Abstract
Claudins are involved in the formation of tight junctions in epithelial and endothelial cells. Claudins form a family of 24 members displaying organ- and tissue-specific patterns of expression. In the present study, we evaluated the specificity of the claudin-2 expression in various normal human tissues and gastrointestinal cancers by quantitative reverse transcriptase–polymerase chain reaction and immunohistochemistry. In 14 various normal tissues, claudin-2 mRNA was expressed in the kidney, liver, pancreas, stomach, and small intestine; the highest level of which was detected in the kidney. Colorectal cancers (CRCs) expressed claudin-2 mRNA at high levels. Immunohistochemical analysis of claudin-2 in 146 gastric cancers (GCs) and 99 CRCs demonstrated claudin-2 expression in 2.1% of GCs and 25.3% of CRCs, respectively. There was no obvious correlation between claudin-2 expression and clinicopathological parameters of CRCs. These results suggest that the expression of claudin-2 may involve organ specificity, and increased expression of claudin-2 may participate in colorectal carcinogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Claudin-1 and claudin-2 were the first members of the transmembrane tetraspan family of proteins identified as being involved in tight junction formation with the recruitment of occludin [4] and binding to other tight junction constituents [8]. Claudins form a family of at least 24 members displaying organ- and tissue-specific patterns of expression [9, 17]. Among the claudin family members, expression of claudin-2 is found in the liver, pancreas, and gut in normal rat tissues [17]. Claudin-2 expression is ubiquitous in the epithelial cells at the crypts of the small intestine but restricted to the undifferentiated cell compartment of the colon in rats [17]. Claudin-2 is also known to be expressed in mouse nephron [3, 9]. However, the expression pattern of claudin-2 remains to be elucidated in normal human tissues.
Gastrointestinal cancers including gastric cancer (GC) and colorectal cancer (CRC) are the most common malignancies worldwide. A better knowledge of changes in gene expression during gastrointestinal carcinogenesis may lead to new paradigms and possible improvements in diagnosis, treatment, and prevention. On the other hand, relatively little is known about the expression of claudins in human tumors, and only little information is available on the influence of claudin expression on tumor behavior. It was reported that the expression of claudin-7 was decreased in high-grade breast cancer [10]. Overexpression of claudin-4 has been found in pancreatic adenocarcinoma and its precursor lesions [15, 22], while overexpression of claudin-3 and claudin-4 has been found in prostate and ovarian carcinomas [7, 13]. Concerning the expression of claudin-2 in tumor tissue, it has been reported that claudin-2 expression was detected in 98 (52%) of 188 breast carcinomas [20]. There is one report showing claudin-2 expression in gastrointestinal tumors, but the sample number was small [21].
In the present study, the expression of claudin-2 was investigated in various normal tissues, GCs, and CRCs by quantitative reverse transcriptase–polymerase chain reaction (RT-PCR) and immunohistochemistry. The aim of this study is to clarify whether claudin-2 expression is specific for cancer by comparing the expression level of claudin-2 in various normal tissues with that in cancer tissues.
Materials and methods
Tissue samples
For quantitative RT-PCR, five GCs and nine CRCs were used. The samples were obtained at the time of surgery at the Hiroshima University Hospital and affiliated hospitals. We confirmed microscopically that the tumor specimens consisted mainly (>50%) of carcinoma tissue. Samples were frozen immediately in liquid nitrogen and stored at −80°C until use. Noncancerous samples of the heart, lung, stomach, small intestine, colon, liver, pancreas, kidney, bone marrow, peripheral leukocytes, spleen, skeletal muscle, brain, and spinal cord were purchased directly from Clontech (Palo Alto, CA, USA).
For immunohistochemical analysis, we used archival formalin-fixed, paraffin-embedded tissues from 245 patients who had undergone surgical excision or removal of the tumor by polypectomy for GC (n=146) and CRC (n=99). The 146 GCs were either histologically classified (n=85) or poorly (n=61) differentiated. Ninety-nine CRCs were either histologically classified (n=47) or moderately (n=45) or poorly (n=7) differentiated. Tumor staging was carried out according to the tumor–node–metastasis (TNM) staging system [12]. Because written informed consent was not obtained, for strict privacy protection, identifying information for all samples was removed before analysis; the procedure was in accordance with the Ethical Guidelines for Human Genome/Gene Research enacted by the Japanese Government.
