Abstract
Transcriptional E-cadherin down-regulation can be mediated by Snail, a zinc finger transcription factor. To be able to examine nuclear Snail immunoreactivity in archival human cancers, we established a monoclonal antibody against the purified human Snail protein. The specificity of the selected rat antibody Sn9H2 was demonstrated by Western blot analysis using extracts from different cell lines and by immunofluorescence and immunohistochemistry of primary tissues. Subsequently, a series of 340 adenocarcinomas of the upper gastrointestinal tract, including tumours from the oesophagus (n=154), cardia (n=102) and stomach (n=84), arranged in tissue microarrays, were examined for Snail expression and were correlated to E-cadherin expression and clinico-pathological parameters. Nuclear Snail immunoreactivity was seen in 27 tumours (7.9%) and tended to be more frequent in oesophageal adenocarcinomas (11.1%) than in cardiac (6.9%) or gastric (3.6%) carcinomas (p=0.0428). In 35% of the Snail-positive cases, E-cadherin immunoreactivity was lost. No correlation was found for nuclear Snail expression and tumour grade, Lauren’s classification, WHO classification, tumour stage and tumour size. The pattern of Snail expression observed with our new hybridoma, Sn9H2, which is currently the only antibody that reacts with endogenous nuclear (active) Snail, suggests only a minor role of Snail in tumours of the upper gastrointestinal tract.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cell adhesion molecule and tumour suppressor gene E-cadherin [online Mendelian Inheritance in Man (OMIM) 192090] is well known to play a crucial role during the progression of human cancer [24]. E-cadherin function is required for the maintenance of normal epithelial tissue architecture and the stabilization of adherens junctions [36]. Its inactivation in carcinomas can disrupt these junctional complexes, resulting in marked phenotypic changes and the acquisition of invasive growth [15, 49]. Immunohistochemical studies have demonstrated that reduced or absent E-cadherin immunoreactivity is common in most cancers, including carcinomas of the oesophagus and stomach [8, 24, 42]. Direct transcriptional repression by zinc finger transcription factors such as Snail [3, 12], Slug [10, 21], Zeb1 (deltaEF1; [20]), Zeb2 (Sip1; [13]) or the basic helix–loop–helix transcription factor E12/E47 [35] is one of the mechanisms that may be responsible for reduced E-cadherin immunoreactivity.
Here, we focus our analysis on the transcription factor Snail, which was first described in Drosophila in 1984 [19]. Snail homologues were subsequently found in other species as well, including mouse and human. Snail triggers epithelial–mesenchymal transition (EMT) during embryonic development. Its role during gastrulation and in the delamination of the neural crest from the neural tube has been well established in the mouse (reviewed in [32]).
Since EMT is frequently observed in human tumours [23, 46], Snail has been analysed in carcinoma cells in culture if there is evidence of a functional role during tumour invasion and metastasis. Indeed, it has been shown that Snail directly binds to the E-boxes present in the E-cadherin promoter, resulting in the down-regulation of promoter activity in vitro. Moreover, the transfection of Snail in E-cadherin-positive carcinoma cells induced a full EMT with the down-regulation of E-cadherin and other epithelial marker genes, such as occludin [25], and the up-regulation of mesenchymal markers, including vimentin and fibronectin [3, 12]. Importantly, Snail-expressing cells became invasive after transfection, supporting its role in tumour progression. Snail not only induces invasion but also blocks the cell cycle and confers resistance to cell death [48].
On the mRNA level, Snail is expressed in invasive cells of tumours induced in the mouse skin [12]. In humans, Snail mRNA expression has been detected in biopsies or resected tissue samples from patients with breast cancer [9, 17], gastric cancer [39] and hepatocellular carcinomas [27, 45] and in melanoma cell lines [37]. In colon cancer, Snail mRNA was either found to be expressed [33] or to be absent [40]. Because no Snail-specific antibodies suitable for immunohistochemical analysis of the active, i.e. nuclear localized, molecule have been available so far, evidence for functional Snail protein expression in primary human tumours and tissues is missing. Until now, a direct cellular comparison between E-cadherin down-regulation and endogenous nuclear Snail expression at the protein level in cancer tissues has not been possible.
To analyse nuclear Snail immunoreactivity in human cancers, we established a monoclonal antibody (mAb) in rats against purified human Snail protein. The specificity of the antibody was demonstrated by Western blot, immunofluorescence and immunohistochemistry of cells and primary human tissues. Subsequently, we analysed a series of 340 adenocarcinomas from the upper gastrointestinal tract, i.e. primary carcinomas of the oesophagus, the gastric cardia and the stomach, arranged in tissue microarrays (TMA), for nuclear Snail expression. We compared Snail and a variety of histopathological and clinical parameters and found that Snail expression is significantly more frequent in oesophageal than in cardiac or gastric adenocarcinomas. In 7 out of 20 Snail-positive cases, the expression of Snail in tumour cells was associated with a concomitant loss of E-cadherin expression. No correlation was found between the expression of Snail and tumour grade, tumour stage and histological tumour type according to Lauren’s and WHO classification.
Materials and methods
Cell lines
The following cell lines were used: MCF7 (mammary gland, breast epithelium), HTB-135 (stomach carcinoma) and GC2957 (stomach carcinoma). MCF7 and HTB-135 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM), 10% foetal calf serum (FCS) and 2.5 ml penicillin/streptomycin (#15140-122, Gibco; 10,000 units/ml penicillin G sodium/10,000 μg/ml streptomycin sulphate). The E-cadherin-positive cell line GC2957 was cultured in RPMI 1640 (#31870-025, Gibco), 20% FCS, 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and 2.5 ml penicillin/streptomycin. MCF7 cells have been reported to express both E-cadherin and Snail [14]. HTB-135 cells are E-cadherin negative and Snail positive (E. Rosivatz, unpublished observation).
