK) and CD34. Between the tumor cells, there were CD68 positive macrophages. The tumor cells showed frequently positive for p53 to an extent of 25% level (Fig. 3a). The MIB-1 index of the tumor cells was 19% of the cells (Fig. 3b). In case 2, the spindle cells were also positive for vimentin and negative for epithelial, neural, and lymphoma markers, including ALK and follicular dendritic cell markers. Single spindle cells were positive for smooth muscle actin (SMA) and HHF35 but negative for CD34. They were positive for p53 at a level of 9% and the MIB-1 index was 7% of the cells (Fig. 3c,d). CD68 positive macrophages were present. In case 3, the spindle cells were positive for vimentin but negative for CD34, cytokeratin, EMA, S-100, myoglobin, or desmin. P53 was negative (Fig. 3e). The MIB-1 index was less than 1% (Fig. 3f).
p53 and MIB-1 expression in pancreatic MFH (case 1), IMT (case 2), and IPT (case 3). Case 1: large pleomorphic tumor cells show positive reactions to p53 at a level of 25% (a). They also show a high level (19%) of MIB-1 expression (b). Case 2: similar to Case 1, the tumor cells show strong p53 positivity in some areas reaching 9% (c). They also show high MIB-1 index up to 7% level, but the reaction is less intense compared to case 1 (d). Case 3: in contrast to the above cases, p53 is negative (e). The MIB-1 index is also very low (<1%) (f)
Scattered IgG4-positive plasma cells were consistently detected in all three cases but most conspicuous in case 3.
Genetic analysis
Because nuclear p53 expression was found in cases 1 and 2, direct DNA sequencing for p53 exon 5–8 was carried out (Fig. 4). In case 1, two point mutations were detected: G-to-A transversion at codon 238, resulting in an amino acid change from cysteine to tyrosine, and T-to-C transversion at codon 272, resulting in an amino acid change from valine to alanine. In case 2, one point mutation was found, G-to-T transversion at codon 294, resulting in an amino acid change from glutamate to asparatate. On the other hand, the tumors in both cases 1 and 2 carried a wild-type K-ras oncogene.
Discussion
In this study, we analyzed the immunohistochemical and molecular features of three pancreatic tumors that shared a microscopic spindle-cell pattern with abundant collagen production admixed with inflammatory cells. This analysis revealed that individual features of the three tumors allowed them to be typed as MFH, IMT, and AIP-associated IPT.
The first step in the investigation of these tumors was to distinguish them from undifferentiated carcinomas, which may show a sarcomatoid pattern [16, 43]. As our tumors were negative for cytokeratin, this possibility could be easily excluded. The next tumor to be ruled out was a sarcomatous nodule in association with a mucinous cystic neoplasm [49, 53]. We, therefore, examined multiple sections but failed to find any evidence of a cystic lesion. The diagnoses we finally considered were MFH, IMT, and IPT.
The diagnosis of MFH, which was made in the first patient, was based on the tumor’s microscopic features (i.e., anaplastic spindle cells arranged in a storiform pattern), its sole positivity for vimentin, and the immunohistochemical exclusion of any other tumor, such as malignant peripheral nerve sheath tumor, by the negativity for the respective tumor markers. So far, only nine cases of MFH of pancreatic origin have been reported [1, 3, 12, 14, 23, 26, 27, 33, 46] (Table 3). A review of these cases revealed that their features compare well with those of our case. In addition, we detected nuclear p53 expression and a p53 mutation. This finding is in accordance with the 35% positivity rate for nuclear p53 expression recently reported in MFHs of various origins [9]. In our case, the demonstration of a p53 mutation clearly established the neoplastic nature of the tumor and distinguished it from any non-neoplastic pseudotumor.
In the second patient, we diagnosed an IMT. This is a low-grade tumor composed of fibroblasts and myofibroblasts in association with inflammatory cells, whose molecular pathogenesis was partially elucidated recently. In approximately 50% of IMTs, various gene aberrations including the anaplastic lymphoma kinase gene at chromosome 2p23 have been identified [13, 15, 32, 38, 45]. In our case, no ALK gene abnormality was found. However, an ALK gene abnormality is more often seen in children or young adults than in elderly people. This may explain why we did not detect an ALK gene abnormality in our case [6, 22]. The diagnosis of an IMT was, therefore, based on the microscopic diagnostic criteria such as fasciitis-like, fascicular, and sclerosing areas with a prominent chronic inflammatory infiltrate. In addition, we found nuclear p53 expression and a p53 mutation, findings that have also been reported in IMTs [51].
