Abstract
GRAS family transcription factors (TF) are involved in multiple biological processes in plants. In recent years among the 54 identified GRAS proteins, only few have been studied functionally in tomato (Solanum lycopersicum). In the present study, a novel and previously uncharacterized member of tomato GRAS transcription factors family SlGRAS15 was isolated and functionally characterized. It was observed that SlGRAS15 preferably expressed in roots, followed by young leaves, stem, and comparatively low transcripts levels were noticed in all other tissues. To explore the SlGRAS15 function in detail, an RNA interference (RNAi) vector targeting SlGRAS15 was constructed and transformed into tomato plants. The transgenic plants carrying SlGRAS15-RNAi displayed pleiotropic phenotypes associated with multiple agronomical traits including reduced plant height and small leaf size with pointed margins, increased node number, lateral shoots, and petiolules length. In addition, transcriptional analysis revealed that silencing SlGRAS15 altered vegetative growth by downregulating gibberellin (GA) biosynthesis genes and stimulating the GA deactivating genes, thus lowering the endogenous GA content in tomato transgenic lines. Moreover, the GA signaling downstream gene (SlGAST1) was downregulated but the negative regulator of GA signaling (SlDELLA) was upregulated by SlGRAS15 silencing. The root and hypocotyl length in SlGRAS15-RNAi lines showed reduced growth under normal conditions (Mock) as compared with the wild type (WT) control plants. Taken together, these findings enhanced our understanding that suppression of SlGRAS15 lead to a series of developmental processes by modulating gibberellin signaling and demonstrate an association between the SlGRAS15 and GA signaling pathway during vegetative growth in tomato.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
GRAS proteins are plant-specific transcription factors that play critical roles in plant development and signal transduction pathways, including gibberellin biosynthesis and signaling (Lee et al. 2002), auxin signaling (Gao et al. 2004; Sánchez et al. 2007), phytochrome signaling (Torres-Galea et al. 2006), lateral shoot formation (Li et al. 2003), and gametogenesis (Morohashi et al. 2003). Typically, members of the GRAS (named after GAI, RGA, SCR) family show high sequence similarity to each other in their C-terminus, where motifs comprising of LHRI, LHRII, PFYRE, VHIID, and SAW can be seen (Bolle 2004; Pysh et al. 1999). In distinction, N-terminus of GRAS TF differs in sequence and length, which appears like the foremost contributor to the specific function of every gene (Sun et al. 2011; Tian et al. 2004).
SlGRAS15 belongs to SCR subfamily and is more closely correlated to AtSCR in Arabidopsis, StSCR in Solanum tuberosum, and CaSCRL in Capsicum annum than any other protein, but the function of these three proteins except SlGRAS15 is well known (Cui et al. 2007). Tomato GRAS proteins genome wide analysis has identified 54 GRAS proteins (Niu et al. 2017). However, only eight GRAS proteins that have been functionally characterized, which comprise of SlLS, SlDELLA, SlGRAS2, SlGRAS6, SlGRAS26, SlGRAS24, SlGRAS38, SlGRAS7, and SlGRAS40. Upregulation of two homologous genes SlGRAS24 and SlGRAS40, belongs to HAM subfamily in Arabidopsis developed same phenotypes, such as dwarf plant height, delay in flowering time, fewer number of flowers, lower seed number, and lower percentage of fruit set (Huang et al. 2017; Liu et al. 2017). The SlFSR (SlGRAS38) controls fruit shelf life by altering the fruit cell wall metabolism (Zhang et al. 2018), while downregulation of SlGRAS26 play a vital role in the initiation of lateral and inflorescence meristems in tomato (Zhou et al. 2018).
As an important growth regulator, gibberellin (GA) plays an essential role in plant growth and development processes including endosperm mobilization, seed germination, stem elongation, root development, leaf expansion, flowering, pollen maturation, and fruit set (Claeys et al. 2014; Hedden and Phillips 2000). Recently, many studies contribute to the understanding of GA biosynthesis. For instance, severe GA biosynthesis defective mutants (such as the deletion of the GA1 gene in ga1-3) show defects in development, and gibberellin deficient dwarf phenotypes are restored to WT by applying exogenous gibberellic acid (GA3) (Magome et al. 2004; Olimpieri et al. 2011). In comparison, mutants impaired in gibberellin signaling present similar phenotypes but these are not rescued by exogenous GA3 (Harberd et al. 2009).
The function of GRAS genes has been well studied in various plants species, it is essential to study the role of all other GRAS proteins in tomato. The GRAS24 gene is involved in downstream GA signaling (Huang et al. 2016), while GRAS2 function in the upstream GA biosynthesis (Li et al. 2018). This different role of GRAS24 and GRAS2 in GA signaling pathway further confirms the importance of GRAS gene in GA stability. The numerous functions of gibberellin are a result of the periodic and spatial regulation of gibberellin biosynthesis, perception, and subsequent signal transduction (Olszewski et al. 2002). AtSCL3 acts as an integrator of DELLAs and SHR-SCR, a complex in which two types of GRAS proteins mediates GA dependent elongation of cells in the root endodermis. The SHR-SCR complex combined with auxin influx carriers LAX3 and AUX1 has synergetic effects on primary/lateral root development in Arabidopsis (Della Rovere et al. 2015).
Tomato is a very important crop for human diet and model species due to its significance as the availability of molecular tool and efficient transformation. Arabidopsis sleepy1 (sly1) and potato photoperiod responsive 1 (phor1) are some GA-deficient mutants, which are insensitive to exogenous GA application due to the deficiency in the GA signal transduction pathway (Amador et al. 2001; McGinnis et al. 2003). Though the rest of GA-deficient mutants responsible for the inhibition of GA biosynthesis pathway, such as semi dwarf 1 (sd1), can be restored by the GA application (Neeraja et al. 2009). In tomato, a putative DELLA mutant procera (pro) displays a well-characterized constitutive GA response phenotype (Bassel et al. 2008) and homeostatic GA responses are regulated by both DELLA-dependent and independent pathways (Livne et al. 2015). Identifying the role of other GRAS family genes in crops like tomato would help to clarify the molecular mechanisms regulating developmental process and possibly assist the breeding of efficient varieties.