Cloning of human Snail cDNA
The cDNA coding for human Snail (SNAI1, OMIM 604238, RefSeq 005985) was amplified by reverse transcription-polymerase chain reaction (RT-PCR) from MDA-MB-435S human mammary carcinoma cells known to express Snail mRNA [3, 12]. Recently, evidence was provided that this cell line is of melanocytic origin [38]. The upstream (5′-CAT ATG CCG CGC TTT CCT CGT CAG G-3′) and downstream (5′-AAG CTT TGC GGG GAC ATC CTG AGC AG-3′) primers used for the amplification of a 802-bp sequence were designed to contain an NdeI site and a HindIII site, respectively. After gel purification of the amplification product, the Snail sequence was cloned using the NdeI and HindIII sites and standard methods into a modified expression vector, pHAT, in-frame with the so-called histidine affinity tag (HAT) sequence (BD Biosciences Clontech, Palo Alto, USA). The original pHAT vector was modified (Jörg Mages, Technical University Munich, Germany, unpublished data) to contain the His tag at the C terminus, instead of the N terminus, of the recombinant protein. The construct was sequenced to exclude sequence alterations that may have occurred during amplification. The recombinant vector, now termed pSnail1HAT, was expressed in bacteria, and the resultant His-tagged protein was purified from inclusion bodies using a talon metal resin (BD Biosciences Clontech) under denaturing conditions using 8 M urea. For the expression of recombinant His-tagged Snail in human tumour cell lines, full-length 6× His-tagged Snail from pSnail1HAT flanked by the XbaI/KpnI restriction sites were ligated into the pBK-CMV vector (#212209, Stratagene; GenBank accession no. U37573).
Cell transfection
The day before transfection, 1×105 cells per well of a six-well plate were seeded in 2 ml of appropriate growth medium. Cells were grown to 70% confluency for approximately 24 h. For the preparation of solution A, 1 μg plasmid DNA was diluted in 100 μl Optimem (#31985, Invitrogen) for each transfection and mixed gently. For the preparation of solution B, 15 μl Lipofectamine (#18324-012, Invitrogen) reagent was diluted in 100 μl Optimem for each transfection and mixed. Solutions A and B were combined, mixed gently and incubated at room temperature for 45 min to allow the formation of DNA–liposome complexes. For each transfection, 800 μl Optimem was added to the DNA–liposome complexes and gently mixed. Growth medium was aspirated from the cells, and the diluted transformation mix was added drop by drop. The cells were incubated with the complexes for 6 h at 37°C in a CO2 incubator. Following incubation, 1 ml of growth medium (containing twice the normal concentration of serum) was added without removing the transfection mixture.
Twenty-four hours following the start of transfection, the medium was replaced with normal growth medium. An assay for transient gene expression was carried out 48 h after the start of transfection. Alternatively, 72 h after the start of transfection, cells were passaged 1:10 into a selective medium to achieve stable clones.
Quantitative real-time RT-PCR
RNA extraction from the cell lines, RT, quantitative real-time PCR and quantitation of Snail and E-cadherin mRNA expression were done as previously described [39] and were used here without modification. The primers and probes were designed to span an intron to exclude annealing to genomic DNA. Amplicon size was kept below 100 bp. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was included as a housekeeping gene control to correct for equal RNA amounts. We then calculated relative amounts of mRNA by the standard curve method in relation to GAPDH levels. The TaqMan runs were done in triplicate and repeated in an independent experiment.
Western blot
For Western blot analysis using hybridoma supernatant Sn9H2 to detect Snail protein, extracts from the MCF7 and GC2957 cells, as well as extracts from fresh, frozen human placenta, were prepared using a 1:1 mixture of 2× sodium dodecyl sulfate (SDS) electrophoresis buffer (120 mM Tris pH 6.8, 4% SDS, 16% glycerol, 2% beta-mercaptoethanol) and tissue protein extraction reagent (T-PER, Pierce, Rockford, IL, USA). Twenty micrograms of total protein was used for SDS-polyacrylamide gel electrophoresis (PAGE). Protein detection was performed using a peroxidase-coupled secondary antibody (ECL-Western, Amersham, Germany).
Immunofluorescence analysis of HTB-135 gastric cancer cells
Cells were grown on glass cover slips for 24 h and subsequently rinsed briefly in phosphate-buffered saline (PBS). For cell fixation, cover slips were immersed in freshly prepared 3.7% para-formaldehyde for 30 min, followed by 3× washes in PBS. The cover slips were incubated in 0.25% Triton X-100/PBS for 5 min at room temperature for cell permeabilization and then placed cell-side up in a petri dish and covered with blocking buffer [1% bovine serum albumin (BSA) in PBS] for 30 min. After removing the blocking buffer, primary antibodies [Sn9H2, 1:10 in blocking buffer; anti-His-tag (#70796-3, Novagen), 1:500 in blocking buffer] were distributed on the cover slip and incubated at 4°C for 16 h (Sn9H2) or at room temperature for 2 h (anti-His-tag), followed by 3× washes with PBS. Secondary antibodies conjugated to a fluorochrome [anti-rat fluorescein isothiocyanate (FITC), #112-069-044, Jackson laboratories; anti-mouse tetramethyl rhodamine isothiocyanate (TRITC), #115-026-044, Jackson Laboratories] were distributed on the cover slips and incubated for 1 h at room temperature. Cover slips were washed 3× with PBS and inverted onto slides containing 10 μl of mounting medium from Antifade kit (#S-2828, MoBiTec GmbH, Germany). Excess mounting medium was removed, and the edges were sealed and dried before the analysis of immunofluorescence using a Zeiss Axiovert microscope.
