Abstract
Inflammatory and neoplastic disease processes of the abdominal cavity are frequently associated with disruption of the integrity of the peritoneal mesothelium. In the present study, we analyzed the effects of the pro-inflammatory cytokines interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) on the morphology and expression of adhesion molecules of human peritoneal mesothelial cells (HPMC). Treatment of HPMC with IL-1β and TNF-α resulted in a time- and dose-dependent alteration of the normal cobblestone morphology of the mesothelium with loss of polarization, cellular retraction and exposure of the submesothelial matrix. The effect was already observable after 6 h of treatment and was most pronounced at a dose of 10 ng/ml of IL-1β or TNF-α. These morphological alterations were associated with a significant rearrangement of the expression of mesothelial adhesion molecules as detected by flow cytometry. IL-1β and TNF-α both led to a loss of the expression of the hemidesmosomal integrin subunits α6 (P<0.01 and P<0.001) and β4 (P<0.01) and an increased expression of the integrin subunit α5 (P<0.001 and P<0.01). IL-1β furthermore upregulated the expression of the integrin subunits α1, α2 and the adhesion molecule CD44 while the latter was downregulated by TNF-α. Our data indicate that IL-1β and TNF-α may significantly affect disease processes of the abdominal cavity by their potential to disrupt the mesothelial basal cell-matrix adhesion and, thus, the integrity of the peritoneal mesothelial cell lining.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The peritoneal mesothelium is a highly specialized monolayer of polarized flat epithelial cells that covers the entire surface of the abdominal cavity. It serves as a protective anatomical barrier, as a non-adhesive frictionless interface for the movement of abdominal organs and is involved in the formation and turnover of abdominal fluid [7, 27]. The integrity of the mesothelial cell lining is a prerequisite for these functions.
Injury to the abdominal cavity is a common phenomenon. It can be caused by trauma, surgery, infections, or various neoplasms. Both inflammatory and neoplastic disease processes involving the abdominal cavity are associated with marked structural alterations of peritoneal mesothelium, which include loss of polarization, cellular retraction with exposure of the submesothelial extracellular matrix (ECM) and frequently detachment and suspension of mesothelial cells in peritoneal effusions [7, 27, 39]. The precise pathomechanisms involved in the disruption of the mesothelial integrity are still poorly defined but are likely to involve alterations of the expression of mesothelial adhesion molecules [4, 6, 33].
Mesothelial cells, like other epithelial cells, adhere to the ECM by various adhesion molecules. Of particular importance are integrins, a group of transmembraneous heterodimeric glycoproteins composed of α and β subunits [11, 28]. There are at least 16 α and 8 β subunits with intracellular domains associated with the cytoskeleton and extracellular domains that bind to the ECM. The classical fibronectin receptor (α5β1), the αVβ1 fibronectin receptor, and the α6β1 laminin receptor appear to have specific ligands [28]. Other members of the integrin subfamiliy can exhibit different ligand specificities, depending on the cell type by which they are expressed. In addition to their role as adhesion molecules, integrins function as signaling receptors and have been demonstrated to modulate cellular differentiation, growth, and migration as well as cell shape and cytoskeletal organization [5, 11]. An important integrin-independent mechanism of the adhesion of mesothelial cells to the ECM is provided by CD44 [12].
Inflammatory as well as malignant peritoneal effusions contain a variety of cytokines, which may affect and alter the functions and the structure of the peritoneal mesothelial cell lining [16, 20, 26, 34, 41]. Studies from our group and others suggest that the pro-inflammatory cytokines interleukin (IL)-1β and tumor necrosis factor-α (TNF-α) are key regulators of the pathobiological functions of the peritoneal mesothelium. They stimulate human peritoneal mesothelial cells (HPMC) to a markedly increased secretion of various cytokines such as IL-6, IL-8, transforming growth factor-β (TGF-β), basic fibroblast growth factor (bFGF), and vascular endothelial cell growth factor (vEGF) and, thus, seem to induce an "activated" mesothelial cell phenotype [9, 19, 25, 35, 36].
In the present study, we investigated the effects of IL-1β and TNF-α on the morphology, adhesive properties, and expression of adhesion molecules of HPMC.