Here, we report the function of SlGRAS15 (Solyc10g074680), a member of SCR subfamily and aimed to discover more about its function in tomato plant development. The analysis of expression profile showed that SlGRAS15 is highly expressed in young leaves, stem, and root. Moreover, the expression level of SlGRAS15 can be increased by external application of GA, ABA, and IAA. The promoter of SlGRAS15 was analyzed and found to be associated with both gibberellin- and auxin-related cis-acting elements. To further apprehend the role of SlGRAS15 in tomato growth and development, we prepared a specific RNAi vector to generate SlGRAS15-RNAi lines, and pleiotropic phenotypes associated with multiple agronomical traits were observed. The short height of plants was rescued by exogenous application of GA3 further supporting the observation that SlGRAS15 transgenic lines are GA sensitive. Phenotypic and gene expression analyses are reported here to disclose the probable bases of all these phenotypes, which would help to increase our knowledge of GRAS proteins in the regulation of plant growth and developmental processes.
Materials and methods
Plant materials and growth conditions
In this study the WT tomato (Solanum lycopersicum Mill. cv. Ailsa Craig AC++) and transgenic lines were grown in a controlled greenhouse standard condition (25 °C/18 °C temperature day/night, day night cycle 16 h/8 h, light intensity of 250 μmol m−2 s−1, and humidity 80%) managed and watered regularly. For experiments of tissue culture, seeds of tomato were surface sterilized using sodium hypochlorite solution, then immersed and shaked in sterilized distilled water up to 3 days to germinate and sowed on 1/2 MS solid agar medium (pH ¼ 5.8). Transgenic tomato lines of the first generation (T0) came from tissue culture, second generation (T1), and third generation (T2) from seeds respectively, were used in this study.
For tissues/organs specific expression profiling of SlGRAS15 gene, roots, stems, young leaves, mature leaves, senescent leaves, flowers (tagged at anthesis), sepals, and tomato fruits of five developmental stages were collected. The fruit at following development stages were collected; IMG (immature green at 20 days post anthesis (DPA), MG (mature green at 35 DPA), B (breaker with the start of change in color from green to yellow), B4 (4 days after breaker stage), and B7 (7 days after breaker) (Xie et al. 2014). The fifth leaf from the top and young stem of the consistent developmental stage of 90 days old plants were collected and used for analysis of gene expressions. All the tissues were gathered in the start of July, flowers were collected early in the morning while rest of the tissues were collected at noon, frozen in liquid nitrogen immediately and stored at − 80 °C until required.
Sequence, structure, and phylogenetic analysis
The cDNA and protein data of SlGRAS15 gene were retrieved from NCBI (http://www.ncbi.nlm.nih.gov/) and Sol Genomics Network (SGN) (http://solgenomics.net/). The DNAMAN 6.0 software was used to carry out multiple sequence alignments of SlGRAS15 with other proteins, while Scan-Prosite (http://prosite.expasy.org/scanprosite/) was used for the annotation of conserved functional domains. For phylogenetic study, the MEGA 6.06 software (http://megasoftware.net/) was used. A total of 18 GRAS peptide sequences were used to construct an unrooted neighbor-joining phylogenetic tree with the bootstrap set at 1000 replicates. GenBank and Sol Genome Network genes accession numbers for multiple sequence alignment and phylogenetic study are as follow: SlGRAS15 (Solyc10g074680), StSCR (XM_006365258.2), CaSCRL (XM_016690185.1), NtSCRL (XM_016630625.1), AtSCR (NM_115282.4), BnSCR-like (XM_022708083.1), GmSCR (XM_003551583.4), OsSCR1-like (XM_015759916.2), SlGRAS43 (Solyc09g066450.1), SlGRAS38 (Solyc08g080400.1.1), AtSCL4 (Solyc02g085340.1.1), SlGRAS2 (Solyc07g063940.1.1), AtPAT1 (Solyc11g012510.1.1), SlLS (Solyc07g066250.1.1), SlDELLA (Solyc11g011260.1.1), ZmGA1-like (NM_001319732.2), SlGRAS24 (Solyc01g090950.2.1), SlGRAS37 (Solyc08g078800.1.1), and SlGRAS40 (Solyc08g078800.1). For the analysis of putative cis-elements in the promoter sequence of SlGRAS15 (2000 bp at 5′ upstream end) were predicted against the promoter database PLACE (http://www.dna.affrc.go.jp/PLACE/signalscan.html) (Higo et al. 1999) (Table S2).
Plasmid construction and plant transformation
For SlGRAS15-RNAi plasmid construction, the pBIN19 vector was used. A 429-bp-long fragment of SlGRAS15 DNA at the mRNA 5′-end was used in the hairpin and amplified by using the SlGRAS15-F/R specific primers (Table S1). The construct was transformed into tomato cv. (AC++) Ailsa Craig by Agrobacterium tumefaciens (strain LBA4404) (Chen et al. 2004). MS medium containing kanamycin was used to screen positive transformed lines, which were analyzed by genomic PCR to determine the existence of T-DNA using the primers NPTII-F/R (Table S1).
Plant treatments
The tomato seedlings at five leaf stage were used for treatments and each treatment is repeated three times. WT seedlings of tomato were sprayed with IAA 50 μM, GA3 50 μM, ABA 100 μM, or distilled water used as control (mock). After treatment the third leaves of the seedlings at 0, 1, 2, 4, 8, 12, and 24 h were harvested, instantly kept in liquid nitrogen and stored at − 80 °C. The dwarf transgenic plants were sprayed with 100 μM GA3 on every 3rd day up to 2 weeks, starting from 30 days after sowing while the non-treated plants were used as control.