Generation of a rat mAb, Sn9H2, against human Snail
Purified His-tagged human Snail protein (50 μg) was injected both intraperitoneally and subcutaneously into Lou/C rats using CPG2006 (TIB MOLBIOL, Berlin, Germany) as adjuvant. After an 8-week interval, a final boost was given intraperitoneally and subcutaneously 3 days before fusion. The fusion of the myeloma cell line P3X63–Ag8.653 with the rat immune spleen cells was performed essentially as described [28]. Hybridoma supernatants were tested in a solid-phase immunoassay using the His-tagged protein that was used for immunization (10 μg/ml) adsorbed to polystyrene microtiter plates. An irrelevant His-tagged protein served as a negative control. Bound rat mAbs were detected with a cocktail of biotinylated mouse mAbs against the rat immunoglobulin G (IgG) heavy chains [α-IgG1, α-IgG2a and α-IgG2b (ATCC, Manassas, VA, USA) and α-IgG2c (Ascenion, Munich, Germany)]. The biotinylated mAbs were visualized with peroxidase-labelled Avidin (Alexis, Grünberg, Germany) and o-phenylenediamine as chromogen in the peroxidase reaction. The clone designated Sn9H2 (rat IgG2) was stably subcloned and used for further analysis.
Immunohistochemical analysis
After standard pressure-cooker-based antigen retrieval with citric acid pretreatment, 2-μm sections were incubated in goat serum to block nonspecific reactivity. For the detection of Snail-specific immunoreactivity, the specimens were incubated with diluted Sn9H2 hybridoma supernatant (1:20 in 1% BSA in PBS) for 2 h at room temperature. Bound antibodies were detected using the avidin–biotin complex (ABC) peroxidase method (ABC Elite Kit, Vector, Burlingame, CA, USA). Final staining was developed with the Sigma FAST DAB peroxidase substrate kit (Sigma, Deisenhofen, Germany). Haemalaun was used for counterstaining. In addition, E-cadherin immunoreactivity was detected using the mAbs HECD-1 (Alexis) or clone 34 (#C37020, Transduction Laboratories, Lexington, KT, USA), both of which are known to react with human E-cadherin and to work on archival material. Subsequently, adenocarcinomas from the upper gastrointestinal tract arranged in TMA (see below) were analysed in exactly the same way. For the analysis of cancer samples, non-tumourous epithelium was used as positive control for E-cadherin immunoreactivity. Sections of placenta tissue were used as positive control for Sn9H2.
Immunohistochemical analysis of upper gastrointestinal adenocarcinomas
The analysis was based on 266 patients with adenocarcinomas of the upper gastrointestinal tract who underwent surgical resection without prior radio- and/or chemotherapy between January 1983 and January 2003 at the University of Düsseldorf, Germany. Additionally, 101 patients with oesophageal adenocarcinomas who underwent resection without prior radio- and/or chemotherapy between January 1991 and January 2004 at the Technical University of Munich, Germany, were included. Of the patients, 292 (79.6%) were men. The age of the patients ranged between 29 and 90 years (median 63 years). Together, the series consisted of 158 oesophageal, 108 cardiac and 101 gastric adenocarcinomas. Differentiation between oesophageal and cardiac adenocarcinomas done by a senior pathologist (MS) based on a review of the original macroscopic pathologic descriptions of the operation specimens according to standard criteria [43]. Tumours that had their epicenters in the oesophagus were regarded as oesophageal in origin, and tumours that had their epicenters in the cardia were regarded as cardiac in origin. Staging was performed according to the current tumor, node and metastasis (TNM) classification [44]. Histological typing included Lauren’s classification [29] and the current WHO classification for gastric carcinomas [22]. Grading was performed according to the current WHO classification. Signet-ring cell carcinomas and mucinous carcinomas were categorized as G3.
Accordingly, 116 tumours (31.6%) were in stage I, 105 in stage II (28.6%), 110 in stage III (30.0%) and 36 in stage IV (9.8%). According to Lauren’s classification, 26 tumours (7.1%) belonged to the diffuse type, 274 (74.7%) to the intestinal type and 67 (18.3%) to the mixed type. According to the WHO classification, this series included 56 signet-ring cell carcinomas (15.3%), 4 papillary adenocarcinomas (1.1%), 7 mucinous adenocarcinomas (1.9%) and 300 tubular adenocarcinomas (81.7%). Twelve tumours were graded as G1 (3.3%), 131 as G2 (35.7%) and 224 as G3 (61.0%). Tumour size (largest diameter) ranged between 5 and 185 mm (median 50).
Construction of TMA
For each of the 367 adenocarcinomas, one paraffin block was selected, and representative, non-necrotic tumour areas were marked on Haemalaun–Eosin-stained slides from these blocks. Three tissue cylinders with a diameter of 0.6 mm per tumour were punched from these areas and brought into a recipient paraffin block using a tissue arraying instrument (Beecher Instruments, Silver Spring, MD). In the case of signet-ring cell carcinomas, six, instead of three, biopsies were sampled. A sample template designating the sample location by case number was created. Four-micrometer sections from the TMA were used for the subsequent immunohistochemical investigations.
The immunohistological evaluation of Snail expression in TMAs was performed by a senior pathologist (MS). A tumour was considered positive for Snail expression if, in at least one tumour, a sample nuclear expression of Snail was detectable. A positive immunoreactivity was confirmed using whole-tissue sections from the corresponding donor block.
Statistical analysis
The analysis of the expression of Snail and a variety of clinico-pathological parameters, such as tumour stage, nodal status, tumour grade, tumour size, tumour type (according to Lauren and WHO) and location of the primary tumour, was performed according to the chi-square exact test.
