Abstract
Helicobacter pylori (H. pylori) is a “slow” bacterial pathogen, which induces several gastroduodenal diseases. Varying degrees of inflammation can be present in the gastric mucosa of patients infected with H. pylori. The case presented here is a male patient suffering from dyspepsia and nausea. His upper gastrointestinal endoscopy revealed pan gastritis. Histological examination of multiple gastric biopsies taken from the body and antrum showed a rare morphological expression of H. pylori gastritis characterized by diffuse plasma cell infiltration with extensive Russell body formation. Diffuse infiltration of plasma cells with Russell bodies in gastric mucosa can cause difficulties in differentiation from neoplastic processes. However, immunohistochemically, the infiltrating cells in the gastric mucosa stained negatively with cytokeratins while they expressed both kappa and lambda light chains showing their polyclonal nature. The presence of diffuse plasma cells with Russell bodies in the gastric mucosa may represent a different presentation of H. pylori gastritis. There are only two case reports of similar presentation and both have been called “Russell body gastritis”.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Helicobacter pylori (H. pylori) is a major etiological agent in a variety of gastroduodenal diseases including chronic gastritis, peptic ulcer, gastric cancer, and lymphoma [1, 2, 8, 9]. Varying degrees of mucosal inflammation and atrophy can be present in gastric biopsies infected with this microorganism. H. Pylori infection causes damage to both superficial and glandular epithelial cells. Cellular injury may be inflicted directly by the bacteria or by mechanisms mediated by inflammation or apoptosis [6]. Bacterial attachment and superficial cell activation results in the rapid recruitment of neutrophils in the gastric mucosa, followed by lymphocytes, and later by plasma cells [4, 6]. Plasmacytic infiltrate is the hallmark of chronic inflammation of gastric mucosa. However, plasma cells containing Russell bodies, which are thought be accumulations of condensed intercisternal immunoglobulin (Ig) in the cytoplasm of plasma cells [7], are extremely rare in H. pylori gastritis. There are only two case reports of plasma cells with Russell bodies infiltrating the gastric mucosa in the English literature [3, 11].
The case presented here has characteristic features of so-called “Russell body gastritis” associated with H. pylori infection.
Clinical history
A 70-year-old male patient suffering from dyspepsia and nausea for the last 6 months was admitted to the Department of Gastroenterology, Ankara University Medical Faculty. He had a history of essential hypertension and benign prostatic hyperplasia. His physical examination was normal except for slight tenderness in the epigastric area. Routine blood tests revealed hypochromic microcytic anemia, consistent with iron deficiency. Upper gastrointestinal endoscopy revealed flattened gastric folds and edema, which were suggestive of pan gastritis. Multiple gastric biopsies from the body and antrum were taken for histopathological examination.
Materials and methods
Biopsy samples were fixed in 10% formalin, processed routinely, and embedded in paraffin. Sections (4 μm thick) were taken from the paraffin blocks and stained with hematoxylin and eosin (H&E) for light microscopic examination. Immunohistochemical examination was performed using a streptavidin-biotin peroxidase technique (Zymed, US) and AEC as chromogen. Monoclonal antibodies used included pan-cytokeratin (pan CK; clone 5D3+LP34, Neomarkers, California), CD20 (clone L26, Novocastra, UK), CD79a (clone JCB117, Dako, California), CD68 (clone PG-M1, Dako, California), plasma cell antigen (PCA; clone VS38c, Dako, California), kappa (clone HP-6054, Novocastra, UK) and lambda (clone kp-53, Novocastra, UK) light chains.
Results
Histopathological findings
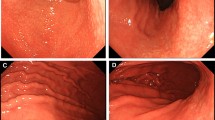
Histopathological examination of the gastric biopsies revealed atrophy with moderate mononuclear cellular infiltration in the lamina propria and polymorphonuclear leukocyte infiltration of glandular epithelium in the gastric antrum. In the lamina propria of the corpus mucosa, in addition to the findings found in antral mucosa, a diffuse infiltration of cells—with large eosinophilic cytoplasm and eccentric nuclei somewhat resembling plasma cells—was observed (Fig. 1a, b). On higher magnification, these cells had numerous round eosinophilic intracytoplasmic inclusions, so-called Russell bodies (Fig. 1c). Colonization by H. pylori was observed on the surface of foveolar cells in H&E and modified Giemsa stains.
a, b Gastric biopsy showing diffuse infiltration of lamina propria by monomorphous cells with large eosinophilic cytoplasms and eccentric nuclei (hematoxylin and eosin; ×100 and ×200, respectively). c High-power view of expanded lamina propria by the infiltrating plasma cells and numerous round eosinophilic intracytoplasmic inclusions, so-called Russell bodies (hematoxylin and eosin; oil immersion)
Immunohistochemical findings
Immunohistochemically, the infiltrating cells were positive with CD79a (Fig. 2a) and PCA, while no staining was observed with CD20, CD68 or pan CK. The cells expressed both kappa (Fig. 2b) and lambda (Fig. 2c) light chains showing their polyclonal nature. The case was diagnosed as H. pylori gastritis with extensive Russell body formation.
Following this diagnosis, immune and protein electrophoreses together with a bone marrow trephine biopsy were performed in order to exclude a plasma cell neoplasia. No abnormalities were found in either test while bone marrow biopsy showed a normal plasma cell ratio. Follow-up of the patient since the diagnosis has shown him to be well; however, he refused to be re-examined endoscopically after H. pylori treatment.