Cell lines
Eight cell lines derived from human GC were used. The TMK-1 cell line was established in our laboratory [16]. Five GC cell lines of the MKN series were kindly provided by Dr. T. Suzuki. KATO-III and HSC-39 cell lines were kindly provided by Dr. M. Sekiguchi and Dr. K. Yanagihara [25], respectively. All cell lines were maintained in RPMI 1640 (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) containing 10% fetal bovine serum (Whittaker, Walkersville, MA, USA) in a humidified atmosphere of 5% CO2 and 95% air at 37°C.
Quantitative RT-PCR analysis
Total RNA was extracted with an RNeasy Mini kit (Qiagen, Valencia, CA, USA), and 1 μg of total RNA was converted to cDNA with a First-Strand cDNA Synthesis kit (Amersham Pharmacia, Little Chalfont, UK). PCR was performed with an SYBR Green PCR Core Reagents kit (Applied Biosystems, Foster City, CA, USA). Real-time detection of the emission intensity of SYBR green bound to double-stranded DNA was performed with an ABI PRISM 7700 Sequence Detection System (Applied Biosystems) as described previously [11]. Claudin-2 cDNA and internal control cDNAs [β-actin gene (ACTB) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH)] were PCR-amplified separately. Relative gene expression was determined by the threshold cycles for the claudin-2 and ACTB or GAPDH genes. Reference samples (GC cell line, KATO-III) were included on each assay plate to verify plate-to-plate consistency. Plates were normalized to each other by these reference samples. PCR amplification was performed according to the manufacturer’s instructions in 96-well optical trays with caps with a 25-μl final reaction mixture. Quantitative RT-PCRs were performed in triplicate for each sample primer set, and the mean of the three experiments was used as the relative quantification value. Claudin-2 primer sequences are forward primer 5′-TCCCCAAACCCACTAATCACA-3′ and reverse primer 5′-CCAACCTCAGCCAGAGAGAGG-3′. ACTB primer sequences were 5′-TCACCGAGCGCGGCT-3′ and 5′-TAATGTCACGCACGATTTCCC-3′ [11]. GAPDH primer sequences were 5′-GGTGAAGGTCGGAGTCAACG-3′ and 5′-AGAGTTAAAAGCAGCCCTGGTG-3′. The units are arbitrary, and we calculated claudin-2 mRNA expression by standardization to 1.0 μg total RNA from KATO-III as 1.0. We found a similar result in both quantitative RT-PCR analyses of claudin-2 and ACTB or GAPDH of 8 GC cell lines, 14 various normal tissues, 5 GC tissues, and 9 CRC tissues. Therefore, throughout this article, we will describe and discuss the results obtained using ACTB as an internal control in quantitative RT-PCR analysis.
Western blot analysis
Preparation of whole cell lysates from GC cell lines was made and Western blotting was performed as described previously [26]. Protein concentrations were determined by Bradford protein assay (Bio-Rad, Hercules, CA, USA) with bovine serum albumin used as the standard. Lysates (20 μg) were solubilized in Laemmli’s sample buffer by boiling and then subjected to 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by electrotransfer onto a nitrocellulose filter. Anti-claudin-2 polyclonal antibody was purchased from Zymed (South San Francisco, CA, USA), and anti-β-actin mouse monoclonal antibody was purchased from Sigma (USA). Peroxidase-conjugated anti-rabbit IgG and anti-mouse IgG was used in the secondary reaction, respectively. The immunocomplex was visualized with an ECL Western Blot Detection System (Amersham Pharmacia Biotech).
Immunohistochemistry
A Dako LSAB kit (Dako, Carpinteria, CA, USA), which is based on the LSAB method, was used for the immunohistochemical analysis. In brief, microwave pretreatment in citrate buffer was performed for 15 min to retrieve antigenicity. After blocking the peroxidase with 3% H2O2–methanol for 10 min, sections were incubated with antibody/rabbit polyclonal anti-claudin-2, 1:100 (Zymed).