Results
To analyse Snail protein expression in human cell lines and tissues, and for the determination of intracellular localization, mAbs were produced in rats against a purified His-tagged human Snail protein. Hybridoma supernatants were tested in a solid-phase immunoassay against the specific protein. Hybridoma Sn9H2 of rat IgG2a subclass was selected for further analysis.
Antibody specificity
Hybridoma supernatant Sn9H2 detected a single protein band at approximately 32 kDa in the Escherichia coli inclusion body preparation that was used for protein purification and in the E-cadherin-negative gastric cancer cell line HTB-135 (Fig. 1a). A protein band of very similar molecular weight was seen using a mAb against the tag (anti-His) in the inclusion body preparation that is absent in the HTB-135 cell extract. After the transient transfection of human Snail cDNA fused to the histidine affinity tag sequence in HTB-135 gastric cancer cells, hybridoma supernatant Sn9H2, indirectly detected by an FITC-labelled secondary anti-rat antibody, reacted with a protein that is located in the cytoplasm and strongly in the nucleus (Fig. 1b). Using the anti-His antibody and a rhodamine-labelled secondary antibody, immunofluorescence was exclusively seen in the nucleus, suggesting that exogenously expressed His-tagged Snail is retained within the nucleus while endogenously expressed Snail is localized also in the cytoplasm.
Analysis of hybridoma supernatant Sn9H2. a Western blot. Inclusion body preparation from the transfected E. coli strain used to purify Snail protein and a lysate from an E-cadherin-negative gastric cancer cell line, HTB-135, were examined for Snail expression. Protein bands at approximately 32 kDa were observed in both lysates using hybridoma supernatant Sn9H2. An antibody against the His tag, anti-His, detected only a protein band in the E. coli lysate. b Intracellular localization of endogenously and exogenously expressed Snail. The distribution of Snail in Snail-His-transfected HTB-135 cells was observed by immunocytochemical staining with hybridoma supernatant Sn9H2 or anti-His antibody, followed by fluorescein isothiocyanate (FITC)-labelled or tetramethyl rhodamine isothiocyanate (TRITC)-labelled secondary antibodies, respectively. Immunofluorescence was visualized and captured with fluorescence microscopy. Endogenously expressed Snail was detected within the nucleus and the cytoplasm; exogenously expressed Snail was seen only in the nucleus
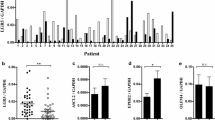
To prove a functional effect of Snail in E-cadherin-positive gastric cancer cells and to provide additional evidence for the specificity of the novel hybridoma supernatant Sn9H2, we analysed GC2957 gastric cancer cells. Using anti-Snail antibody Sn9H2, we were able to detect a band of about 32 kDa in GC2957 cells, suggesting the expression of low levels of endogenous Snail (Fig. 2a). Using real-time quantitative RT-PCR, we were able to detect only traces of Snail mRNA in GC2957 cells, whereas high levels of Snail mRNA were found in HTB-135 gastric cancer cells (data not shown). After the overexpression of Snail in GC2957 cells, the 32-kDa band increased in intensity, and E-cadherin levels were reduced. Alpha-tubulin expression was used to show equal protein loading. Despite low but detectable levels of Snail mRNA and protein, GC2957 cells formed cellular contacts and grew in epithelial-like structures. The overexpression of Snail, however, resulted in cell scattering (Fig. 2b).
Hybridoma supernatant Sn9H2 detects a functional Snail protein. E-cadherin-positive gastric cancer cells GC2957 (a, b) and MCF7 mammary cells (c, d) were transfected with vector as control or with Snail-His. a Western blot demonstrating the overexpression of Snail and the reduction of E-cadherin in the Snail-transfected cells. Alpha-tubulin was used as protein loading control. b Snail overexpression resulted in cell scattering. c Western blot showing the reduction of E-cadherin after Snail overexpression. No change is seen for alpha-tubulin. d Quantitative real-time RT-PCR demonstrates the reduction of E-cadherin expression by 95% after 3.7-fold Snail overexpression at the mRNA level. Results are representative of two independent transfection experiments done in triplicates and measured 48 h after transfection. The mRNA levels of vector-transfected cells are set to 100%; the mean ± standard deviation is shown
After the transfection of another E-cadherin-positive cell line, MCF7 mammary cells, the 32-kDa band detected by hybridoma supernatant Sn9H2 became stronger, less E-cadherin protein was present, and levels for alpha-tubulin did not change significantly (Fig. 2c). Quantitative real-time RT-PCR demonstrated 95% reduced E-cadherin and 3.7-fold increased Snail mRNA levels upon transfection with the Snail cDNA (Fig. 2d).
These results indicate that the overexpression of a functional Snail protein resulting in E-cadherin down-regulation and cell scattering can be visualized using hybridoma supernatant Sn9H2.
Immunohistochemistry using human placenta as test system
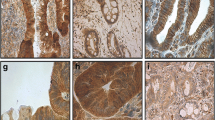
After having rigorously demonstrated antibody specificity by Western blot and immunofluorescence, we tested if hybridoma supernatant Sn9H2 may react with formalin-fixed and paraffin-embedded tissues. As a test system, we used human placenta, as this tissue was reported to be Snail-mRNA-positive using Northern blot analysis [34]. Using Western blot analysis, we detected a protein band at about 32 kDa in extracts of human placenta, confirming Snail expression in this tissue on the protein level but without providing information about which cell types are Snail positive (Fig. 3a). In human placenta, two morphologically distinct cell populations that differentiate from cytotrophoblast stem cells can be identified: (a) villous cytotrophoblasts are immotile cells that fuse to form a syncytium overlying the cytotrophoblast stem cells, and (b) extravillous cytotrophoblasts become invasive and invade the endometrium and, eventually, its arterial system. Thus, in placenta, both invasive (extravillous cytotrophoblasts) and non-invasive (cytotrophoblast stem cells) cells are present (Fig. 3b). These different cell types may be optimally suited for the determination of Snail immunoreactivity using formalin-fixed and paraffin-embedded archival material and, consequently, for the demonstration of the usefulness of hybridoma supernatant Sn9H2 for immunohistochemical analysis. As can be seen in Fig. 3c, hybridoma supernatant Sn9H2 exclusively reacts with invasive extravillous trophoblasts (EVT), while trophoblast stem cells are not stained. The immunoreactivity is clearly nuclear, a finding that is in line with Snail’s function as a transcriptional repressor. In contrast, membranous E-cadherin immunoreactivity is seen preferentially in trophoblast stem cells; invasive trophoblasts only show a very faint reaction or they are E-cadherin negative. These very encouraging results enabled us to perform the first analysis for nuclear Snail immunoreactivity of a larger series of primary human carcinomas.