Materials and methods
Isolation and culture of HPMC
HPMC were obtained from omental tissue of consenting patients undergoing elective abdominal surgery as described previously [25]. In every case small pieces of the omental specimens were fixed in formalin, embedded in paraffin and used to control the morphology of the mesothelium on hematoxylin & eosin-stained sections (Fig. 1E, F). The rest of the omental tissue (approximately 1–3 cm3) was rinsed in phosphate-buffered saline (PBS, Seromed, Biochrom, Berlin, Germany) at room temperature and incubated in a collagenase I solution (0.88–1.76 mg/ml, 220 U/ml; Seromed) for 1 h at 37°C. Subsequently, the collagenase solution containing the detached mesothelial cells was filtered through a 70 µm cell strainer and centrifuged at 500 g for 10 min. The cell pellet was resuspended in RPMI-1640 medium (Seromed) supplemented with 10% fetal calf serum (Sebak, Suben, Austria), penicillin/streptomycin (200 U/ml, 200 µg/ml; Seromed) and l-glutamine (2 mM; Seromed) and grown to confluence in 25-cm2 tissue culture flasks (Greiner, Kremsmuenster, Austria) at 37°C in a humidified atmosphere of 5% CO2 in air. Primary cultures of HPMC were characterized by flow cytometry using monoclonal antibodies (mAbs) against cytokeratin subtypes 8 and 18 (MAb CAM 5.2; Becton Dickinson, Mountain View, CA) and vimentin (MAb V9; Dakopatts, Glostrup, Denmark) and the demonstration of a uniform coexpression of both intermediate filaments. Passage 2 cultures were used for experiments.
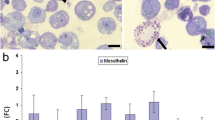
A Confluent human peritoneal mesothelial cells (HPMC) under control conditions. HPMC are organized in monolayers of flat polygonal cells with close cell–cell contacts giving a cobblestone appearance. Phase contrast microscopy ×200. B Confluent mesothelial cells after treatment with interleukin (IL)-1β (10 ng/ml) for 48 h. HPMC show retraction of the cytoplasms, loss of cell–cell contacts and extensive exposure of the submesothelial matrix. Phase contrast microscopy ×200. C HPMC under control conditions. Cells are flat and connected to each other by fine intercellular bridges. There is almost no exposure of the submesothelium. Scanning electron microscopy; bar 10 µm. D HPMC treated for 48 h with IL-1β (10 ng/ml). The cells reveal plump cellular processes and show a rounded, cuboidal shape. There is extensive exposure of the submesothelial matrix (stars). Scanning electron microscopy; bar=10 µm. E Omental specimen used for one of the experiments prior to isolation of HPMC. The mesothelium is flat and resting on a layer of submesothelial matrix. Hematoxylin & eosin ×40. F Omental specimen with cellular retraction and cuboidalization, presumably due to early inflammation. Note the similarity to the mesothelial changes depicted in B and D. The cells isolated from this specimen were not used for experiments
Treatment of HPMC with pro-inflammatory cytokines
For experiments, HPMC were detached with trypsine/versene (0.05%/0.02%), washed once in medium and seeded in 12.5-cm2 falcon flasks in a volume of 2.5 ml. After confluency, the cultures were washed twice with fresh culture medium and then incubated for 0–48 h in the absence or presence of the appropriate cytokines. Recombinant IL-1β and interferon (IFN)-γ were purchased from R&D Systems (Minneapolis, USA). TNF-α was obtained from Sigma (Munich, Germany). Incubation of cells with cytokines did not have any significant effect on cell viability as shown by trypan blue exclusion and a cell analyzer system CASY1 (Schärfe System, Reutlingen, Germany) and did not induce apoptosis as demonstrated by flow cytometry (see below). All experiments were done in duplicate.
