Abstract
Rb1-inducible coiled-coil 1 (Rb1cc1) expressed at high levels is associated with the maturation of human embryonic musculoskeletal cells. To clarify the molecular role of Rb1cc1 in muscular differentiation, we investigated the expression of Rb1cc1 and other genes that regulate differentiation in murine embryonic tissues and in C2C12 myoblasts. We also evaluated the effects of RNA interference (RNAi)-mediated Rb1cc1 knockdown on C2C12 myoblast differentiation. After Rb1cc1, Rb1 and myosin heavy chain (Myhc) were expressed in mouse embryonic muscles. The synchronous expression of Rb1cc1 and Rb1 predicted Myhc expression during C2C12 myoblast differentiation. RNAi-mediated knockdown of Rb1cc1 led to Rb1 suppression, and C2C12 myoblasts failed to differentiate. These results indicated that Rb1cc1 is a potent regulator of the Rb1 pathway and a novel mediator that plays a crucial role in muscular differentiation. Rb1cc1 expression is, thus, a prerequisite for myogenic differentiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During differentiation, skeletal muscle undergoes terminal withdrawal from the cell cycle, the activation of coordinated gene expression, and the fusion of myoblasts into multinucleated myotubes. These events are largely dependent on muscle-specific transcription factors and the retinoblastoma 1 (Rb1) tumor suppressor. The MyoD family includes MyoD, myf5, myogenin, and MRF4 [3, 11, 22], among which MyoD plays particularly pivotal roles in the activation of muscle-specific gene expression and cell-cycle arrest [7, 19]. However, the roles of these myogenic factors apparently depend on Rb1, which might mediate both terminal withdrawal from cell cycle and the upregulation of tissue-specific genes associated with terminal differentiation [12, 23]. Myotubes deficient in Rb1 do not differentiate and, in fact, re-enter the cell cycle in response to mitogens [21]. Moreover, introducing MyoD into Rb1-deficient mouse embryonic fibroblasts cannot induce late markers of myogenic differentiation and cell-cycle arrest, indicating that Rb1 is required for MyoD-mediated myogenic function [8, 18]. Although Rb1 is important to muscular differentiation, the precise mechanisms remain obscure.
We recently identified a novel molecule, Rb1-inducible coiled-coil 1 (Rb1cc1), which is expressed at high levels in human embryonic musculoskeletal and cultured osteosarcoma cells. Rb1cc1 induces Rb1 expression in a variety of cultured cells [5, 6], and the expression of both molecules is synchronous [5]. We also demonstrated that Rb1cc1 contributes to the maturation of human embryonic musculoskeletal cells [6]. These findings implied that Rb1cc1 is involved in muscular differentiation.
To define the relationship between Rb1cc1 function and muscle differentiation, we used the murine C2C12 myoblast model, which has been widely studied as an in vitro model to understand the regulation of myogenic differentiation [10, 13]. Switching C2C12 cell lines to low-serum medium induces their differentiation. This process closely resembles myogenic differentiation in vivo. The expression of both Rb1cc1 and Rb1 is similarly upregulated in the model. Furthermore, Rb1cc1 knockdown led to the downregulation of Rb1 expression, and late markers of myogenic differentiation were not expressed. The present study showed that Rb1cc1 is vitally involved in skeletal muscle differentiation.
Materials and methods
Northern blots
Mouse embryo full stage blots (Seegene, Seoul, Korea) were hybridized with gene-specific probe nucleotides (nt) 1536–3500 of Rb1cc1 (GenBank accession number, AB070619), 336–675 of Rb1 (GenBank accession number, NM000321), and 2695–2719 of myosin heavy chain (Myhc) (GenBank accession number, NM144961). The probes were labeled with [α-32P] dCTP (3000 Ci/mmol; Amersham, Piscataway, NJ) using random priming and hybridized with the blot membrane at 42°C overnight in 50% formamide containing 1% sodium dodecyl sulfate (SDS), 1 mol/l NaCl, 200 mg/ml sonicated herring sperm DNA, and 10% dextran sulfate. The blots were then washed twice at 42°C in 2×standard saline citrate (SSC) containing 1% SDS and once with 0.2×SSC containing 1% SDS for 5 min at 65°C. Blots were visualized using autoradiography with a Kodak BioMax and intensifying screens (Kodak, New Haven, CT).