Quantitative real-time PCR analysis
The total RNA was extracted from various tissues of WT and SlGRAS15-RNAi lines (T2) by using RNAiso Plus (Takara) following manufacturer’s instructions. For reverse transcription reaction we used 1 μg of RNA and the M-MLV reverse transcriptase kit (Promega). The GoTaq qPCR Master Mix (Promega) and the CFX96™ Real-Time System (Bio-Rad) were used for qRT-PCR analysis. An internal reference gene the Clathrin Adaptor Complex, SlCAC (accession number: SGN-U314153) (Expósito-Rodríguez et al. 2008; González-Aguilera et al. 2016) was used in this study for data normalization. All the experiments were performed in triplicate. The specific primers of every gene being used for qRT-PCR are detailed in Table S1.
Measurement of plant architecture parameters
To relate the morphological variances of RNAi lines and WT, several parameters, such as height of plant, length, and width of leaf, leaf shape index (length to width ratio), internode length, petiolules length, and branches number (≥ 0.5 cm) were analyzed. Moreover, height of the plant was measured at the 20th day after germination and once each week up to 2 months. For all parameters of RNAi lines and WT, a minimum of ten plants were used from each line.
Histological observation
Leaf and mature apical leaflets from the similar position under the first inflorescence of WT and SlGRAS15-silenced lines were harvested and dissected. For root microscopy, transverse section of primary root from WT and SlGRAS15-RNAi lines were selected. The harvested samples were instantly fixed by 70% FAA (ethanol/acetic acid/formaldehyde 18:1:1) followed by dehydration, fixation, sectioning, dewaxing, and staining with safranin and fast green. Then analyzed under a light microscope (OLYMPUS IX71, Japan) and photographs were captured.
GA3 contents quantification
Mature leaves of WT and SlGRAS15-RNAi lines were collected and frozen immediately in liquid nitrogen for GA3 content determination. For the extraction and purification of gibberellin (GA3) gibberellin (GA3) kit (GA-4-Y Comin Biotechnology Co., Ltd., China) was used following manufacturer’s guidelines. The High Performance Liquid Chromatography (HPLC) analyses were performed to measure the total volume of biologically active endogenous gibberellin (GA3) in the mature leaves of WT and SlGRAS15-RNAi lines.
Statistical analysis
The mean values of data were evaluated from three replicates and standard error (SE) of the means was calculated. Statistical analysis of data was performed using Sigmaplot 12.1. (SYSTAT and MYSTAT Products, USA, and Canada) and t test (SAS 9.2) was used for measuring significant differences between the means.
Results
SlGRAS15 is a member of SCR subfamily in tomato
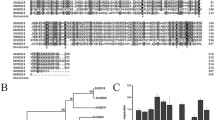
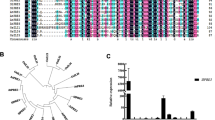
To explore the potential function of SlGRAS15 gene in tomato, we isolated a putative SlGRAS15 gene from WT tomato fruits based on a cDNA clone. The analysis of nucleotide sequence showed that SlGRAS15 was consistent with the cDNA clone, contained an ORF (open reading frame) of 2481-bp-long coding an 827 amino acid putative protein having a typical region of GRAS domain at their C-terminus. To examine the GRAS domain of SlGRAS15 gene in more detail, the full length amino acid sequence of 18 proteins including SlGRAS15, StSCR, CaSCRL, NtSCRL, AtSCR, BnSCR-like, GmSCR, OsSCR1-like, SlGRAS43, SlGRAS38, AtSCL4, SlGRAS2, AtPAT1, SlLS, SlDELLA, ZmGA1-like, SlGRAS24, SlGRAS37, and SlGRAS40 were considered for multiple sequence alignments (Fig. S1). The results showed five conserved motifs are present including LHRI, LHRII, VHIID, PFYRE, and SAW at their C-terminus. Though, these proteins vary in sequence and length at N-terminus. To study phylogenetic relationships among GRAS proteins in Arabidopsis, tomato, and other plant species, we used the amino acid sequences to construct an unrooted neighbor-joining phylogenetic tree (Fig. 1a). From the analysis of the phylogenetic tree, our newly identified SlGRAS15 protein belonged to the growth-related SCR subfamily of GRAS proteins according to their relative functions. Additionally, the results also showed that most of the SCR proteins with similar functions could be classified into the same subgroups on the basis of similarities in their GRAS domains, thus indicating that it may also have a crucial role in modulating gibberellin signaling.
Phylogenetic analysis and relative endogenous expression of SlGRAS15 in various tissues at different development stages in wild type (WT) tomato plants. a Phylogenetic analysis of SlGRAS15 with other proteins was constructed by the neighbor-joining approach by MEGA 6.06. Values above the branches are bootstrap percentages analysis of 1000 replicates. Sl (Solanum lycopersicum), St (Solanum tuberosum), Ca (Capsicum annuum), Nt (Nicotiana tabacum), At (Arabidopsis thaliana), Bn (Brassica napus), Gm (Glycine max), Os (Oryza sativa), and Zm (Zea mays). b The expression pattern of SlGRAS15 in WT tomato. RT, root; ST, stem; YL, young leaf; ML, mature leaf; SL, senescent leaf; SE, sepal of flower in anthesis; F, flower; IMG, immature green fruit; MG, mature green fruit; B, breaker fruit; B4, 4 days after breaker fruit; B7, 7 days after breaker fruit. The relative expression levels in ST were set to 1. Each value represents the mean ± SD of three independent biological replicates (n = 3). Asterisks indicate a significant difference (P < 0.05) between ST and other tissues
Expression pattern analysis of SlGRAS15 gene in different tomato tissues
To further understand the role of SlGRAS15 gene in tomato growth and development, the transcript accumulation of SlGRAS15 gene was analyzed by qRT-PCR in different plant parts, including vegetative tissues such as young leaves (YL), mature leaves (ML), senescent leaves (SL), stems (ST), roots (RT), and reproductive tissues including sepals (SE), flowers (FL), and fruits at different developmental stages such as immature green (IMG), mature green (MG), Breaker (B), 4 days after breaker (B4), and 7 days after breaker (B7) (Fig. 1b). The results revealed that SlGRAS15 gene shows high expression in roots, followed by young leaves, stem, and comparatively low transcripts levels were detected in all remaining tissues, which reveals the tissue specific expression in tomato. This gradual ascending expression of SlGRAS15 in tomato indicates that SlGRAS15 may contribute in the development of roots, leaf, and stem but not in developing fruits.