In the third patient, the tumor expressed neither SMA, S100 and CD34 nor nuclear p53. Together with its histological features, we concluded that the tumor represented an IPT. IPTs that arise in the course of AIP must be clearly distinguished from MFHs or IMTs, considering their different pathogenesis and therapy (i.e., its treatment with steroids). However, many previous reports of IMTs in the pancreas did not give any consideration to IPTs arising in the course of AIP (Table 4). Judging from the published illustrations and the descriptions, we believe that many previous cases of IMTs and IPTs arose in the setting of AIP [2, 8, 10, 17, 19, 20, 24, 28, 31, 32, 34–36, 39, 41, 42, 44, 47, 48, 50, 52] (see Table 4).
IMTs and IPTs associated with AIP have many clinicopathological features in common. First, both are mass-forming lesions which commonly focus on the pancreas head and cause obstructive jaundice. Therefore, such patients are often suspected to suffer from pancreatic ductal adenocarcinoma [18, 19]. Second, involvement of the distal bile duct, as reported in many IMTs, is a common finding in patients with AIP [18, 19]. Third, a lymphoplasmacytic infiltrate and myofibroblasts arranged in a storiform pattern are seen in both lesions [10, 26, 48, 50].
It has been reported that IgG4-positive plasma cells are abundant in AIP which help to establish its diagnosis [18, 54]. In this study, the demonstration of IgG4-positive plasma cells was not useful for the differential diagnosis as these cells were also found in both the MFH and the IMT. Nevertheless, constant presence of IgG4 cells in these tumors might indicate non-incidental occurrence of mesenchymal tumors with background of AIP.
In summary, we report three cases of tumorous spindle cell lesions of the pancreas, two of which were found to be neoplasms and the other an inflammatory process. p53 expression and gene mutation provided an important clue for distinguishing the two true neoplasms from the pseudotumor.
References
Allen KB, Skandalakis LJ, Brown BC, Gray SW, Skandalakis JE (1990) Malignant fibrous histiocytoma of the pancreas. Am Surg 56:364–368
Abrebanel P, Sarfaty S, Gal R, Chaimoff C, Kessler E (1984) Plasma cell granuloma of the pancreas. Arch Pathol Lab Med 108:531–532
Bastian D, Ramaswamy A, Barth PJ, Gerdes B, Ernst M, Bartsch D (1999) Malignant fibrous histiocytoma of the pancreas: a case report with genetic analysis. Cancer 85:2352–2358
Baylor SM, Berg JW (1973) Cross-classification and survival. Characteristics of 5,000 cases of cancer of the pancreas. J Surg Oncol 5:335–358
Chutaputti A, Burrell NI, Boyer J (1995) Pseudotumor of the pancreas associated with retroperitoneal fibrosis: a dramatic response to corticosteroid therapy. Am J Gastroenterol 90:1155–1158
Coffin CM, Patel A, Perkins S, Elenitoba-Johnson KS, Perlman E, Griffin CA (2001) ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod Pathol 14:569–576
Dehner LP (2004) Inflammatory myofibroblastic tumor. The continued definition of one type of so-called inflammatory pseudotumor. Am J Surg Pathol 28:1652–1654
Dudiak KM (1993) Inflammatory pseudotumor of the pancreas. Am J Roentgenol 160:1324–1325
Engellau J, Persson A, Bendahl PO, Akerman M, Domanski HA, Bjerkehagen B,Lilleng P, Weide J, Rydholm A, Alvegard TA, Nilbert M (2004) Expression profiling using tissue microarray in 211 malignant fibrous histiocytomas confirms the prognostic value of Ki-67. Virchows Arch 445:224–230
Esposito I, Bergmann F, Penzel R, di Mola FF, Shrikhande S, Büchler MW, Friess H, Otto HF (2004) Oligoclonal T-cell populations in an inflammatory pseudotumor of the pancreas possibly related to autoimmune pancreatitis: an immunohistochemical and molecule analysis. Virchows Arch 444:119–126