Sections were treated consecutively at room temperature with primary antibody for 2 h, followed by sequential 10 min incubation with biotinylated anti-rabbit IgG and peroxidase-labeled streptavidin. Staining was completed after 10 min incubation with the substrate–chromogen solution. The sections were counterstained with 0.1% hematoxylin. The results of staining with each antibody were evaluated with reference to the percentage of stained cancer cells. The results of immunohistochemistry were graded as follows: “−,” 0% to 25% of tumor cells showed immunoreactivity; “+,” 25–50% of tumor cells showed immunoreactivity; “++,” more than 50% of tumor cells showed immunoreactivity. We regarded “++” as positive throughout this report.
Statistical methods
Associations between clinicopathologic parameters and claudin-2 expression were analyzed by Fisher’s exact test. P values less than 0.05 were considered statistically significant.
Results
Measurement of mRNA expression of claudin-2 in various normal tissues, GCs, and CRCs by quantitative RT-PCR
To measure the expression levels of claudin-2 mRNA, we performed quantitative RT-PCR on 5 GCs, 5 CRCs, and 14 normal tissue samples (heart, lung, stomach, small intestine, colon, liver, pancreas, kidney, bone marrow, peripheral leukocytes, spleen, skeletal muscle, brain, and spinal cord). The results are shown in Fig. 1. In general, the expression levels in CRCs were higher than those in GCs and normal tissues. Among the 14 normal tissues mentioned, the highest level of expression was detected in the kidney. Although obvious expression of claudin-2 was also detected in the liver, pancreas, stomach, and small intestine, it was not as high as compared with the kidney. In the lung and skeletal muscle, a faint expression of claudin-2 was found. There was no expression in the remaining normal tissues. Expression levels of claudin-2 were not so different between normal stomach and GCs. While no expression of claudin-2 was found in normal colon, two of five CRCs expressed claudin-2 at significantly higher levels (more than 5 arbitrary units).
Quantitative RT-PCR analysis of claudin-2 in 14 various normal tissues as well as in 5 GC and 5 CRC samples. Among the various normal tissues, the highest level of claudin-2 expression was found in the kidney; low expression was detected in the stomach, small intestine, liver, and pancreas; and faint expression was seen in the lung and skeletal muscle. In GCs, the expression levels were not so different from that in normal stomach. In CRCs, the expression levels were higher than those in normal colon
Expression and localization of claudin-2 in cancer cell lines
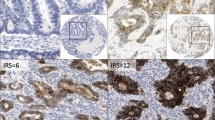
To confirm the mRNA expression of claudin-2 in cancer cells, we performed quantitative RT-PCR in eight GC cell lines. As shown in Fig. 2a, an obvious expression of claudin-2 was detected in MKN-45, MKN-74, and KATO-III, whereas only a low level of expression was seen in HSC-39; there was no expression detected in the remaining four GC cell lines. The anti-claudin-2 antibody detected an approximately 22-kDa band on Western blot of cell extracts from MKN-45, MKN-74, and KATO-III (Fig. 2b). We also confirmed these results by immunohistochemical staining in MKN-28 and MKN-45 cell lines. Claudin-2 staining was detected in cell membranes in MKN-45 cells but not in MKN-28 cells (Fig. 2c). Thus, this antibody was considered to be useful in the detection of claudin-2 protein in situ.