Hybridoma supernatant Sn9H2 detects a nuclear protein in invasive trophoblasts in placenta. a Western blot showing Snail expression in placenta; the molecular weight is compared with the purified protein and Snail-transfected GC2957 gastric cancer cells. b Diagram of human placenta demonstrating the different cell types, e.g. non-invasive trophoblast stem cells and invasive EVT. c Immunoreactivity of Snail and E-cadherin in formalin-fixed and paraffin-embedded placenta. Nuclear Snail immunoreactivity is seen in invasive EVT; E-cadherin is localized at the cell membrane of trophoblast stem cells and reduced or absent in invasive EVT. (The arrow indicates the direction of invasion.)
Analysis of nuclear Snail immunoreactivity in primary adenocarcinomas of the upper gastrointestinal tract
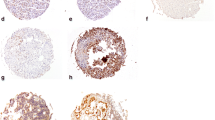
According to the immunohistochemical analyses based on TMAs, 27 tumours were considered positive for Snail expression while 313 were considered negative. Immunoreactivity for Snail in the tumour cells was found exclusively nuclear. Twenty-seven cases had to be excluded from further analysis because either only non-cancerous tissue was present in all three punches or all tissue samples had been lost during immunohistochemical staining. Besides cancer cells, immunoreactivity for Snail was found consistently in a small subset of stromal cells (inside and outside of the tumours), i.e. fibroblasts and endothelial cells. This expression tended to be accentuated in areas of mucosal erosion or ulceration.
To validate immunohistochemical analyses based on TMAs, whole-tissue sections from the donor blocks of the 27 Snail-positive carcinomas were immunohistochemically stained for Snail. Analysis of whole-tissue sections confirmed Snail expression in all cases considered to be positive based on TMA analysis. Figure 4 shows examples of some of these cases. Immunohistochemical analysis of whole-tissue sections allowed the semiquantitative assessment of Snail expression. In 14 tumours, 5–25% of the tumour cells were Snail positive; in 4 tumours, 26–50% of the tumour cells were Snail positive; in 1 tumour, the percentage of Snail-positive tumour cells was 51–75%, and in 1 tumour, the percentage was 76–100%.
Snail and E-cadherin immunoreactivity in cancers of the upper gastrointestinal tract. a This undifferentiated tumour shows an inverse correlation of Snail and E-cadherin expression. The rectangles indicate areas that are shown at higher magnification below the upper panel. Upper panel, ×100; lower panel, ×200. b Tumour cells in this tumour show coexpression of Snail and E-cadherin.
To analyse the correlation between the expression of Snail and E-cadherin, serial whole-tissue sections of only 20 Snail-positive carcinomas were also immunohistochemically stained for E-cadherin. Of these 20 Snail-positive carcinomas, only 7 showed a loss of E-cadherin expression in the tumour area where Snail expression was detectable (Fig. 4), indicating no clear correlation between Snail up-regulation and E-cadherin down-regulation.
Correlation of Snail expression and clinico-pathological parameters
The expression of Snail in adenocarcinomas from the upper gastrointestinal tract was not correlated with a variety of clinico-pathological parameters, such as tumour grade, Lauren’s classification, WHO classification, tumour stage and tumour size (Table 1). However, the expression of Snail was significantly more frequent in oesophageal adenocarcinomas (11.1%) than in cardiac (6.9%) or in gastric (3.6%) carcinomas (p=0.0428).
Discussion
A hallmark in disease progression of epithelial cancers is the functional loss of the homophilic cell adhesion molecule E-cadherin, altering both the physical attachment of cells and the regulatory signals transmitted through cytoplasmic adapter molecules. Reduced or lack of E-cadherin immunoreactivity has been observed in many types of cancers and correlated with histopathological and clinical features, such as tumour dedifferentiation, invasive growth, lymph node metastasis and a worse patient prognosis [24]. The main mechanisms resulting in a loss of E-cadherin function include loss of heterozygosity (LOH) in combination with gene mutations [50], promoter hypermethylation [18, 31], enhanced degradation due to HAKAI, a c-Cbl-like protein [16], down-regulation by the RON tyrosine kinase [2], down-regulation by the transmembrane molecule dysadherin [26] and proteolytic degradation by matrix metalloproteases [30]. While E-cadherin gene mutations are found in diffuse-type gastric cancer [4, 5] and in invasive lobular breast cancer [6], they are rare in other cancers [7] and may not account for the majority of E-cadherin inactivations seen in human cancer tissues.
Recent reports highlighted the significance of EMT regulators that directly repress E-cadherin transcription. Several transcription factors, including the zinc finger transcription factor Snail [3, 12], the Snail-related molecule Slug [10, 21], Zeb2 (Sip1; [13]), Zeb1 [20] and E12/47 [35], may bind to E-box elements present in E-cadherin’s promoter, resulting in transcriptional down-regulation. In various types of cancers and cell lines, the increased expression of Snail mRNA has been correlated with the loss of or reduced E-cadherin expression, although in several systems, a coexpression of Snail and E-cadherin was observed [14]. In the current study, we transfected two different cell lines with human Snail and found a concomitant reduction in E-cadherin levels, indicating the expression of a functional Snail molecule.