Fletcher CD (1992) Pleomorphic malignant fibrous histiocytoma: fact or fiction? A critical reappraisal based on 159 tumors diagnosed as pleomorphic sarcoma. Am J Surg Pathol 16:213–228
Garvey JF, Ng A, England JF, Sheldon DM (1989) Malignant fibrous histiocytoma of the pancreas. HPB Surg 1:233–237
Griffin CA, Hawkins AL, Dvorak C, Henkle C, Ellingham T, Perlman EJ (1999) Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res 59:2776–2780
Haba R, Kobayashi S, Hirakawa E, Miki H, Okino T, Kurokawa T, Yamamoto S (1996) Malignant fibrous histiocytoma of the pancreas. Pathol Int 46:515–519
Hojo H, Newton Jr WA, Hamoudi AB, Qualman SJ, Wakasa H, Suzuki S, Jaynes F (1995) Pseudosarcomatous myofibroblastic tumor of the urinary bladder in children: a study of 11 cases with review of the literature. An Intergroup Rhabdomyosarcoma Study. Am J Surg Pathol 19:1224–1236
Hoorens A, Prenzel K, Lemoine NR, Klöppel G (1998) Undifferentiated carcinoma of the pancreas: analysis of intermediate filament profile and K-ras mutations provides evidence of a ductal origin. J Pathol 185:53–60
Johnson RL, Page DL, Dean RH (1983) Pseudotumor of the pancreas. South Med J 76:647–649
Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, Okamoto A, Egawa N, Nakajima H (2003) A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol 38:982–984
Klöppel G, Lüttges J, Löhr M, Zamboni G, Longnecker D (2003) Autoimmune pancreatitis: pathological, clinical, and immunological features. Pancreas 27:14–19
Kroft SH, Stryker SJ, Winter JN, Ergun G, Rao MS (1995) Inflammatory pseudotumor of the pancreas. Int J Pancreatol 18:277–283
Kutok JL, Pinkus GS, Dorfman DM, Fletcher CD (2001) Inflammatory pseudotumor of lymph node and spleen: an entity biologically distinct from inflammatory myofibroblastic tumor. Hum Pathol 32:1382–1387
Lawrence B, Perez-Atayde A, Hibbard MK, Rubin BP, Dal Cin P, Pinkus JL, Pinkus GS, Xiao S, Yi ES, Fletcher CD, Fletcher JA (2000) TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic tumors. Am J Pathol 157:377–384
Liu DM, Jeffrey RB, Mindelzun RE (1999) Malignant fibrous histiocytoma presenting as cystic pancreatic mass. Abdom Imaging 24:299–300
Liu TH, Consorti ET (2000) Inflammatory pseudotumor presenting as a cystic tumor of the pancreas. Am Surg 66:993–997
Lüttges J, Pierre E, Zamboni G, Weh G, Lietz H, Kussmann J, Klöppel G (1997) Malignant non-epithelial tumors of the pancreas. Pathologe 18:233–237
Mai G, Baer HU, Mittler M, Uhl W, Buchler MW, Rodriguez RU, Ruchti C (2002) Malignant fibrous histiocytoma of the pancreas. Pancreas 25:320–324
Margules RM, Allen RE, Dunphy JE (1976) Pancreatic tumor of mesenchymal origin presenting as obstructive jaundice. Am J Surg 131:357–359
McClain MB, Burton EM, Day DS (2000) Pancreatic pseudotumor in an 11-year-old child: imaging findings. Pediatr Radiol 30:610–613
Meis-Kindblom JM, Kjellstrom C, Kindblom LG (1998) Inflammatory fibrosarcoma: update, reappraisal, and perspective on its place in the spectrum of inflammatory myofibroblastic tumors. Semin Diagn Pathol 15:133–143
Meis JM, Enzinger FM (1991) Inflammatory fibrosarcoma of the mesentery and retroperitoneum. A tumor closely simulating inflammatory pseudotumor. Am J Surg Pathol 15:1146–1156
Morris-Stiff G, Vujanic GM, Al-Wafi A, Lari J (1998) Pancreatic inflammatory pseudotumour: an uncommon childhood lesion mimicking a malignant tumor. Pediatr Surg Int 13:52–54
Palazzo JP, Chang CD (1993) Inflammatory pseudotumor of the pancreas. Histopathology 23:475–477
Pascal RR, Sullivan L, Hauser L, Ferzli G (1989) Primary malignant fibrous histiocytoma of the pancreas. Hum Pathol 20:1215–1217
Petter LM, Martin JK Jr, Menke DM (1998) Localized lymphoplasmacellular pancreatitis forming a pancreatic inflammatory pseudotumor. Mayo Clin Proc 73:447–450
Pungpapong S, Geiger XJ, Raimondo M (2004) Inflammatory myofibroblastic tumor presenting as a pancreatic mass: a case. JOP 5:360–367