SlGRAS15 contributes to morphogenesis during vegetative growth in tomato
To ascertain the role of SlGRAS15 in tomato, a 429-bp fragment of SlGRAS15 near the N-terminal was cloned to suppress the endogenous expression levels of SlGRAS15 through RNAi silencing. Nine transgenic independent RNAi lines were obtained. The silencing efficiency of SlGRAS15 transgenic line 1, 3, and 4 were 79%, 85%, and 95% respectively, and were labeled as RNAi1, RNAi3, and RNAi4 (Fig. 2a) and were selected for further study. Under the same growth conditions these RNAi lines showed the same phenotypes in vegetative growth and development when observed visually. The height and length of first four internodes were measured. We observed that SlGRAS15-RNAi lines showed a reduction in plant height compared with the WT plants (Fig. 2b–d).
Phenotypes and expression analysis of SlGRAS15 in wild type (WT) and transgenic lines. a The expression levels of SlGRAS15 in transgenic and WT plants, examined by real-time qRT-PCR, using young leaves of the plants as tissue sample. The expression data of WT plants were normalized to 1. b The growth rate of WT and SlGRAS15-RNAi lines. The plants height was measured every 7 days starting from 20 days to 2 months. c The SlGRAS15-RNAi plants (RNAi1, RNAi3, and RNAi4) were dwarfed; the picture was taken at 2 months after colonization. d Vertical view of WT and transgenic plants. Each value represents the mean ± SD of three independent biological replicates (n = 3). Bar = 1 cm. Asterisks indicate a significant difference (P < 0.05) between WT and transgenic lines
The number of nodes and mature compound leaves number were significantly increased while length and width of mature leaflets decreased in SlGRAS15-RNAi lines as compared with the WT. However, the leaf shape indexes remained unchanged. The length of petiolules in SlGRAS15-RNAi lines were all longer than WT plants, while the 4 internodes length in SlGRAS15-RNAi line were smaller than WT plants. This demonstrates that the dwarf transgenic lines depicted the shortening of internodal length (Fig. 3a–h). Besides, in SlGRAS15-RNAi lines mature leaves were smaller in size than WT, we also observed that the margins of leaflets of transgenic lines were pointed (Fig. 3a). Two genes GOB (Goblet) (Berger et al. 2009) and NAM2 (Hendelman et al. 2013) of NAC TF family are considered to be involved in the maintenance and establishment of leaf margins. Therefore, we measured the expression of these two genes and significant increase in the expression levels of these genes were observed in RNAi lines than in WT (Fig. S2). Furthermore, microscopic analyses of the transverse section of leaf of RNAi lines displayed reduced leaf thickness, which can be attributed to the spongy mesophyll that seems thicker in the WT compared with the RNAi line. The spongy mesophyll of RNAi line contain less cell layers and are more disorganized than in the WT (Fig. 4a, b). In addition, microscopic study of roots showed that SlGRAS15-RNAi lines also exhibited less cell layers and more disorganized structure as compared with WT plants (Fig. 4c).
Phenotypes of wild type (WT) and SlGRAS15-silenced lines. a Phenotypes of leaf number, petiolules length, and leaf size of WT and SlGRAS15-RNAi lines. b Node number of WT and SlGRAS15-RNAi lines. c Number of leaves starting from 30 to 50 days of SlGRAS15-RNAi lines and WT plants. d Leaf lenght. e Leaf width of WT and SlGRAS15-silenced lines. The fourth leaf from the top of 2-months-old transgenic and non-transgenic plants was used for leaf size measurement. f Leaf shape index of WT and SlGRAS15-RNAi lines. g Length of petiolules from SlGRAS15-RNAi lines and WT plants. h Node length of SlGRAS15-RNAi lines and WT plants. Each value represents the mean ± SD of three independent biological replicates (n = 3). Bar = 1 cm. Asterisks indicate a significant difference (P < 0.05) between WT and transgenic lines
Microscopy of leaf and roots from wild type (WT) and SlGRAS15-RNAi lines. a Transverse section of leaf from WT (left) and RNAi transgenic line (right); bars = 9 μm. b Cell layers from WT (left) and RNAi transgenic line (right); bars = 9 μm. The samples were prepared from 60-days-old plants. c Transverse section of root from WT (left) and RNAi transgenic line (right). The samples were prepared from 8-days-old seedlings
Effect of various phytohormones on expression of SlGRAS15 gene
The plant growth hormones play vital roles in various environmental signals and developmental processes. Therefore, the transcript accumulation level of SlGRAS15 under various hormones treatments was analyzed by qRT-PCR. The qRT-PCR analysis revealed that the transcript accumulation of SlGRAS15 was significantly increased by exposure to ABA in all treatments except at 2 h, but exhibiting a peak at 24 h; IAA induced SlGRAS15 expression significantly in all treatments but peaked at 12 h. Moreover, the GA3 treatment reduced SlGRAS15 expression in all treatments except at 12 h and 24 h where its transcript accumulation was significantly induced (Fig. 5a–c). The transcript accumulation of SlGRAS15 was reduced by ACC (ethylene precursor) but showed significant increase only at 12 h stage. Indicating that downregulation of SlGRAS15 weakens the responsiveness to ethylene. In addition, the MeJA reduced SlGRAS15 expression in all treatments except at 2 h and 8 h where its transcript accumulation was significantly induced (Fig. 5d, e). These findings suggest that SlGRAS15 may be involved in phytohormones biosynthesis or signaling.