Histology and immunohistochemistry
Pregnant female C57BL6 mice were sacrificed by cervical dislocation, and embryos were transferred to 10% buffered formalin and fixed overnight. The embryos were washed several times in 70% ethanol, embedded in paraffin, and serially sliced into 4-µm sections. Deparaffinized sections were immersed in 0.3% H202, autoclaved at 120°C for 1 min, and rinsed with 1×Tris-buffered saline (TBS) prior to incubation overnight at 4°C with the following primary antibodies: anti-Rb1cc1 rabbit antiserum (α-Rb1cc1–1104) [2, 5], anti-Rb1 monoclonal antibody (G3–245; PharMingen, San Diego, CA), and anti-Myhc monoclonal antibody (RNMy2/9D2; Novo Castra, Newcastle, UK). The sections were rinsed with 1×TBS and incubated with secondary antibody (Simple Stain MAX-PO; Nichirei, Japan) at room temperature for 1 h. The sections were then stained with 3,3′-diaminobenzidine tetrahydrochloride and counter-stained with hematoxylin.
Cell culture
C2C12 mouse skeletal myoblasts were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (growth medium). To induce muscular differentiation, the cells were rendered quiescent by serum withdrawal for 48 h and stimulated to differentiate by adding 2% horse serum [differentiation medium (DM)].
RNAi and transfection
Three kinds of small interfering RNA (siRNA) plasmid vectors (608, 1993 and 4525) corresponding to different targeting sites (nt.608–626, nt. 1993–2013 and nt. 4525–4545, respectively) of Rb1cc1 mRNA (GenBank accession number, AB070619) were synthesized in vitro by inserting artificial oligonucleotides into pRNAT-H1.1/Hygro (GenScript, Piscataway, NJ). We introduced each siRNA or control vector into C2C12 cells using FuGENE6 according to the supplier’s recommendations (Roche Applied Science).
Reverse-transcription polymerase chain reaction
Total RNA was isolated from C2C12 cells using TRIzol (Gibco-BRL), and first-strand cDNA was synthesized using Superscript III reverse transcriptase and Oligo d(T)12–18 primer (Gibco-BRL). The gene primer sequences were as follows: murine Rb1cc1: forward, 5′- TGCTGCACAAGACTCTCACA and reverse, 5′- CAGCATTTCCTTCTGCTGTG; Rb1: forward, 5′- CACGTGTAAATTCTGCTGCAA and reverse, 5′- CCTGG TGGAGGCATACTGTAA; Gapdh: forward, 5′- CATGACAACTTTGGCATTGTG and reverse, 5′- GTTGAAGTCGCAGGAGACAAC; Myhc: forward, 5′- AAGG AACTTGAAGAAAAGATGGTG and reverse, 5′- TCAAGTTTTCTCTTG GCTCTTTCT; cyclin D3: forward, 5′- TAGGCGCCTGCTCTATGTCT and reverse, 5′-ATCTGTGGGAGTGCTGGTCT; p21: forward 5′-GTCCAATCCTGGTGATGT CC and reverse, 5′- CAGGGCAGAGGAAGTACTGG. The PCR protocols consisted of 26 cycles for Rb1cc1, Rb1 and p21, 21 for Gapdh, 38 for Myhc, and 36 cycles for cyclin D3 of a 20-s denaturation at 95 C, 20-s annealing at 55 C, and 30-s extension at 72°C.
Immunocytochemical analysis of C2C12 cells
The above-mentioned primary antibodies were labeled with Alexa Fluor 555 (Molecular Probes, Eugene, OR) according to the supplier’s protocol. The C2C12 cells treated with siRNA were fixed, incubated with the appropriate labeled antibody, and evaluated using fluorescent microscopy.
Results
Rb1cc1 predicts Rb1 and Myhc
To determine the importance of Rb1cc1 in muscular differentiation, we analyzed Rb1cc1 expression in murine embryos. Northern blotting showed that Rb1cc1 mRNA was constitutively expressed in the embryos and predicted Rb1 and Myhc. Rb1 mRNA was obviously induced in E10.5 embryos and upregulated at E13.5–14.5 and E16.5–17.5. Following Rb1cc1 and Rb1 expression, Myhc, a late marker of myogenic differentiation, was expressed in E13.5 embryos and obviously induced at E16.5 (Fig. 1).