Scanning electron microscopy
For scanning electron microscopy, HPMC were grown to confluency on glass slides placed in 12-well culture dishes and treated with the respective cytokines as described above. They were then fixed in 3% glutaraldehyde in 0.1 mol/l (molar) sodium cacodylate, pH 7.5 for 30 min, postfixed in 1% OsO4, in 0.1 mol/l cacodylate, rinsed in water, and dehydrated through graded ethanol. Thereafter, the specimens were critical-point dried from absolute ethanol in a critical point dryer using CO2 as transition medium. The dried preparations were coated with 20 nm gold in a sputter coater, and viewed on a Philips SEM 515 scanning electron microscope at 15 kv or 30 kv.
Analysis of adhesion molecule expression by fluorescence activated cell sorting
Analysis of adhesion molecules was conducted in cells that were incubated at the various culture conditions for 48 h. At the end of the incubation period, HPMC were washed in PBS and harvested by gentle treatment with 0.05% versene (Biochrom, Berlin, Germany) as described previously [2]. Cell numbers were determined by a cell counter and analyzer (CASY1). After centrifugation, cells were resuspended in PBS supplemented with 1% BSA. Thereafter, 100,000 cells were incubated with the appropriate monoclonal antibodies in a volume of 100 µl at 4°C for 30 min. Antibodies used were fluorescein-isothiocyanate (FITC)-conjugated mouse anti-human integrin α1 (CD49a), integrin α2 (CD49b), integrin α3 (CD49c), integrin α4 (CD49d), integrin α5 (CD49e), integrin α6 (CD49f), integrin β1 (CD29) and anti-CD44 from Serotec (Oxford, Great Britain; Table 1). A monoclonal antibody against human β4 integrin (CD104) was purchased from Calbiochem (Novabiochem, La Jolla, USA; Table 1). After incubation, the cells were washed three times in PBS, suspended in a volume of 200 µl PBS and stored at 4°C until fluorescence activated cell sorting (FACS) analysis. For analysis of integrin β4, the cells were incubated with anti-mouse rabbit IgG conjugated with FITC (Calbiochem-Novabiochem) at 4°C for 30 min at a dilution of 1:15. Immediately before FACS analysis, cells were stained with propidium iodide (20 µg/ml) to determine cell viability. To rule out apoptosis parallel samples (100,000 cells) for each probe were collected and apoptosis was determined according to Nicoletti et al. [22]. HPMC incubated with mouse anti-human IgG1-HCG as primary antibody and FITC-conjugated F(ab′)2 rabbit anti-mouse IgG as secondary antibody served as negative controls. Fluorescence intensity was calculated with the CellQuest Software (Becton Dickinson). Overlap of spectral signals when double staining the samples for viability (PI) and integrin/CD44 expression (FITC) was excluded by appropriate compensations. Since the yield of median fluorescence intensities differed between the donors of HPMC, results are expressed as percentage of controls that were set as 100%. Human umbilical vein endothelial cells were used as a control to analyze adhesion molecule expression. They were isolated as described previously [2]. For experiments they were treated in a similar fashion as HPMC.
Statistical analysis
All experiments were performed at least five times in duplicates. Statistical analysis was performed using the Student's t-test for paired data with Excel for MS Windows. Fluorescence intensities are presented as median values ± median absolute deviation in relation to controls, which were set as 100% [29]. A P value under 0.05 was considered significant.
Results
Disruption of the mesothelial cell integrity by IL-1β and TNF-α
Under control conditions, confluent HPMC formed a monolayer of flat polygonal, polarized cells exhibiting a typical cobblestone morphology. The cells were connected to each other by tight cell junctions and covered the whole submesothelial matrix without any visible intercellular clefts (Fig. 1A). These observations were also confirmed by scanning electron microscopy that revealed multiple fine cellular processes between the flat mesothelial cells (Fig. 1C).