Expression profile analysis of SlGRAS15 in wild type (WT) tomato plants by qRT-PCR under various phytohormone treatments. The response of SlGRAS15 expression to phytohormones a ABA, b IAA, c GA3, d ACC, and e MeJA. The relative expression levels were normalized to 1 in untreated (mock) (sprayed with distilled water) plants (0 h). Each value represents the mean ± SE of three independent biological replicates (n = 3). Asterisks indicate a significant difference (P < 0.05) between untreated plants and treated plants
To further understand the sensitivity of SlGRAS15-RNAi lines to plant growth hormones, exogenous ABA, IAA, GA3, ACC, and MeJA were applied to WT and SlGRAS15-RNAi seedlings to investigate whether downregulation of SlGRAS15 altered other aspects of ABA, IAA, GA3, ACC, and MeJA dependent plant growth. The SlGRAS15-RNAi lines showed significant reduction in hypocotyl length except the RNAi1 line which showed non-significant reduction in hypocotyl length under normal conditions (Mock) as compared with the WT control plants. While the root length in SlGRAS15-RNAi lines were non-significantly reduced under normal conditions (Mock) compared with the WT (Fig. S3). In the presence of 2 and 5 μM ABA treatment, hypocotyl and root growth was inhibited in SlGRAS15-RNAi seedlings than the WT plants. The exposure of SlGRAS15-RNAi seedlings to 5 and 10 μM IAA caused slightly short hypocotyl and root length than WT seedlings, indicating that seedlings of SlGRAS15-RNAi lines were somehow sensitive to auxin when SlGRAS15 was downregulated. Similarly, SlGRAS15-RNAi lines were grown on MS media supplemented with 2 and 5 μM GA3 inhibited the hypocotyl and root growth as compared with the WT, reducing the initial difference in length (Fig. S3). These results suggest that SlGRAS15 is involved in ABA, IAA, and GA3, dependent pathways to regulate plant growth and development. Moreover, the transcript accumulation of SlGRAS15 was in part regulated by ABA, IAA, and GA3. Analysis of promoter sequence confirmed that there are various cis-elements related to plant hormones present in the promoter region of SlGRAS15 gene, including ABA, IAA, GA3, MeJA, and Ethylene responsive (Table S2). The presence of these cis-elements showed that they have possible and important function in phytohormones response (Fig. 5). Our study emphasized that the SlGRAS15 expression is hormone inducible and produce a signaling cascade during growth and development in tomato plants.
SlGRAS15-RNAi lines exhibits increased lateral shoot number in tomato
Besides the dwarf plant and small leaf, we also observed that the number of lateral shoots in SlGRAS15-RNAi lines was much higher than that of WT, weaker apical dominance and reduced plant height may also be attributed to the higher lateral shoots number. To find out the possible reason for this phenotype of RNAi lines, we studied the expression level of two genes Blind (SlBL) (Schmitz et al. 2002) of class R2R3 from Myb TF family act as a positive regulator in the development of lateral meristems and BRC1b/TCP8 (Martin-Trillo et al. 2011) related to the development of axillary shoots, in WT and SlGRAS15-RNAi lines. The SlBL gene expression level is induced in SlGRAS15-RNAi lines while the SlBRC1b, a TCP transcription factor family member controls cell proliferation and elongation in the lateral organs and meristems of tomato (Martin-Trillo et al. 2011) were significantly downregulated in transgenic lines (Fig. S2).
Silencing SlGRAS15 regulates the transcripts accumulation of gibberellins-related genes
To find out the effect of SlGRAS15 silencing on gibberellin metabolism, the expression level of GA biosynthetic genes in RNAi lines and WT stems were determined. Two genes CPS and KAO in the GA biosynthetic pathway involved in the preliminary steps of GA biosynthesis were significantly downregulated in the SlGRAS15-RNAi lines (Fig. 6a, b). Furthermore, GA3oxs are able to catalyze the last step to crop bioactive endogenous GAs (GA1, GA3, and GA4) (Hedden and Kamiya 1997; Yamaguchi 2008). In SlGRAS15 silenced lines, the transcript accumulation of GA3ox1 and -2 was significantly reduced, indicating that the GA biosynthetic pathway was inhibited in the transgenic plants (Fig. 6c, d). The expression level of SlGA2ox1, and -2 coding for GA2oxs (the central GA catabolic enzyme) were assessed by qRT-PCR. These two genes were induced in the SlGRAS15-RNAi lines as compared with WT plants (Fig. 6e, f). This revealed that downregulation of SlGRAS15 might halt the GA biosynthesis pathway but stimulates the pathway involved in GA inactivation resulting in GA deficiency. However, the transcript level of DELLA protein, a negative regulator of gibberellin signaling pathway, was increased in RNAi lines (Fig. 6g). Expression levels of tomato gibberellin stimulated transcripts1 (SlGAST1); a GA responsive downstream gene (Ding et al. 2013) in tomato was significantly suppressed in RNAi lines (Fig. 6h). This showed that downregulation of SlGRAS15 alters GA biosynthesis, signal transduction, and its catabolism.