Expression and localization of claudin-2 in GC cell lines. Claudin-2 expression was analyzed at the mRNA level by quantitative RT-PCR (a) and at the protein level by Western analysis (b) and immunohistochemistry (c); claudin-2 immunoreactivity was evident in the cell membranes of MKN-45 cells but not MKN-28 cells
Expression and localization of claudin-2 in CRC tissues
To predict the sensitivity of anti-claudin-2 rabbit polyclonal antibody in immunohistochemistry, we examined the mRNA expression and protein expression of claudin-2 in an additional four CRC tissues. Firstly, we performed quantitative RT-PCR to detect claudin-2 mRNA expression level and found an obvious expression of claudin-2 (more than 5 arbitrary units) in three of four examined CRC tissue samples (Fig. 3a). The anti-claudin-2 antibody detected an approximately 22-kDa band on Western blot of protein extracts from the three CRC tissues, which showed obvious high expression in quantitative RT-PCR analysis (Fig. 3b). We also confirmed these results by immunohistochemical staining. Claudin-2 staining was detected in cell membranes of the same three CRC tissue samples, which expressed claudin-2 at a significantly higher level in both quantitative RT-PCR and Western blotting analyses (Fig. 4c). Therefore, in these CRC tissue samples, we found a good correlation between mRNA and protein levels. This result also suggests that in immunohistochemical analysis, our antibody may detect claudin-2 protein expression at levels higher than 5 arbitrary units measured by quantitative RT-PCR analysis.
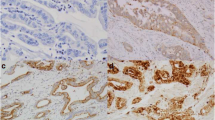
Immunohistochemical analysis of claudin-2 in normal kidney (as a control) as well as in GC and CRC tissues. Staining for claudin-2 was observed in the basal membranes of the proximal tubule of the kidney (a), the cellular membrane of GC cells forming a tubular structure (b), and the cellular membrane of CRC cells forming a papillotubular structure (c). c is case number 1, which expressed claudin-2 at a high level (more than 5 arbitrary units measured by quantitative RT-PCR), as shown in Fig. 3a,b. Original magnification was ×400
Expression of claudin-2 protein in GCs and CRCs by immunohistochemistry
We then examined the expression of claudin-2 protein in 146 GC and 99 CRC samples by immunohistochemistry. Immunostaining was also performed in normal kidney to serve as a positive control because our real-time RT-PCR revealed a high expression level and a previous report showed claudin-2 expression in mouse nephron. In the kidney, claudin-2 positivity was found strongest in the basal membranes of the proximal tubule, which is consistent with the result of previous reports [3, 9] (Fig. 3a). No obvious staining of claudin-2 was found in normal liver, stomach, and small and large intestines (data not shown). Of the 146 cases of GC, only 3 (2.1%) were positive for claudin-2. Immunoreactivity of claudin-2 was mainly observed in the cell membranes of GC cells forming a tubular structure (Fig. 3b). In CRCs, 25 (25.3%) of 99 cases were positive for claudin-2. Claudin-2 was mainly localized in the cell membranes of tumor cells forming a papillotubular structure (Fig. 3c).
We analyzed the relation between the expression of claudin-2 and clinicopathologic characteristics of CRC. There was no clear correlation between claudin-2 staining and clinicopathological parameters such as location, T grade, N grade, M grade, stage, and histologic differentiation (Table 1).
Discussion
Cellular tight junctions are structures that help preserve the integrity of cellular layers and regulate their permeability [23, 24]. It may be hypothesized that changes in expression of tight junctional proteins can lead to cellular disorientation and detachment, which are commonly seen in neoplasia. In this study, we demonstrated for the first time the expression of claudin-2 mRNA in normal human kidney, liver, pancreas, and so on. Among these, the highest level of expression was detected in the kidney. In addition, an obvious expression of claudin-2 was also detected in the stomach and small intestine. These results are consistent with a previous report in rat and mice; claudin-2 is expressed in nephron, liver, pancreas, and gut [3, 17]. We found a basal expression of claudin-2 in kidney tubules, although claudin-2 is not a known basal membrane protein and tight junctions are not known to be in the region of basal membrane. We could not explain well this unusual expression. However, it has been already published by Kiuchi-Saishin et al. [9] that claudin-2, claudin-10, and claudin-11 were clearly concentrated at the tight junction of epithelial cells of the proximal tubules. Among these, only claudin-2 seemed to be distributed along basal plasma membranes in addition to tight junction. This staining is specific for claudin-2 because the kidneys of claudin-2-deficient mice demonstrated no such staining (Furuse et al., unpublished data) [9].