Most studies analysing Snail expression have been performed at the mRNA level, possibly because only a few commercial antibodies that react with human Snail protein are available. Using an in-house-made polyclonal antibody against mouse Snail, Dominguez et al. [14] detected a doublet band in Western blot analysis (15% gel) at about 32 kDa in extracts from Snail-transfected HTB-29 and Madin-Darby canine kidney (MDCK) cells (80 μg of a cell extract per lane was used). They postulated a modification of the snail protein (phosphorylation) that may be responsible for the upper band seen in the blot and may regulate nuclear localization. We showed here that in E-cadherin-negative HTB-135 gastric cancer cells, endogenously expressed Snail seems to be localized in the nucleus and in the cytoplasm, whereas exogenously expressed Snail is seen exclusively in the nucleus. When we double our cell lysates from HTB-135 cells (40 μg total protein instead of 20 μg) and extend film exposure time (10 s instead of 5 s), a second smaller band is visible in Western blots (not shown). In extracts from human placenta, however, only one band is seen, migrating at the same position as the purified human protein and most likely corresponding to the “upper” band. In this tissue, Snail immunoreactivity appeared exclusively nuclear (Fig. 3).
When we used commercial anti-Snail antibodies used by others (e.g. [47, 48]), they did not work (data not shown). In addition, none of those could be applied for analysis of Snail nuclear immunoreactivity in archival human tissues so far. Therefore, we generated a rat mAb, termed Sn9H2, against human Snail that works excellently in Western blotting and can be used for immunohistochemical analysis of human tissue samples and, although not yet tested, possibly for immunoprecipitation and chromatin immunoprecipitation studies. The specificity of the antibody was demonstrated by Western blot analysis, immunofluorescence and immunohistochemical analysis of appropriate test systems, such as transfected cells in a culture expressing functional Snail and invasive trophoblasts from human placenta. With our new antibody, a direct correlation between E-cadherin repression and Snail neoexpression can now be analysed in cell lines and tissues, even after formalin fixation. In some cell lines, there is a coexpression of E-cadherin and Snail. A forced overexpression of Snail, however, resulted in the reduction of E-cadherin, suggesting some dosage effect. This may explain the failure to find a concomitant reduction of E-cadherin in every Snail-positive case (see “Results”).
To start with an evaluation of Snail immunoreactivity in human cancers, we chose adenocarcinomas of the upper gastrointestinal tract, i.e. tumours that arise in the oesophagus, in the cardia or in the stomach. Today, it is generally accepted that the majority of carcinomas of the upper gastrointestinal tract develop through an accumulation of somatic genetic and epigenetic changes, such as point mutations and/or LOH in the p53 gene, promoter methylation of p16ink4A and amplification and overexpression of cyclin D1, epidermal growth factor receptor (EGFR) and c-erbB-2. In addition, the expressions of E-cadherin and adenomatous polyposis coli (APC) were frequently found to be altered (for review, see [41]). We found Snail immunoreactivity in about 8% of the 340 cases analysed. No statistically significant correlation to the pathological and clinical data evaluated was seen. However, Snail immunoreactivity was more frequently detected in oesophageal cancers compared with cardia or gastric cancers, although only with marginal statistical significance. From the 154 analysed cases with oesophageal cancers, 17 (11%) were found to express Snail. In the gastric cancers analysed, only 4% Snail-positive tumours were detected. In a previous study, using quantitative real-time RT-PCR, we found Snail mRNA overexpression preferentially in diffuse-type gastric cancers [39]. However, analysing a much larger series of tumours, as was done in the current study, we did not find a statistical correlation between Snail immunoreactivity and Lauren’s classification (diffuse vs intestinal).
Epithelial–mesenchymal transition mediated by Snail is considered to be a local event occurring at certain sites within an invasive tumour; EMT is typically seen at the invasion front [11]. Interestingly, Snail mRNA was not detected in a series of microdissected colon cancers in one study [40] but was reported to be overexpressed in another [33]. As mentioned in the “Results” section, some fibroblasts and endothelial cells showed Snail immunoreactivity. Even after careful tissue microdissection, it cannot be excluded that non-tumourous cells, such as fibroblasts or endothelial cells, are present in the tissue samples and may have contributed to the results of mRNA studies. Hybridoma Sn9H2 can now aid to solve some discrepancies that may have arisen in quantitative real-time RT-PCR analysis of primary tumours.
Using in situ hybridisation, Blanco et al. [9] found Snail mRNA expression in scarce fibroblastic cells present in stromal regions of breast cancers. In the same study, 9 out of 21 breast cancers expressed Snail mRNA, inversely correlating with the grade of differentiation of the tumours [9]. The authors concluded that Snail may be a marker of metastatic potential. While estrogen receptor signalling via metastasis associated gene 3 (MTA3) up-regulation was demonstrated to play a role in Snail inhibition in breast cancer [17], the mechanisms for Snail regulation in cancers of the upper gastrointestinal tract are currently unknown. Recently, the human Snail promoter was characterized, demonstrating an induction of Snail transcription by integrin linked kinase (ILK) and the oncogenes Ha-ras and v-Akt [1]. In addition, extracellular signal-regulated kinase (ERK) and NFkappaB/p65 also stimulated Snail transcription [1]. In an elegant paper, Zhou et al. [51] demonstrated a role for glycogen synthase kinase-3beta (GSK-3beta) in the regulation of Snail function. The authors found that GSK-3beta binds to and phosphorylates Snail at two consensus motifs to regulate both ubiquitination and subcellular localization. Nuclear immunoreactivity, however, indicative of active Snail was apparently not seen in 134 breast cancer samples analysed. Thus, our hybridoma Sn9H2, which, to our knowledge, is currently the only antibody that reacts with endogenous nuclear Snail, may be a valuable tool in determining the percentage of active Snail in archival human cancers. As soon as antibodies for other active E-cadherin repressors suitable for immunohistochemistry of archival human tissues are available, the relative contribution of each of these repressor molecules in E-cadherin silencing can be analysed in defined sets of human tumours.