Treatment of HPMC with the pro-inflammatory cytokines IL-1β or TNF-α resulted in a dramatic change of the morphology of the mesothelium and a disruption of its integrity. The cells revealed a retraction of their cytoplasms with loss of cell–cell contacts and a consecutive exposure of the submesothelial matrix. This phenomenon appeared to occur rapidly and in a time- and dose-dependent fashion. The maximal effect was seen at doses of 10 ng/ml of either IL-1β or TNF-α. At this dose, the first signs of cellular retraction became already evident after only 6 h of treatment. Over time, the morphological alterations developed in a progressive manner with a maximum effect after 24–48 h of incubation. At this time, the mesothelial cell monolayer showed a complete disorganization with loss of cellular polarization (Fig. 1B). The adhesion of the cells to the submesothelial matrix seemed to be only granted by residual thin finger-like cellular processes and there was a denudation of large areas of the submesothelial matrix (Fig. 1D). Similar, but less pronounced effects were already noticed at doses of 0.1 ng/ml of both pro-inflammatory cytokines. Doses of 0.01 ng/ml were, however, not effective in changing the morphology of the mesothelium. The effects induced by IL-1β and TNF-α were consistently observed in independent experiments in HPMC derived from ten different donors, both male and female. Treatment with IFN-γ was not associated with any structural alterations of the mesothelium.
Analyses of mesothelial adhesion molecules
The disruption of the structural integrity of the mesothelium by IL-1β and TNF-α was associated with a significant alteration of the expression of mesothelial adhesion molecules. Under control conditions, the mesothelial cells showed a distinct expression of the integrin subunits α2, β1, β4 and CD44 and a comparably lower expression of integrin subunits α1, α5 and α6. The integrin subunits α3 and α4 were not detectable.
Treatment of HPMC with IL-1β (10 ng/ml) for 48 h resulted in a significant increase of the expression of the integrins α1, α2, and α5 (Fig. 2A). The median fluorescence levels of the subunits α1, α2, and α5 were increased by 35.7±16.3% (P<0.05), 75.7±22.3 (P<0.01), and 28.7±6.5% (P<0.001), respectively. In addition, IL-1β induced a significant upregulation of the CD44 molecule with median fluorescence levels being 40.5±12.5% (P<0.001; Fig. 3) over control values. In contrast, IL-1β induced a loss of the expression of the laminin receptor α6 integrin. The respective median fluorescence intensity showed a significant reduction to 77±8.6% (P<0.01) of the control levels (Fig. 2A). The expression of β4 was also reduced by IL-1β, but this effect did not reach statistical significance. Interestingly, treatment with TNF-α (10 ng/ml) also led to a loss of the α6β4 integrin. Under these conditions, the median fluorescence levels of the α6 chain decreased to 79.3±4.8% (P<0.001) and of the β4 chain to 47.2±13.3% (P<0.01) of controls (Fig. 2B). As after IL-1β treatment, the integrin subunit α5 was also upregulated (+22.9±9.6%, P<0.01) after incubation with TNF-α (Fig. 2B). However, in contrast to IL-1β, TNF-α induced a decrease of the expression of the CD44-molecule (−18.1±7.0%, P<0.01; Fig. 3).
Regulation of the expression of mesothelial integrins by pro-inflammatory cytokines. The 10th, 25th, 50th (median), 75th and 90th percentiles of the percentage increase/decrease of the expression of α and β integrin subunits after treatment with interleukin (IL)-1 β (Α), tumor necrosis factor (TNF)-α (Β) or interferon (IFN)-γ (C) (each 10 ng/ml) are presented. Untreated controls were set as 100%. Results are derived from duplicate cultures of human peritoneal mesothelial cells isolated from five separate donors. *P<0.05, **P<0.01, ***P<0.001
Regulation of the expression of CD44 by pro-inflammatory cytokines. The 10th, 25th, 50th (median), 75th and 90th percentiles of the percentage increase/decrease of the expression of CD44 after treatment with interleukin (IL)-1β, tumor necrosis factor (TNF)-α and interferon (IFN)-γ are presented. Untreated controls were set as 100%. Results are derived from duplicate cultures of human peritoneal mesothelial cells isolated from five separate donors. **P<0.01, ***P<0.001
IFN-γ treatment of HPMC (10 ng/ml) resulted in a reduced expression of CD44 (−22.7±8.5%, P<0.01; Fig. 3) and a downregulation of the β4 chain (−25.0±8.0%, P<0.01; Fig. 2C). All other investigated mesothelial integrin subunits (α1–6, β1) remained unaffected by IFN-γ.
The downregulation of the α6β4 integrin and disruption of the integrity of the mesothelium by IL-1β and TNF-α was also associated with a marked decline of the adhesive properties of HPMC. Under control conditions, a 10-min incubation with collagenase-I was required to detach the HPMC from the submesothelial matrix. This time span was significantly reduced to 4 min and 6 min in cells pre-treated with IL-1β or TNF-α (data not shown).