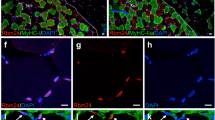
Immunohistochemical staining with Rb1cc1, Rb1, and Myhc in murine embryos also showed Rb1cc1, Rb1, and Myhc expression in developing murine muscular tissues (Fig. 2). Rb1cc1 was expressed predominantly in the cytoplasm, and Rb1 was located in the nucleus. Myhc was expressed throughout 18 dpc mouse muscles.
Expression of Rb1cc1 and Rb1 is associated with muscular differentiation
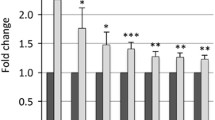
To investigate the Rb1cc1-mediated molecular mechanism in muscle differentiation, we used the murine C2C12 myoblast system. Rb1cc1 and Rb1 gene expression was induced in C2C12 cells by DM. Thereafter, differentiated C2C12 cells also expressed high levels of Myhc, a late marker of myogenic differentiation, compared with proliferating C2C12 cells. More p21 expression was induced in differentiating than in proliferating C2C12 cells (Fig. 3).
Serial expressional analysis of genes regulating muscle differentiation. Expression levels of Rb1cc1, Rb1, Myhc, p21, and Gapdh in C2C12 cells incubated in differentiation medium (DM) for indicated periods, or maintained in growth medium (GM) were determined by semi-quantitative reverse-transcription polymerase chain reaction. More Rb1cc1 and Rb1 genes were induced by DM. Following expression of these genes, Myhc was obviously inducted in differentiated C2C12 cells
Rb1cc1-specific knockdown disturbs myoblast differentiation
To study the functions of endogenous Rb1cc1 during the differentiation of C2C12 myoblasts, we knocked down Rb1cc1 expression using siRNAs. This process led to Rb1 repression, as well as failed Myhc expression and myoblast differentiation. The expression of a series of genes, including p21 and cyclin D3, was similar during the experimental process for the differentiation (Fig. 4). Immunocytochemically, C2C12 cells with siRNA vectors failed to synthesize Rb1cc1 and expressed low levels of Rb1 and Myhc (Fig. 5). Exposure to several siRNAs corresponding to different regions of Rb1cc1 mRNA similarly caused C2C12 myoblast differentiation to fail (data not shown).
Effects of Rb1cc1 knockdown in C2C12 cells. C2C12 cells treated with siRNA vector (4525) were incubated in differentiation medium (DM). Expression levels of Rb1cc1, Rb1, Myhc, p21, cyclin D3 and Gapdh were determined using semi-quantitative reverse-transcription polymerase chain reaction. Rb1cc1 siRNA inhibited Rb1 expression and resulted in failed Myhc expression. Expression of p21 and cyclin D3 was not affected
Effects of Rb1cc1 knockdown on C2C12 myoblasts differentiation. C2C12 cells treated with siRNA vector (4525) were incubated in differentiation medium (DM). Fluorescent immunocytochemistry of Rb1cc1, Rb1, and Myhc demonstrated that the C2C12 cells treated with Rb1cc1 siRNA (green: GFP) failed to synthesize Rb1cc1 (Red, Alexa Fluor 555; A) in the cytoplasm, and they expressed neither Rb1 (Red, Alexa Fluor 555; B) nor Myhc (Red, Alexa Fluor 555; C)
Discussion
Rb1cc1 is a novel gene that is associated with multidrug resistance to anti-cancer agents. The expression of both Rb1cc1 and Rb1 is synchronized in various cancer cell lines and in normal human tissues. Moreover, the introduction of wild-type Rb1cc1 induces Rb1 expression in human leukemic cells [5]. Rb1cc1 expressed at high levels contributes to the maturation of human embryonic musculoskeletal cells [6]. To our knowledge, Rb1cc1 might interact with Stathmin [17], the Listeria monocytogenesis surface protein ActA [20], or with focal adhesion kinase [1]. However, other molecules that associate with Rb1cc1 remain unknown, and the molecular mechanisms of Rb1cc1-mediated cellular functions are obscure.