Qanadli SD, d’Anthouard F, Cugnec JP, Frija G (1997) Plasma cell granuloma of the pancreas: CT finding. J Comput Assist Tomogr 21:735–736
Scarpa A, Capelli P, Mukai K, Zamboni G, Oda T, Iacono C, Hirohashi S (1993) Pancreatic adenocarcinomas frequently show p53 gene mutations. Am J Pathol 142:1534–1543
Sciot R, Dal Cin P, Fletcher CD, Hernandez JM, Garcia JL, Samson I, Ramos L, Brys P, Van Damme B, Van den Berghe H (1997) Inflammatory myofibroblastic tumor of bone: report of two cases with evidenceof clonal chromosomal changes. Am J Surg Pathol 21:1166–1172
Scott L, Blair G, Taylor G, Dimmick J, Fraser G (1988) Inflammatory pseudotumors in children. J Pediatr Surg 23:755–758
Scully RE, Mark EJ, McNeely BU (1982) Weekly clinicopathological exercises. Case 6-1982. N Engl J Med 306:349–358
Shankar KR, Losty PD, Khine MM, Lamont GL, McDowell HP (1998) Pancreatic inflammatory tumour: a rare entity in childhood. J R Coll Surg Edinb 43:422–423
Slavotinek JP, Bourne AJ, Sage MR, Freeman JK (2000) Inflammatory pseudotumour of the pancreas in a child. Pediatr Radiol 30:801–803
Solcia E, Capella C, Klöppel G (1997) Atlas of tumor pathology. Tumors of pancreas. AFIP series #20, Washington, DC, pp 211–213
Stringer MD, Ramani P, Yeung CK, Capps SN, Kiely EM, Spitz L (1992) Abdominal inflammatory myofibroblastic tumours in children. Br J Surg 79:1357–1360
Su LD, Atayde-Perez A, Sheldon S, Fletcher JA, Weiss SW (1998) Inflammatory myofibroblastic tumor: cytogenetic evidence supporting clonal origin. Mod Pathol 11:364–368
Suster S, Philipps M, Robinson MJ (1989) Malignant fibrous histiocytoma (giant cell type) of the pancreas. Cancer 64:2303–2308
Uzoaru I, Chou P, Reyes-Mugica M, Shen-Schwarz S, Gonzalez-Crussi F (1993) Inflammatory myofibroblastic tumor of the pancreas. Surg Pathol 5:181–188
Walsh SV, Evangelista F, Khettry U (1998) Inflammatory myofibroblastic tumor of the pancreaticobiliary region: morphologic and immunocytochemical study of three cases. Am J Surg Pathol 22:412–418
Wilentz RE, Albores-Saavedra J, Hruban RH (2000) Mucinous cystic neoplasms of the pancreas. Semin Diagn Pathol 17:31–42
Wreesmann V, van Eijck CH, Naus DC, van Velthuysen ML, Jeekel J, Mooi WJ (2001) Inflammatory pseudotumour (inflammatory myofibroblastic tumour) of the pancreas: a report of six cases associated with obliterative phlebitis. Histopathology 38:105–110
Yamamoto H, Oda Y, Saito T, Sakamoto A, Miyajima K, Tamiya S, Tsuneyoshi M (2003) p53 mutation and MDM2 amplification in inflammatory myofibroblastic tumours. Histopathology 42:431–439
Yamamoto H, Watanabe K, Nagata M, Tasaki K, Honda I, Watanabe S, Soda H, Takenouti T (2002) Inflammatory myofibroblastic tumor (IMT) of the pancreas. J Hepatobiliary Pancreat Surg 9:116–119
Zamboni G, Scarpa A, Bogina G, Iacono C, Bassi C, Talamini G, Sessa F, Capella C, Solcia E, Rickaert F, Mariuzzi GM, Klöppel G (1999) Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol 23:410–422
Zen Y, Harada K, Sasaki M, Sato Y, Tsuneyama K, Haratake J, Kurumaya H, Katayanagi K, Masuda S, Niwa H, Morimoto H, Miwa A, Uchiyama A, Portmann BC, Nakanuma Y (2004) IgG4-related sclerosing cholangitis with and without hepatic inflammatory–pseudotumor, and sclerosing pancreatitis-associated sclerosing cholangitis: do they belong to a spectrum of sclerosing pancreatitis? Am J Surg Pathol 28:1193–1203
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mizukami, H., Yajima, N., Wada, R. et al. Pancreatic malignant fibrous histiocytoma, inflammatory myofibroblastic tumor, and inflammatory pseudotumor related to autoimmune pancreatitis: characterization and differential diagnosis. Virchows Arch 448, 552–560 (2006). https://doi.org/10.1007/s00428-006-0157-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-006-0157-x