Transcript levels of genes involved in GA biosynthesis, catabolism, and signal transduction pathway in wild type (WT) and SlGRAS15-silenced plants. a–h Expression levels of SlCPS, SlKAO, SlGA3ox1, GA3ox2, SlGA2ox1,SlGA2ox2, SlDELLA, and SlGAST1 in WT and transgenic lines, respectively. Each value represents the mean ± SD of three independent biological replicates (n = 3). Asterisks indicate a significant difference (P < 0.05) between WT and transgenic lines
Bioactive endogenous GA3 contents in WT and SlGRAS15-RNAi lines were also measured, the results confirmed that GA3 contents was decreased in SlGRAS15-RNAi lines than WT (Fig. 7a), indicating that the dwarf phenotypes may be due to reduced endogenous bioactive GA3. To further confirm this notion, exogenous application of 100 μM GA3 on every 3rd day for 2 weeks were adopted to recover the dwarf plant phenotypes of SlGRAS15-RNAi lines and confirmed that RNAi plant were able to restore their height to WT levels (Fig. 7b, c).
Active GA3 is lower in SlGRAS15-RNAi lines than in wild type (WT) plants; dwarf phenotype can also be rescued by spraying with 100 μM GA3 and plant height of GA3-treated plants. a The GA3 content of WT and SlGRAS15-silenced lines. b The dwarf phenotype of SlGRAS15 lines was rescued by spraying with 100 μM GA3 on every 3rd day starting from 30 days after sowing up to 2 weeks; pictures were taken 14 days after treatment. c The plant height of GA3-treated plants. Bars = 1 cm. Each value represents the mean ± SD of three independent biological replicates (n = 3). Asterisks indicate a significant difference (P < 0.05) between WT and transgenic lines
Discussion
Here, SlGRAS15 gene a member of SCR subfamily has been identified and studied. Among the three members of this subfamily none of them have been studied in tomato. Expression analysis of SlGRAS15 in different parts of tomato plant showed that it highly accumulates in roots, young leaves, and stem. This led us to hypothesize that SlGRAS15 may function during the growth and development of tomato. Suppression of SlGRAS15 in tomato altered plant morphological features, response to phytohormones treatment ABA, IAA, and GA3, phase transition and significantly decreased the plant height and leaf size, extensive branching, increases leaf number, and petiolules length.
In our study, we observed that the transcript accumulation of SlGRAS15 was affected by phytohormones treatment and had higher sensitivity to ABA, IAA, and GA3 than the WT plants at seedlings stage (Fig. S3). The plant growth hormone ABA play key role in altering several aspects of plant developmental processes, like seed germination, seed dormancy, and growth of seedlings. ABA can also function to increase leaf abscission, senescence of leaf, and flower (Zhou et al. 2009). The qRT-PCR analysis showed that the transcripts of SlGRAS15 mRNA gradually increased at the beginning, while increased slightly at 12 h after ABA treatment. These results shows that silencing SlGRAS15 plays an important role in regulating plant responses to ABA during seedlings growth. Furthermore, the major constituents such as TFs, which are especially involved in the ABA signaling pathway can affect the sensitivity to ABA exposure (Waseem et al. 2019). The data revealed the involvement of SlGRAS15 in the growth of roots, leaf, stem, and mediating hormone signaling.
The endogenous GA3 contents were decreased in SlGRAS15-RNAi lines than WT (Fig. 7a). In SlGRAS15-RNAi lines, the 4 internodes length were smaller than WT plants, demonstrating that the dwarf transgenic lines depicted the shortening of internodal length (Fig. 3a–h), indicating that the dwarf phenotypes may be due to reduced endogenous bioactive GA3 and short internodal length. To further confirm this phenotype, exogenous application of 100 μM GA3 restored the plant height to WT levels (Fig. 7b, c). Therefore, we supposed that the low concentration of GAs might be responsible for these phenotypes. For the confirmation of this hypothesis, qRT-PCR analyses were performed to evaluate the transcript level of genes related to GAs production in the stem of SlGRAS15-RNAi lines and WT. The results revealed that the expression level of genes related to GA biosynthesis comprising KAO, CPS, GA3ox1, and GA3ox2 were inhibited significantly in SlGRAS15-RNAi lines. Moreover, the SEDILLA- a negative regulator of GAs signaling was induced but SIGAST1 (Ding et al. 2013) (tomato gibberellin stimulated transcript1) transcript accumulation reduced in SIGRAS15-RNAi lines. Conversely, we also evaluated the transcript accumulation of genes related to GA inactivation (SlGA2ox1, –2); prominent upregulation was observed in SlGRAS15-RNAi lines (Fig. 6). These results point out that SlGRAS15-downregulation may affect the tomato DELLA metabolism, afterwards effects the development of cell and consequently led to the GA unresponsive short plant phenotype of SlGRAS15-RNAi lines. It is well reported that the molecular phenomenon underlying the feedback regulation of GA metabolism is operated by DELLA proteins (Hou et al. 2008). DELLAs positively regulate the expression of GA 3-oxidases genes; however, GA 2-oxidase genes expression is suppressed. DELLAs expression level is also exposed to negative feedback mechanism by their own protein products (Middleton et al. 2012). In SlGRAS15-silenced plants, we witnessed the same phenomena, including reduced transcripts of SlGA3ox1 mRNA accumulation, increased levels of DELLA and SlGA2ox1 transcripts. There by we speculate that downregulation of SlGRAS15 might suppress the DELLA protein degradation in tomato plants, which is involved in changing the expression of genes related to gibberellin metabolism by the mechanism of positive and negative feedback. So, our results shows that silencing of SlGRAS15 reduces expansion of leaf by lowering the transcript level of main genes involved in the biosynthesis of GA and triggering the genes involved in GA deactivation. On the other side the bioactive endogenous GA3 in transgenic lines is lower as compared with WT plants (Fig. 7a). These results suggest that SlGRAS15 plays an important role in tomato growth and development through interaction with the promoter region of GA biosynthesis genes in the GA signaling pathway, but whether these interactions are direct or indirect remains to be determined. In the present work, downregulation of SlGRAS15 altered the responsiveness to IAA, led to auxin sensitivity, and altered the abundance of transcripts related to auxin biosynthesis and signaling (Figs. 5b and S3). This is compelling evidence that SlGRAS15 acts as a regulator of auxin and influences auxin and gibberellin homeostasis.