To determine the function of Rb1cc1, the present study investigated the roles of Rb1cc1 on skeletal muscle differentiation. Northern and immunohistochemical analyses of mouse embryos showed that Rb1cc1 expression predicted that of Rb1 and Myhc. Although we demonstrated that the expression of Rb1cc1 and Rb1 is synchronized in musculoskeletal tissues of human embryos [6], Rb1cc1 was expressed more ubiquitously and earlier in mouse than in human embryos. Further, the intracellular localization of Rb1cc1 could change according to the developing status, and Rb1cc1 is involved in the murine developmental process [2]. More importantly, Rb1cc1 and Rb1 expression closely correlated and led to Myhc expression during C2C12 myoblast differentiation. These results suggest that Rb1cc1 is intimately involved in muscle differentiation in vivo and in vitro. Rb1 protein plays crucial roles in the induction of late markers during myogenic differentiation [8, 18]. Rb1cc1 predicted Rb1 during mouse myogenic differentiation and might play an important role in the expression of Rb1 and subsequent Myhc, a late marker of this process.
The RNAi-mediated knockdown of Rb1cc1 reduced Rb1 expression at the transcriptional level, and Myhc was not induced during C2C12 myoblast differentiation. The findings suggest that Rb1cc1 acts in the same pathway as Rb1 and plays a crucial role in myoblast differentiation upstream of Rb1 rather than downstream.
Although many proteins are implicated as critical modulators of myogenic differentiation, the complexity of this process suggests that more remain to be discovered. Here, we postulate that Rb1cc1 is a novel modulator of myogenic differentiation. We found that Rb1cc1 appears to be primarily required for muscular differentiation and that its suppression causes failed maturation of myoblasts largely due to the loss of Rb1 expression. Rb1cc1 is thought to induce Rb1 expression [5]. Rb1cc1 regulated Rb1 expression at the transcriptional level, although many proteins are involved in Rb1 regulation by affecting its phosphorylation status [9, 14, 21, 23]. Rb1cc1 might directly bind the Rb1 gene promoter and induce its expression. Although Rb1cc1 predicted Rb1, such concordance was incomplete during mouse embryonic development. These findings indicate that Rb1cc1 indirectly rather than directly regulates Rb1 expression. Otherwise, Rb1cc1 might associate with Rb1. The Rb1cc1 protein contains a consensus nuclear localization signal (KPRK) [5], and the protein was localized in both the nucleus and cytoplasm. In addition, intracellular sublocalization of Rb1cc1 changes in accordance with developmental status [2]. Rb1cc1 may play functional roles by altering its intracellular sublocalization. Thus, Rb1cc1 might cooperate in nuclear Rb1 expression.
The present study demonstrated that Rb1cc1-siRNAs decreased the expression of Rb1 and subsequently Myhc but not that of p21 and cyclin D3. MyoD, a pivotal factor for myogenic differentiation, can activate muscle-specific gene expression and induce cell-cycle arrest through Rb1-dependent pathways [18, 21]. At the onset of myogenic differentiation, MyoD activates the expression of Rb1, p21, cyclin D3 and induces cell cycle arrest, a process that is referred to as early events [4, 15, 16]. MyoD also co-operates with Rb1 to promote the expression of late markers of muscular differentiation, such as Myhc, which is referred to as a late myogenic event [4]. Therefore, the failed differentiation of myoblasts due to Rb1cc1-specific knockdown was largely dependent on Rb1 repression with no change in p21 and cyclin D3, suggesting that Rb1cc1 is a crucial regulator of late, rather than early myogenic events.
Here, we reported that Rb1cc1 is a novel modulator of myogenic differentiation. The Rb1cc1-Rb1 pathway is primarily required for myogenic differentiation, and elucidating the components and precise mechanisms of the pathway during this process will help to clarify how Rb1cc1 functions under physiological conditions.