Discussion
Russell bodies, first described by Russell in 1890 [10], are considered to represent aggregates of Igs resulting from secretory disturbance of plasma cells [7]. Plasma cells with Russell bodies can be seen in a variety of mucosal surfaces, including gastrointestinal tract in association with chronic inflammation such as H. pylori gastritis.
Recently, two case reports of chronic gastritis showing plasma cell infiltration with extensive Russell body formation have been published. In the first report, Tazawa and Tsutsumi presented a 53-year-old male showing localized accumulation of plasma cells containing Russell bodies in association with infection of H. pylori and defined this pathology as “Russell body gastritis” [11]. The most recent case presented by Erbersdobler et al., was an 80-year-old female with non-H. pylori-associated gastritis, characterized by a diffuse plasma cell infiltration with Russell bodies [3]. Both cases had a focal nature of plasma cell accumulation detected either endoscopically or histologically [3, 11], while the current case is characterized by diffuse and extensive accumulation of Russell bodies presenting as a diffuse pan gastritis on endoscopy.
H. pylori stimulates cytokine production by epithelial cells that recruit and activate immune and inflammatory cells in the underlying lamina propria, causing chronic active gastritis. Although epidemiological evidence shows that infection generally occurs in children, the inflammatory changes progress throughout life [5, 9]. H. pylori has also been associated with gastroduodenal ulcers and gastric cancer [5]. These more severe manifestations of the infection usually occur later in life and only in a minority of infected subjects [1, 2]. In addition, the stimulation of cytokine production by epithelial cells and activation of the immune system lead to the formation of lymphoid aggregates, which may show progression to MALTomas [8].
The presence of diffuse plasma cell infiltration with extensive Russell body formation is suggestive of an interaction between H. pylori and gastric immune system. It could be speculated that the formation of Russell bodies may result from over-stimulation of plasma cells by mucosal pathogens such as H. pylori, leading to accumulation of excess amounts of Igs in the cytoplasm. In support of this view, H. pylori has been detected both in the present case and in the case reported by Tazawa and Tsutsumi [11]. However, the presence of Russell bodies in H. pylori gastritis seems to be a very rare finding, thus a direct role of this pathogen in Russell body formation is highly unlikely.
Though the clinical significance (if any) of Russell bodies in H. pylori gastritis is not known, histopathologically extensive infiltration of plasma cells with Russell bodies in gastric mucosa can cause confusion with neoplastic processes such as signet ring cell carcinoma, malignant lymphoma and solitary plasmacytoma. Interestingly, the low-power view of our case strongly suggested diffuse infiltration of signet ring cells; whereas, eosinophilic round intracytoplasmic inclusions within the infiltrating cells were easily detected on higher magnification. The benign nature of infiltrating plasma cells demonstrated by immunohistochemistry and the lack of lymphoepithelial lesion-like features excluded a malignant lymphoma or plasmacytoma, while negative staining with cytokeratins helped to differentiate the lesion from a carcinoma.
The present case is a good example of an unusual presentation of ordinary H. pylori gastritis, which can either easily be missed or misinterpreted during daily microscopy routine. However, we believe that such histopathological pictures need to be recognized and even deserve to be called by distinct names.
References
Bolin TD, Hunt TR, Korman MG, Lambert JR, Lee A, Talley NJ (1995) Helicobacter pylori a gastric neoplasia: evolving concepts. Med J Aust 163:235–255
Chan AO, Chu KM, Yuen ST, Leung SY, Lam SK, Wong J (2001) Synchronous gastric adenocarcinoma and mucosa-associated lymphoid tissue lymphoma in Helicobacter pylori infection; comparing reported cases between the east and west. Am J Gastroenterol 96:1922–1924
Erbersdobler A, Petri S, Lock G (2004) Russell body gastritis. An usual, tumor-like lesion of the gastric mucosa. Arch Pathol Lab Med 128:915–917
Ernst PB, Crowe SE, Reyes VE (1997) How does Helicobacter pylori cause mucosal damage? The inflammatory response. Gastroenterology 113:S35–S42
Ernst PB, Gold BD (2000) The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu Rev Microbiol 54:615–640
Genta RM (1997) Role of Helicobacter pylori infection in cancer. Helicobacter pylori, inflammation, mucosal damage, and apoptosis: pathogenesis and definition of gastric atrophy. Gastroenterology 113:S51–S55
Hsu SM, Hsu PL, McMillan PN, Fanger H (1982) Russell bodies: a light and electron microscopic immunoperoxidase study. Am J Clin Pathol 77:26–31
Isaacson PG (1990) Lymphomas of mucosa-associated lymphoid tissue (MALT). Histopathology 16:617–619
Kokoska ER, Kauffman GL Jr (2001) Helicobacter pylori and the gastroduodenal mucosa. Surgery 130:13–16
Russell W (1890) An address on a characteristic organism of cancer. BMJ 2:1356–1360
Tazawa K, Tsutsumi Y (1998) Localized accumulation of Russell body-containing plasma cells in gastric mucosa with helicobacter pylori infection: “Russell body gastritis”. Pathol Int 48:242–244
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ensari, A., Savas, B., Okcu Heper, A. et al. An unusual presentation of helicobacter pylori infection: so-called “Russell body gastritis”. Virchows Arch 446, 463–466 (2005). https://doi.org/10.1007/s00428-005-1215-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-005-1215-5