Discussion
Disruption of the mesothelial integrity is a characteristic pathological phenomenon almost invariably occurring in disease processes of the abdominal cavity. Various pathogenic factors are thought to be involved. These include trauma and surgery, exposure to air and gases or the action of cytokines and free oxygen radical species released by invading inflammatory cells or tumor cells [3, 14, 21, 31, 38]. The detailed pathomechanisms are, however, still rather poorly understood. Recent studies have identified IL-1β and TNF-α as key regulators in the mesothelial response to peritoneal injury by "activating" mesothelial cells to increase their release of various cytokines [9, 19, 24, 25, 35, 36, 41].
In the present paper, we demonstrated that IL-1β and TNF-α can also cause profound morphological changes of the mesothelium, resulting in the loss of its integrity. The alterations induced by IL-1β and TNF-α were characterized by cellular retraction, loss of cellular polarization and wide-spread exposure of the submesothelial ECM and closely mimicked the alterations that can be observed in injured mesothelium in vivo (Fig. 1E, F). The morphological alterations were associated with marked alterations of the expression of mesothelial adhesion molecules and a significant reduction of the adhesive properties of the cells.
Our study provides the first analysis of the regulation of integrins and CD44 in HPMC by flow cytometry. In normal HPMC, we observed the expression of the β1 integrin subunits α1 and α2 (collagen and laminin receptors), α5 (fibronectin receptor), α6 (laminin receptor), and the integrin subunit β4 (laminin receptor). The α4 chain (fibronectin receptor) was not detectable. These data are in agreement with in vitro and in vivo studies recently published on resting peritoneal mesothelial cells [33, 40]. However, in contrast to our results, these studies additionally revealed expression of the integrin subunit α3 (collagen, fibronectin, and laminin receptor) a difference that may be due to different culture conditions or cell preparations used by these investigators.
The most remarkable alteration of the integrin-expression profile induced by IL-1β and TNF-α was the strong downregulation and loss of the laminin receptor subunits α6 and β4. The α6β4 integrin is a hemidesmosomal transmembrane molecule that is crucial for the maintenance of the basal cell-matrix adhesion of epithelial cell [1, 5, 10, 11, 28]. Our data thus support the hypothesis that both IL-1β and TNF-α may disrupt the mesothelial integrity by the downregulation of the α6β4 integrin [1, 10]. Molecular defects and/or a functional downregulation of the normal α6β4 expression have been well described in the epidermis and are thought to be responsible for the formation of blistering skin disorders and the epithelial detachment during keratinocyte maturation [5, 11, 28]. In the peritoneum, the downregulation of α6β4 may finally facilitate the mobilization and detachment of mesothelial cell from the submesothelial matrix. Interestingly, similar mechanisms have been suggested to be involved in the detachment of ovarian carcinoma cells that are derived from a modified mesothelium, i.e., the ovarian surface epithelium. Skubitz et al. [30] demonstrated that exfoliated ovarian carcinoma ascites cells show a loss of the α6β4 expression when compared to solid tumors and proposed that the floating tumor cells may be released from the basement membrane due to their deficit of α6 and β4 subunits.
The molecular mechanisms of the downregulation of the mesothelial cell α6 and β4 subunits are currently unclear but may involve a protein kinase C-dependent mobilization from the basolateral hemidesmosomes as described in other epithelial cells [1, 5, 10]. Detailed studies of the localization and potential redistribution of mesothelial integrins under the influence of IL-1β and TNF-α using confocal and two-photon laser-scanning microscopy would be helpful to further unravel these complex issues [40].
We have previously shown that IL-1β may be an important growth factor for exponentially growing HPMC [24]. Together with its potential exfoliating activity, IL-1β may thus be one of the key regulators of the suspension and proliferation of mesothelial cells in peritoneal effusions. In ascitic fluids, mesothelial cells are frequently found to show cellular clustering. This phenomenon may be enhanced by the concomitant upregulation of the mesothelial α5 fibronectin receptor subunits by IL-1β and TNF-α [8].