References
Abbi S, Ueda H, Zheng C, Cooper LA, Zhao J, Christopher R, Guan JL (2002) Regulation of focal adhesion kinase by a novel protein inhibitor FIP200. Mol Biol Cell 13:3178–3191
Bamba N, Chano T, Taga T, Ohta S, Takeuchi Y, Okabe H (2004) Expression and regulation of RB1CC1 in developing murine and human tissues. Int J Mol Med 14:583–587
Blackwell TK, Weintraub H (1990) Differences and similarities in DNA-binding preferences of MyoD and E2A protein complexes revealed by binding site selection. Science 250:1104–1110
Cenciarelli C, De Santa F, Puri PL, Mattei E, Ricci L, Bucci F, Felsani A, Caruso M (1999) Critical role played by cyclin D3 in the MyoD-mediated arrest of cell cycle during myoblast differentiation. Mol Cell Biol 19:5203–5217
Chano T, Ikegawa S, Kontani K, Okabe H, Baldini N, Saeki Y (2002) Identification of RB1CC1, a novel human gene that can induce RB1 in various human cells. Oncogene 21:1295–1298
Chano T, Saeki Y, Serra M, Matsumoto K, Okabe H (2002) Preferential expression of RB1-inducible coiled-coil 1 in terminal differentiated musculoskeletal cells. Am J Pathol 161:359–364
Crescenzi M, Fleming TP, Lassar AB, Weintraub H, Aaronson SA (1990) MyoD induces growth arrest independent of differentiation in normal and transformed cells. Proc Natl Acad Sci U S A 87:8442–8446
Gu W, Schneider JW, Condorelli G, Kaushal S, Mahdavi V, Nadal-Ginard B (1993) Interaction of myogenic factors and the retinoblastoma protein mediates muscle cell commitment and differentiation. Cell 72:309–324
Halevy O, Novitch BG, Spicer DB, Skapek SX, Rhee J, Hannon GJ, Beach D, Lassar AB (1995) Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science 267:1018–1021
Hlaing M, Shen X, Dazin P, Bernstein HS (2002) The hypertrophic response in C2C12 myoblasts recruits the G1 cell cycle machinery. J Biol Chem 277:23794–23799
Lassar A, Davis R, Wright W, Kadesch T, Murre C, Voronova A, Baltimone D, Weintraub H (1991) Functional activity of myogenic HLH proteins requires hetero-oligomerization with E12/E47-like proteins in vivo. Cell 66:305–315
Lipinski MM, Jacks T (1999) The retinoblastoma gene family in differentiation and development. Oncogene 18:7873–7882
Liu CJ, Ding B, Wang H, Lengyel P (2002) The MyoD-inducible p204 protein overcomes the inhibition of myoblast differentiation by Id proteins. Mol Cell Biol 22:2893–2905
Lundberg AS, Weinberg RA (1998) Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol 18:753–761
Magenta A, Cenciarelli C, De Santa F, Fuschi P, Martelli F, Caruso M, Felsani A (2003) MyoD stimulates RB promoter activity via the CREB/p300 nuclear transduction pathway. Mol Cell Biol 23:2893–2906
Martelli F, Cenciarelli C, Santarelli G, Polikar B, Felsani A, Caruso M (1994) MyoD induces retinoblastoma gene expression during myogenic differentiation. Oncogene 9:3579–3590
Maucuer A, Camonis JH, Sobel A (1995) Stathmin interaction with a putative kinase and coiled-coil-forming protein domains. Proc Natl Acad Sci U S A 92:3100–3104
Novitch BG, Mulligan GJ, Jacks T, Lassar AB (1996) Skeletal muscle cells lacking the retinoblastoma protein display defects in muscle gene expression and accumulate in S and G2 phases of the cell cycle. J Cell Biol 135:441–456
Novitch BG, Spicer DB, Kim PS, Cheung WL, Lassar AB (1999) pRb is required for MEF2-dependent gene expression as well as cell-cycle arrest during skeletal muscle differentiation. Curr Biol 9:449–459
Pfeuffer T, Goebel W, Laubinger J, Bachmann M, Kuhn M (2000) LaXp180, a mammalian ActA-binding protein, identified with the yeast two-hybrid system, co-localizes with intracellular Listeria monocytogenes. Cell Microbiol 2:101–114
Schneider JW, Gu W, Zhu L, Mahdavi V, Nadal-Ginard B (1994) Reversal of terminal differentiation mediated by p107 in Rb−/− muscle cells. Science 264:1467–1471
Weintraub H (1993) The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell 75:1241–1244
Wiman KG (1993) The retinoblastoma gene: role in cell cycle control and cell differentiation. FASEB J 7:841–845
Acknowledgements
This study was supported in part by grants-in-aid for PRESTO, JST; Scientific Research on Priority Areas (no. 16021223) and Young Scientists (A) (no. 15689008), MEXT.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Watanabe, R., Chano, T., Inoue, H. et al. Rb1cc1 is critical for myoblast differentiation through Rb1 regulation. Virchows Arch 447, 643–648 (2005). https://doi.org/10.1007/s00428-004-1183-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-004-1183-1