In conclusion, we generated a mAb against human Snail and showed nuclear expression in a subset of adenocarcinomas of the upper gastrointestinal tract. Snail’s role in these tumours and mechanisms of its regulation, e.g. signalling pathways known to induce EMT, however, have to be established.
References
Barbera MJ, Puig I, Dominguez D, Julien-Grille S, Guaita-Esteruelas S, Peiro S, Baulida J, Franci C, Dedhar S, Larue L, Garcia De Herreros A (2004) Regulation of Snail transcription during epithelial to mesenchymal transition of tumour cells. Oncogene 23:7345–7354
Bardella C, Costa B, Maggiora P, Patane’ S, Olivero M, Ranzani GN, De Bortoli M, Comoglio PM, Di Renzo MF (2004) Truncated RON tyrosine kinase drives tumour cell progression and abrogates cell–cell adhesion through E-cadherin transcriptional repression. Cancer Res 64:5154–5161
Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A (2000) The transcription factor Snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2:84–89
Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, Siewert JR, Hofler H (1994) E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res 54:3845–3852
Becker KF, Kremmer E, Eulitz M, Becker I, Handschuh G, Schuhmacher C, Muller W, Gabbert HE, Ochiai A, Hirohashi S, Hofler H (1999) Analysis of E-cadherin in diffuse-type gastric cancer using a mutation-specific monoclonal antibody. Am J Pathol 155:1803–1809
Berx G, Cleton-Jansen AM, Strumane K, de Leeuw WJ, Nollet F, van Roy F, Cornelisse C (1996) E-cadherin is inactivated in a majority of invasive human lobular breast cancers by truncation mutations throughout its extracellular domain. Oncogene 13:1919–1925
Berx G, Becker KF, Hofler H, van Roy F (1998) Mutations of the human E-cadherin (CDH1) gene. Hum Mutat 12:226–237
Birchmeier W, Behrens J (1994) Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta 1198:11–26
Blanco MJ, Moreno-Bueno G, Sarrio D, Locascio A, Cano A, Palacios J, Nieto MA (2002) Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene 21:3241–3246
Bolos V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, Cano A (2003) The transcription factor slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci 116:499–511
Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, Knuechel R, Kirchner T (2001) Variable beta-catenin expression in colorectal cancers indicates tumour progression driven by the tumour environment. Proc Natl Acad Sci U S A 98:10356–10361
Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA (2000) The transcription factor Snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2:76–83
Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, van Roy F (2001) The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell 7:1267–1278
Dominguez D, Montserrat-Sentis B, Virgos-Soler A, Guaita S, Grueso J, Porta M, Puig I, Baulida J, Franci C, Garcia de Herreros A (2003) Phosphorylation regulates the subcellular location and activity of the Snail transcriptional repressor. Mol Cell Biol 23:5078–5089
Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, Lochner D, Birchmeier W (1991) E-cadherin mediated cell–cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol 113:173–185
Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J, Sommer T, Birchmeier W (2002) Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol 4:222–231
Fujita N, Jaye DL, Kajita M, Geigerman C, Moreno CS, Wade PA (2003) MTA3, a Mi-2/NuRD complex subunit, regulates an invasive growth pathway in breast cancer. Cell 113:207–219
Graff JR, Herman JG, Lapidus RG, Chopra H, Xu R, Jarrard DF, Isaacs WB, Pitha PM, Davidson NE, Baylin SB (1995) E-cadherin expression is silenced by DNA hypermethylation in human breast and prostate carcinomas. Cancer Res 55:5195–5199
Grau Y, Carteret C, Simpson P (1984) Mutations and chromosomal rearrangements affecting the expression of Snail, a gene involved in embryonic patterning in Drosophila melanogaster. Genetics 108:347–360
Guaita S, Puig I, Franci C, Garrido M, Dominguez D, Batlle E, Sancho E, Dedhar S, De Herreros AG, Baulida J (2002) Snail induction of epithelial to mesenchymal transition in tumour cells is accompanied by MUC1 repression and ZEB1 expression. J Biol Chem 277:39209–39216
Hajra KM, Chen DY, Fearon ER (2002) The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res 62:1613–1618
Hamilton SR, Aaltonen LA (eds) (2000) World Health Organisation. Classification of tumours. Pathology and genetics of tumours of the digestive system. Lyon: IARC Press
Hay ED (1995) An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 154:8–20
Hirohashi S (1998) Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol 153:333–339
Ikenouchi J, Matsuda M, Furuse M, Tsukita S (2003) Regulation of tight junctions during the epithelium–mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J Cell Sci 116:1959–1967
Ino Y, Gotoh M, Sakamoto M, Tsukagoshi K, Hirohashi S (2002) Dysadherin, a cancer-associated cell membrane glycoprotein, down-regulates E-cadherin and promotes metastasis. Proc Natl Acad Sci U S A 99:365–370
Jiao W, Miyazaki K, Kitajima Y (2002) Inverse correlation between E-cadherin and Snail expression in hepatocellular carcinoma cell lines in vitro and in vivo. Br J Cancer 86:98–101