We also investigated claudin-2 expression in 146 GC and 99 CRC cases to evaluate their differential expression of claudin-2 in gastrointestinal cancers. In previous studies, type-specific expression of claudin-1, claudin-2, claudin-3, claudin-4, claudin-5, and claudin-7 has been detected in various types of cancers including carcinomas of breast, pancreas, liver, esophagus, etc. [20, 21]. Soini [21] also analyzed claudin-2 expression in a small number of GCs and CRCs by using the monoclonal anticlaudin-2 antibody and reported that 12 of 13 GCs (92%) and 9 of 11 CRCs (82%) were positive for claudin-2. No method other than immunohistochemistry was utilized. In our study, the frequency of claudin-2 expression in GC and CRC was much lower as compared to the above studies, partly due to the different antibody used. However, our immunohistochemistry must be precise even if it was not so sensitive because we have found a good correlation between levels of claudin-2 mRNA expression and protein expression by immunostaining and Western analysis of not only GC cell lines but also CRC tissues, as shown in Figs. 2, 3, and 4c. Our antibody may detect claudin-2 expression at levels higher than 5 arbitrary units, as measured by quantitative RT-PCR. In situ hybridization experiments may help detect the expression of claudin-2 with high sensitivity. We will perform it in a future study of claudin-2.
The results of the present study demonstrated that expression of claudin-2 was detected in CRCs, whereas no expression of claudin-2 was detected in normal colon. We did not find any significantly different expression between GC and normal stomach. Therefore, the participation of claudin-2 in tumorigenesis may involve organ specificity. Claudin species have been found to be the major constituents of tight junction strands [2, 5, 9, 19]. The presence of junctional claudin-2 causes the formation of cation-selective channels sufficient to transform a “tight” junction into a “leaky” one [1]. In carcinogenesis of CRC, claudin-2 may contribute to an easier leak of tight junctions in between neoplastic cells. Although we observed claudin-2 staining mainly in the neoplastic cells forming a tubular or papillary structure in both GCs and CRCs, we did not find any significant correlation between claudin-2 protein expression and histological differentiation. Moreover, we could not find any correlation with tumor advancement. Therefore, increased expression of claudin-2 may participate in the development but not in the progression of CRCs, although the exact mechanism is unknown. It has been shown that human claudin-2 promoter activity is positively regulated by the caudal-related homeobox gene (CDX2), as well as by the hepatocyte nuclear factor-1 alpha isoform (HNF-1α) [18]. A published study has shown the presence of functional cross talk between CDX2 and the Wnt pathway in the positive regulation of claudin-2 expression [14]. These regulations may be the cause of the involvement of claudin-2 in colorectal carcinogenesis. In fact, abnormalities in Wnt signaling, including β-catenin/TCF, have been shown to participate in the pathogenesis of CRC [6]. Further studies are needed to explore the exact mechanism of claudin-2 involvement in gastrointestinal tumor pathology.
References
Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, Schulzke JD, Fromm M (2002) Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci 115:4969–4976
Colegio OR, Van Itallie C, Rahner C, Anderson JM (2003) Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol Cell Physiol 284:C1346–C1354
Enck AH, Berger UV, Yu AS (2001) Claudin-2 is selectively expressed in proximal nephron in mouse kidney. Am J Physiol Renal Physiol 281:F966–F974
Furuse M, Sasaki H, Fujimoto K, Tsukita S (1998) A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol 143:391–401
Furuse M, Sasaki H, Tsukita S (1999) Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol 147:891–903
Gaspar C, Fodde R (2004) APC dosage effects in tumorigenesis and stem cell differentiation. Int J Dev Biol 48(5–6):377–386
Hough CD, Sherman-Baust CA, Pizer ES, Montz FJ, Im DD, Rosenshein NB, Cho KR, Riggins GJ, Morin PJ (2000) Large-scale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res 60(22):6281–6287
Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S (1999) Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol 147:1351–1363
Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S (2002) Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol 13:875–886
Kominsky SL, Argani P, Korz D, Evron E, Raman V, Garrett E, Rein A, Sauter G, Kallioniemi OP, Sukumar S (2003) Loss of the tight junction protein claudin-7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast. Oncogene 22(13):2021–2033
Kondo T, Oue N, Yoshida K, Mitani Y, Naka K, Nakayama H, Yasui W (2004) Expression of POT1 is associated with tumor stage and telomere length in gastric carcinoma. Cancer Res 64:523–529
Lauren P (1965) The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 64:31–49
Long H, Crean CD, Lee WH, Cummings OW, Gabig TG (2001) Expression of Clostridium perfringens enterotoxin receptors claudin-3 and claudin-4 in prostate cancer epithelium. Cancer Res 61(21):7878–7881
Mankertz J, Hillenbrand B, Tavalali S, Huber O, Fromm M, Schulzke JD (2004) Functional crosstalk between Wnt signaling and Cdx-related transcriptional activation in the regulation of the claudin-2 promoter activity. Biochem Biophys Res Commun 314:1001–1007
Michl P, Barth C, Buchholz M, Lerch MM, Rolke M, Holzmann KH, Menke A, Fensterer H, Giehl K, Lohr M, Leder G, Iwamura T, Adler G, Gress TM (2003) Claudin-4 expression decreases invasiveness and metastatic potential of pancreatic cancer. Cancer Res 63(19):6265–6271
Ochiai A, Yasui W, Tahara E (1985) Growth-promoting effect of gastrin on human gastric carcinoma cell line TMK-1. Jpn J Cancer Res 76:1064–1071
Rahner C, Mitic LL, Anderson JM (2001) Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology 120:411–422
Sakaguchi T, Gu X, Golden HM, Suh E, Rhoads DB, Reinecker HC (2002) Cloning of the human claudin-2 5′-flanking region revealed a TATA-less promoter with conserved binding sites in mouse and human for caudal-related homeodomain proteins and hepatocyte nuclear factor-1alpha. J Biol Chem 277:21361–21370
Sasaki H, Matsui C, Furuse K, Mimori-Kiyosue Y, Furuse M, Tsukita S (2003) Dynamic behavior of paired claudin strands within apposing plasma membranes. Proc Natl Acad Sci U S A 100:3971–3976
Soini Y (2004) Claudins 2, 3, 4, and 5 in Paget’s disease and breast carcinoma. Hum Pathol 35:1531–1536
Soini Y (2005) Expression of claudins 1, 2, 3, 4, 5 and 7 in various types of tumors. Histopathology 46:551–560
Terris B, Blaveri E, Crnogorac-Jurcevic T, Jones M, Missiaglia E, Ruszniewski P, Sauvanet A, Lemoine NR (2002) Characterization of gene expression profiles in intraductal papillary-mucinous tumors of the pancreas. Am J Pathol 160(5):1745–1754
Tsukita S, Furuse M (2000) Pores in the wall: claudins constitute tight junction strands containing aqueous pores. J Cell Biol 149:13–16
Tsukita S, Furuse M (2002) Claudin-based barrier in simple and stratified cellular sheets Curr Opin Cell Biol 14(5):531–536
Yanagihara K, Seyama T, Tsumuraya M, Kamada N, Yokoro K (1991) Establishment and characterization of human signet ring cell gastric carcinoma cell lines with amplification of the c-myc oncogene. Cancer Res 51:381–386
Yasui W, Ayhan A, Kitadai Y, Nishimura K, Yokozaki H, Ito H, Tahara E (1993) Increased expression of p34cdc2 and its kinase activity in human gastric and colonic carcinomas. Int J Cancer 53:36–41
Acknowledgements
This work was supported, in part, by Grants-in-Aid for Cancer Research from the Ministry of Education, Culture, Science, Sports, and Technology of Japan and from the Ministry of Health, Labor, and Welfare of Japan. We thank Mr. Masayoshi Takatani and Ms. Mutsumi Ueda for excellent technical assistance and advice. We thank the Analysis Center of Life Science, Hiroshima University, for the use of their facilities. The experiments in this study were in accordance with the Ethical Guidelines for Human Genome/Gene Research enacted by the Japanese government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aung, P.P., Mitani, Y., Sanada, Y. et al. Differential expression of claudin-2 in normal human tissues and gastrointestinal carcinomas. Virchows Arch 448, 428–434 (2006). https://doi.org/10.1007/s00428-005-0120-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-005-0120-2