Moreover, we observed that SlGRAS15-RNAi lines showed comparatively small sized leaves with pointed margins than that of WT but the leaf index (length to width ratio) remained unchanged (fig. 3f). The leaf growth is a complex stepwise process of development under the control of various environmental and developmental factors which are defined by proliferation, expansion, and maturation phases (Falcone et al. 2007). Two genes belong to NAC TFs family SlGOB (Goblet) (Berger et al. 2009) and SlNAM2 (Hendelman et al. 2013), are well known for their function to control the leaf margins. So we quantified the expression level of these two genes in WT and SlGRAS15-RNAi lines. Significant increase of them was recorded in SlGRAS15-RNAi lines when compared with WT plants (Fig. S2). Leaves of tomato endure prolonged morphogenesis even when compared with species having compound leaves, causing elaborate and inconstant leaf forms. Leaf shape in tomato varies among different-cultivars, different plants of the same cultivar, and even unlike leaves on the same plant is interesting (Pattanaik et al. 2014). According to our analysis, SlGRAS15-RNAi plants displayed increased number of leaf and shoot branches, specifying the vigorous vegetative growth of these plants than that of WT (Figs. 3 and S2).
Tomato transgenic plants carrying SlBL-RNAi expression, the origination of lateral meristems is blocked during shoot development and inflorescence, which leads to an intense decrease in the number of lateral axes (Silva et al. 2014). Therefore we assessed the transcript level of SlBL (Mauricio and Rausher 1997; Silva et al. 2014) in WT and SlGRAS15-RNAi lines, where SlGRAS15-RNAi lines showed an increased expression of SlBL in the axillary buds, showing that SlGRAS15 gene may positively regulate the transcript level of SlBL (Fig. S2). In tomato plants the loss of function mutation of SlBRC1b leads to enlarged branch out growth, suggesting that SlBRC1b play a key role in suppression of shoot branch (Silva et al. 2014). The transcript level of SlBRC1b which act as shoot branch suppressor was also reduced in the axillary buds of SlGRAS15-RNAi lines. In angiosperms, shoot branching mainly defines the complete plant architecture and affects the key features of plant life, include plant height, nutrients distribution, and visibility for pollinators.
In conclusion, we have identified an important plant regulator, SlGRAS15 in tomato. Results obtained from studies performed enable us to propose a linkage between SlGRAS15 gene and manipulation of plant architecture, response to growth hormones, plant height, and leaf number by downregulating the main GA biosynthesis genes and stimulating the GA deactivating genes in tomato. Taken together, we highlight that SlGRAS15 is an essential regulator involved in vegetative developmental processes. Our findings provide a valuable opportunity to deepen understanding on the genetic mechanism underlying significant tomato agronomic traits.
References
Amador V, Monte E, García-Martínez J-L, Prat S (2001) Gibberellins signal nuclear import of PHOR1, a photoperiod-responsive protein with homology to Drosophila armadillo. Cell 106:343–354
Bassel GW, Mullen RT, Bewley JD (2008) Procera is a putative DELLA mutant in tomato (Solanum lycopersicum): effects on the seed and vegetative plant. J Exp Bot 59:585–593
Berger Y et al (2009) The NAC-domain transcription factor GOBLET specifies leaflet boundaries in compound tomato leaves. Development 136:823–832. https://doi.org/10.1242/dev.031625
Bolle C (2004) The role of GRAS proteins in plant signal transduction and development. Planta 218:683–692
Chen G, Hackett R, Walker D, Taylor A, Lin Z, Grierson D (2004) Identification of a specific isoform of tomato lipoxygenase (TomloxC) involved in the generation of fatty acid-derived flavor compounds. Plant Physiol 136:2641–2651. https://doi.org/10.1104/pp.104.041608
Claeys H, De Bodt S, Inzé D (2014) Gibberellins and DELLAs: central nodes in growth regulatory networks. Trends Plant Sci 19:231–239
Cui H et al (2007) An evolutionarily conserved mechanism delimiting SHR movement defines a single layer of endodermis in plants. Science 316:421–425
Della Rovere F et al (2015) Arabidopsis SHR and SCR transcription factors and AUX1 auxin influx carrier control the switch between adventitious rooting and xylogenesis in planta and in in vitro cultured thin cell layers. Ann Bot 115:617–628
Ding J, Chen B, Xia X, Mao W, Shi K, Zhou Y, Yu J (2013) Cytokinin-induced parthenocarpic fruit development in tomato is partly dependent on enhanced gibberellin and auxin biosynthesis. PLoS One 8:e70080
Expósito-Rodríguez M, Borges AA, Borges-Pérez A, Pérez JA (2008) Selection of internal control genes for quantitative real-time RT-PCR studies during tomato development process. BMC Plant Biol 8:131
Falcone A, Nelissen H, Fleury D, Van Lijsebettens M, Bitonti MB (2007) Cytological investigations of the Arabidopsis thaliana elo1 mutant give new insights into leaf lateral growth and Elongator function. Ann Bot 100:261–270