The disruption of the mechanical barrier function of the peritoneal mesothelium by IL-1β and TNF-α may be of critical importance for the initiation and progression of inflammatory and neoplastic peritoneal disease processes. The loss of the mesothelial cell junctions may lead to an increased loss of proteins into the peritoneal cavity and subsequently to an increased intraperitoneal oncotic pressure with formation of ascitic fluids [21, 27]. Both, IL-1β and TNF-α may further booster these effects by inducing HPMC to release VEGF and bFGF, which may, in turn, increase the submesothelial capillary permeability [9, 19]. These combined effects on the permeability of peritoneal mesothelial and endothelial cells may also facilitate the transmigration of inflammatory cells into the peritoneal cavity [4, 18, 20]. The loss of the non-adhesive properties of the peritoneal surface in conjunction with an IL-1β-induced secretion of the fibrogenic growth factors bFGF and TGF-β by HPMC may finally be a crucial step in the development of peritoneal adhesions and fibrosis [9, 15, 25].
In malignant peritoneal disease processes, the alterations of the mesothelium may profoundly affect the mechanisms of peritoneal tumor spread. We show that the mesothelial CD44 expression can be upregulated by IL-1β and downregulated by TNF-α and IFN-γ. The interaction of CD44 molecules with hyaluronic acid is supposed to be one of the most important mechanisms of the initial implantation of various cancers, e.g., ovarian cancer, gastric cancer, or pancreatic cancer, to the peritoneal mesothelium. This important step of the peritoneal tumor dissemination may be significantly modified by pro-inflammatory cytokines [13, 17, 23, 37]. However, the loss of the mechanical barrier function of the peritoneal cell lining may be even more important. Tumor cells generally adhere much faster and stronger to the ECM than to the intact mesothelium [17, 32]. The disruption of the mesothelium by IL-1β or TNF-α may thus facilitate the adhesion of floating tumor cells to the submesothelial ECM. In fact, this hypothesis is strongly supported by a recent in vivo model of peritoneal carcinosis in which damage to the mesothelium caused by a pneumoperitoneum resulted in an advanced peritoneal tumor spread and a significant decrease in animal survival [38].
The present data further support the concept that peritoneal mesothelial cells are key players in the regulation of peritoneal diseases. Detailed in situ analyses of the expression of integrins and CD44 in normal peritoneum and various peritoneal diseases will be of great interest.
References
Alt A, Ohba M, Li L, Gartsbein M, Belanger A, Denning MF, Kuroki T, Yuspa SH, Tennenbaum T (2001) Protein kinase C-delta-mediated phosphorylation of alpha6beta4 is associated with reduced integrin localization to the hemidesmosome and decreased keratinocyte attachment. Cancer Res 61:4591–4598
Amberger A, Maczek C, Jurgens G, Michaelis D, Schett G, Trieb K, Eberl T, Jindal S, Xu Q, Wick G (1997) Co-expression of ICAM-1, VCAM-1, ELAM-1 and Hsp60 in human arterial and venous endothelial cells in response to cytokines and oxidized low-density lipoproteins. Cell Stress Chaperones 2:94–103
Andreoli SP, Mallett C, Williams K, McAteer JA, Rothlein R, Doerschuk CM (1994) Mechanisms of polymorphonuclear leukocyte mediated peritoneal mesothelial cell injury. Kidney Int 46:1100–1109
Bittinger F, Schepp C, Brochhausen C, Lehr HA, Otto M, Kohler H, Skarke C, Walgenbach S, Kirkpatrick CJ (1999) Remodeling of peritoneal-like structures by mesothelial cells: its role in peritoneal healing. J Surg Res 82:28–33