Kremmer E, Kranz BR, Hille A, Klein K, Eulitz M, Hoffmann-Fezer G, Feiden W, Herrmann K, Delecluse HJ, Delsol G, Bornkamm GW, Mueller-Lantzsch N, Grassert FA (1995) Rat monoclonal antibodies differentiating between the Epstein-Barr virus nuclear antigens 2A (EBNA2A) and 2B (EBNA2B). Virology 208:336–342
Lauren P (1965) The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. Acta Pathol Microbiol Scand 64:31–49
Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ (1997) Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol 139:1861–1872
Machado JC, Oliveira C, Carvalho R, Soares P, Berx G, Caldas C, Seruca R, Carneiro F, Sobrinho Simoes M (2001) E-cadherin gene (CDH1) promoter methylation as the second hit in sporadic diffuse gastric carcinoma. Oncogene 20:1525–1528
Nieto MA (2002) The Snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol 3:155–166
Palmer HG, Larriba MJ, Garcia JM, Ordonez-Moran P, Pena C, Peiro S, Puig I, Rodriguez R, De La Fuente R, Bernad A, Pollan M, Bonilla F, Gamallo C, De Herreros AG, Munoz A (2004) The transcription factor SNAIL represses vitamin D receptor expression and responsiveness in human colon cancer. Nat Med 10:917–919
Paznekas WA, Okajima K, Schertzer M, Wood S, Jabs EW (1999) Genomic organization, expression, and chromosome location of the human Snail gene (SNAI1) and a related processed pseudogene (SNAI1P). Genomics 62:42–49
Perez-Moreno MA, Locascio A, Rodrigo I, Dhondt G, Portillo F, Nieto MA, Cano A (2001) A new role for E12/E47 in the repression of E-cadherin expression and epithelial–mesenchymal transitions. J Biol Chem 276:27424–27431
Perez-Moreno M, Jamora C, Fuchs E (2003) Sticky business: orchestrating cellular signals at adherens junctions. Cell 112:535–548
Poser I, Dominguez D, de Herreros AG, Varnai A, Buettner R, Bosserhoff AK (2001) Loss of E-cadherin expression in melanoma cells involves up-regulation of the transcriptional repressor Snail. J Biol Chem 276:24661–24666
Rae JM, Ramus SJ, Waltham M, Armes JE, Campbell IG, Clarke R, Barndt RJ, Johnson MD, Thompson EW (2004) Common origins of MDA-MB-435 cells from various sources with those shown to have melanoma properties. Clin Exp Metastasis 21:543–552
Rosivatz E, Becker I, Specht K, Fricke E, Luber B, Busch R, Hofler H, Becker KF (2002) Differential expression of the epithelial–mesenchymal transition regulators Snail, SIP1, and twist in gastric cancer. Am J Pathol 161:1881–1891
Rosivatz E, Becker I, Bamba M, Schott C, Diebold J, Mayr D, Hofler H, Becker KF (2004) Neoexpression of N-cadherin in E-cadherin positive colon cancers. Int J Cancer 111:711–719
Sarbia M, Becker KF, Hofler H (2004) Pathology of upper gastrointestinal malignancies. Semin Oncol 31:465–475
Shiozaki H, Tahara H, Oka H, Miyata M, Kobayashi K, Tamura S, Iihara K, Doki Y, Hirano S, Takeichi M (1991) Expression of immunoreactive E-cadherin adhesion molecules in human cancers. Am J Pathol 139:17–23
Siewert JR, Stein H (1998) Classification of adenocarcinoma of the esophago–gastric junction. Br J Cancer 85:1457–1459
Sobin LH, Wittekind Ch (eds) (2002) TNM classification of malignant tumours, 6th edn. Wiley, New York
Sugimachi K, Tanaka S, Kameyama T, Taguchi K, Aishima S, Shimada M, Sugimachi K, Tsuneyoshi M (2003) Transcriptional repressor Snail and progression of human hepatocellular carcinoma. Clin Cancer Res 9:2657–2664
Thiery JP (2002) Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer 2:442–454
Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J, Aranda S, Palomo S, McCormick F, Izpisua-Belmonte JC, de la Pompa JL (2004) Notch promotes epithelial–mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev 18:99–115
Vega S, Morales AV, Ocana OH, Valdes F, Fabregat I, Nieto MA (2004) Snail blocks the cell cycle and confers resistance to cell death. Genes Dev 18:1131–1143
Vleminckx K, Vakaet L Jr, Mareel M, Fiers W, van Roy F (1991) Genetic manipulation of E-cadherin expression by epithelial tumour cells reveals an invasion suppressor role. Cell 66:107–119
Vos CB, Cleton-Jansen AM, Berx G, de Leeuw WJ, ter Haar NT, van Roy F, Cornelisse CJ, Peterse JL, van de Vijver MJ (1997) E-cadherin inactivation in lobular carcinoma in situ of the breast: an early event in tumourigenesis. Br J Cancer 76:1131–1133
Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC (2004) Dual regulation of Snail by GSK-3beta mediated phosphorylation in control of epithelial–mesenchymal transition. Nat Cell Biol 6:931–940
Acknowledgements
We thank M. Blöchinger and M. Bekesch for excellent technical assistance, J. Mages for providing the modified pHAT vector, and R. Langer for help with some tissues. The E-cadherin-positive gastric cancer cell line GC2957 was kindly provided by R. Seruca, IPATIMUP-Porto. This work is supported by a grant from the Deutsche Krebshilfe (grant no. 10-2012-Be3) to KFB and HH.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosivatz, E., Becker, KF., Kremmer, E. et al. Expression and nuclear localization of Snail, an E-cadherin repressor, in adenocarcinomas of the upper gastrointestinal tract. Virchows Arch 448, 277–287 (2006). https://doi.org/10.1007/s00428-005-0118-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-005-0118-9