Borradori L, Sonnenberg A (1999) Structure and function of hemidesmosomes: more than simple adhesion complexes. J Invest Dermatol 112:411–418
Cannistra SA, Kansas GS, Niloff J, DeFranzo B, Kim Y, Ottensmeier C (1993) Binding of ovarian cancer cells to peritoneal mesothelium in vitro is partly mediated by CD44. Cancer Res 53:3830–3838
Carter D, True L, Otis CN (1997) Serous membranes: histology for pathologists. In: Sternberg SS (ed) Lippincott–Raven, New York, pp 2299–2328
Casey RC, Burleson KM, Skubitz KM, Pambuccian SE, Oegema TR Jr, Ruff LE, Skubitz AP (2001) Beta 1-integrins regulate the formation and adhesion of ovarian carcinoma multicellular spheroids. Am J Pathol 159:2071–2080
Cronauer MV, Stadlmann S, Klocker H, Abendstein B, Eder IE, Rogatsch H, Zeimet AG, Marth C, Offner FA (1999) Basic fibroblast growth factor synthesis by human peritoneal mesothelial cells. Am J Pathol 155:1977–1984
Dowling J, Yu QC, Fuchs E (1996) Beta4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J Cell Biol 134:559–572
Giancotti FG, Ruoslahti E (1999). Integrin signaling. Science 285:1028–1032
Goodison S, Urquidi V, Tarin D (1999). CD44 cell adhesion molecules. Mol Pathol 52:189–196
Hosono J, Narita T, Kimura N, Sato M, Nakashio T, Kasai Y, Nonami T, Nakao A, Takagi H, Kannagi R (1998) Involvement of adhesion molecules in metastasis of SW1990, human pancreatic cancer cells. J Surg Oncol 67:77–84
Ikubo A, Morisaki T, Katano M, Kitsuki H, Anan K, Uchiyama A, Tanaka M, Torisu M (1995) A possible role of TGF-beta in the formation of malignant effusions. Clin Immunol Immunopathol 77:27–32
Klaus A, Margreiter R, Pernthaler H, Klima G, Offner FA (2003) Diffuse mesenterial sclerosis: a characteristic feature of chronic small-bowel allograft rejection. Virchows Arch 442:48–55
Kutteh WH, Kutteh CC (1992) Quantitation of tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 in the effusions of ovarian epithelial neoplasms. Am J Obstet Gynecol 167:1864–1869
Lessan K, Aguiar DJ, Oegema T, Siebenson L, Skubitz AP (1999) CD44 and beta1 integrin mediate ovarian carcinoma cell adhesion to peritoneal mesothelial cells. Am J Pathol 154:1525–1537
Li FK, Davenport A, Robson RL, Loetscher P, Rothlein R, Williams JD, Topley N (1998) Leukocyte migration across human peritoneal mesothelial cells is dependent on directed chemokine secretion and ICAM-1 expression. Kidney Int 54:2170–2183
Mandl-Weber S, Cohen CD, Haslinger B, Kretzler M, Sitter T (2002) Vascular endothelial growth factor production and regulation in human peritoneal mesothelial cells. Kidney Int 61:570–578
Margetts PJ, Kolb M, Yu L, Hoff CM, Holmes CJ, Anthony DC, Gauldie J (2002) Inflammatory cytokines, angiogenesis, and fibrosis in the rat peritoneum. Am J Pathol 160:2285–2294
Nagy JA, Morgan ES, Herzberg KT, Manseau EJ, Dvorak AM, Dvorak HF (1995) Pathogenesis of ascites tumor growth: angiogenesis, vascular remodeling and stroma formation in the peritoneal lining. Cancer Res 55:376–385
Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C (1991) A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods 139:271–279
Nishimura S, Chung YS, Yashiro M, Inoue T, Sowa M (1996) CD44H plays an important role in peritoneal dissemination of scirrhous gastric cancer cells. Jpn J Cancer Res 87:1235–1244
Offner FA, Obrist P, Stadlmann S, Feichtinger H, Klingler P, Herold M, Zwierzina H, Hittmair A, Mikuz G, Abendstein B, Zeimet A, Marth C (1995) IL-6 secretion by human peritoneal mesothelial and ovarian cancer cells. Cytokine 7:542–547
Offner FA, Feichtinger H, Stadlmann S, Obrist P, Marth C, Klingler P, Grage B, Schmahl M, Knabbe C (1996) Transforming growth factor-β synthesis by human peritoneal mesothelial cells. Induction by interleukin-1. Am J Pathol 148:1679–1688
Propst T, Propst A, Herold M, Schauer G, Judmaier G, Braunsteiner H, Stoffler G, Vogel W (1993) Spontaneous bacterial peritonitis is associated with high levels of interleukin-6 and its secondary mediators in ascitic fluid. Eur J Clin Invest 23:832–836
Runyon BA, Hillebrand DJ (1998) Surgical peritonitis and other diseases of the peritoneum, mesentery, momentum, and diaphragm. In: Feldman M, Scharschmidt BF, Sleisenger MH (eds) Gastrointestinal and liver disease. Pathophysiology, diagnosis, management. Saunders, Philadelphia, pp 2035–2046
Ruoslahti E, Vaheri A (1997). Cell-to-cell contact and extracellular matrix. Curr Opin Cell Biol 9:605–607
Sachs L (1997) Angewandte statistik. In: Sachs L (ed) Anwendung statistischer methoden. Springer, Berlin Heidelberg New York, pp 335–337
Skubitz AP, Bast RCJr, Wayner EA, Letourneau PC, Wilke MS (1996) Expression of alpha 6 and beta 4 integrins in serous ovarian carcinoma correlates with expression of the basement membrane protein laminin. Am J Pathol 148:1445–1461
Sriram PS, Mohammed KA, Nasreen N, Hardwick J, Van Horn R, Sanders K, Antony VB (2002) Adherence of ovarian cancer cells induces pleural mesothelial cell (PMC) permeability. Oncol Res 13:79–85
Strobel T, Cannistra SA (1999) Beta1-integrins partly mediate binding of ovarian cancer cells to peritoneal mesothelium in vitro. Gynecol Oncol 73:362–367
Tietze L, Borntraeger J, Klosterhalfen B, Amo-Takyi B, Handt S, Gunther K, Merkelbach-Bruse S (1999) Expression and function of beta(1) and beta(3) integrins of human mesothelial cells in vitro. Exp Mol Pathol 66:131–139
Topley N (1995) The cytokine network controlling peritoneal inflammation. Perit Dial Int 15:35–40
Topley N, Joerres A, Luttmann W, Petersen MM, Lang J, Thierauch KH, Muller C, Coles GA, Davies M, Williams JD (1993) Human peritoneal mesothelial cells synthesize interleukin-6: induction by IL-1β and TNFα. Kidney Int 43:226–233
Topley N, Brown Z, Joerres A, Westwick J, Davies M, Coles GA, Williams JD (1993) Human peritoneal mesothelial cells synthesize interleukin-8: synergistic induction by interleukin-1 beta and tumor necrosis factor-alpha. Am J Pathol 142:1876–1886
Van Rossen ME, Hofland LJ, van den Tol MP, van Koetsveld PM, Jeekel J, Marquet RL, van Eijck CH (2001) Effect of inflammatory cytokines and growth factors on tumour cell adhesion to the peritoneum. J Pathol 193:530–537
Volz J, Volz-Koster S, Kanis S, Klee D, Ahlert C, Melchert F (2000) Modulation of tumor-induced lethality after pneumoperitoneum in a mouse model. Cancer 89:262–266
Whitaker D, Papadimitriou J (1985) Mesothelial healing: morphological and kinetic investigations. J Pathol 145:159–175
Witz CA, Montoya-Rodriguez IA, Miller DM, Schneider BG, Schenken RS (1998) Mesothelium expression of integrins in vivo and in vitro. J Soc Gynecol Invest 5:87–93
Zeimet AG, Widschwendter M, Knabbe C, Fuchs D, Herold M, Muller-Holzner E, Daxenbichler G, Offner FA, Dapunt O, Marth C (1998) Ascitic interleukin-12 is an independent prognostic factor in ovarian cancer. J Clin Oncol 16:1861–1868
Acknowledgements
The excellent technical assistance of Mrs. I. Jehart is gratefully acknowledged. We would also like to thank Mr. J. Pollheimer for helpful comments on statistics.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stadlmann, S., Raffeiner, R., Amberger, A. et al. Disruption of the integrity of human peritoneal mesothelium by interleukin-1β and tumor necrosis factor-α. Virchows Arch 443, 678–685 (2003). https://doi.org/10.1007/s00428-003-0867-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-003-0867-2