Gao M-J, Parkin I, Lydiate D, Hannoufa A (2004) An auxin-responsive SCARECROW-like transcriptional activator interacts with histone deacetylase. Plant Mol Biol 55:417–431
González-Aguilera KL, Saad CF, Chávez Montes RA, Alves-Ferreira M, De Folter S (2016) Selection of reference genes for quantitative real-time RT-PCR studies in tomato fruit of the genotype MT-Rg1. Front Plant Sci 7:1386
Harberd NP, Belfield E, Yasumura Y (2009) The angiosperm gibberellin-GID1-DELLA growth regulatory mechanism: how an “inhibitor of an inhibitor” enables flexible response to fluctuating environments. Plant Cell 21:1328–1339
Hedden P, Kamiya Y (1997) GIBBERELLIN BIOSYNTHESIS: enzymes, genes and their regulation. Annu Rev Plant Physiol Plant Mol Biol 48:431–460. https://doi.org/10.1146/annurev.arplant.48.1.431
Hedden P, Phillips AL (2000) Gibberellin metabolism: new insights revealed by the genes. Trends Plant Sci 5:523–530
Hendelman A, Stav R, Zemach H, Arazi T (2013) The tomato NAC transcription factor SlNAM2 is involved in flower-boundary morphogenesis. J Exp Bot 64:5497–5507. https://doi.org/10.1093/jxb/ert324
Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database. Nucleic Acids Res 27:297–300
Hou X, Hu W-W, Shen L, Lee LYC, Tao Z, Han J-H, Yu H (2008) Global identification of DELLA target genes during Arabidopsis flower development. Plant Physiol 147:1126–1142
Huang W, Xian Z, Hu G, Li Z (2016) SlAGO4A, a core factor of RNA-directed DNA methylation (RdDM) pathway, plays an important role under salt and drought stress in tomato. Mol Breed 36:28
Huang W et al (2017) Overexpression of a tomato miR171 target gene SlGRAS24 impacts multiple agronomical traits via regulating gibberellin and auxin homeostasis. Plant Biotechnol J 15:472–488. https://doi.org/10.1111/pbi.12646
Lee S et al (2002) Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following imbibition. Genes Dev 16:646–658
Li X et al (2003) Control of tillering in rice. Nature 422:618
Li M, Wang X, Li C, Li H, Zhang J, Ye Z (2018) Silencing GRAS2 reduces fruit weight in tomato. J Integr Plant Biol 60:498–513
Liu Y et al (2017) Overexpression of SlGRAS40 in tomato enhances tolerance to abiotic stresses and influences auxin and gibberellin signaling. Front Plant Sci 8:1659. https://doi.org/10.3389/fpls.2017.01659
Livne S et al (2015) Uncovering DELLA-independent gibberellin responses by characterizing new tomato procera mutants. Plant Cell 27:1579–1594
Magome H, Yamaguchi S, Hanada A, Kamiya Y, Oda K (2004) Dwarf and delayed-flowering 1, a novel arabidopsis mutant deficient in gibberellin biosynthesis because of overexpression of a putative AP2 transcription factor. Plant J 37:720–729
Martin-Trillo M et al (2011) Role of tomato BRANCHED1-like genes in the control of shoot branching. Plant J Cell Mol Biol 67:701–714. https://doi.org/10.1111/j.1365-313X.2011.04629.x
Mauricio R, Rausher MD (1997) Experimental manipulation of putative selective agents provides evidence for the role of natural enemies in the evolution of plant defense. Evolution 51:1435–1444
McGinnis KM, Thomas SG, Soule JD, Strader LC, Zale JM, Sun T-p, Steber CM (2003) The arabidopsis SLEEPY1 gene encodes a putative F-box subunit of an SCF E3 ubiquitin ligase. Plant Cell 15:1120–1130
Middleton AM et al (2012) Mathematical modeling elucidates the role of transcriptional feedback in gibberellin signaling. Proc Natl Acad Sci 109:7571–7576
Morohashi K, Minami M, Takase H, Hotta Y, Hiratsuka K (2003) Isolation and characterization of a novel GRAS gene that regulates meiosis-associated gene expression. J Biol Chem 278:20865–20873
Neeraja CN, Vemireddy LR, Malathi S, Siddiq EA (2009) Identification of alternate dwarfing gene sources to widely used Dee-Gee-Woo-Gen allele of sd1 gene by molecular and biochemical assays in rice (Oryza sativa L.). Electron J Biotechnol 12:7–8
Niu Y, Zhao T, Xu X, Li J (2017) Genome-wide identification and characterization of GRAS transcription factors in tomato (Solanum lycopersicum). PeerJ 5:e3955
Olimpieri I, Caccia R, Picarella ME, Pucci A, Santangelo E, Soressi GP, Mazzucato A (2011) Constitutive co-suppression of the GA 20-oxidase1 gene in tomato leads to severe defects in vegetative and reproductive development. Plant Sci 180:496–503
Olszewski N, Sun T-p, Gubler F (2002) Gibberellin signaling: biosynthesis, catabolism, and response pathways. Plant Cell 14:S61–S80
Pattanaik S, Patra B, Singh SK, Yuan L (2014) An overview of the gene regulatory network controlling trichome development in the model plant, Arabidopsis. Front Plant Sci 5:259
Pysh LD, Wysocka-Diller JW, Camilleri C, Bouchez D, Benfey PN (1999) The GRAS gene family in arabidopsis: sequence characterization and basic expression analysis of the SCARECROW-LIKE genes. Plant J 18:111–119
Sánchez C et al (2007) Two SCARECROW-LIKE genes are induced in response to exogenous auxin in rooting-competent cuttings of distantly related forest species. Tree Physiol 27:1459–1470
Schmitz G, Tillmann E, Carriero F, Fiore C, Cellini F, Theres K (2002) The tomato blind gene encodes a MYB transcription factor that controls the formation of lateral meristems. Proc Natl Acad Sci U S A 99:1064–1069. https://doi.org/10.1073/pnas.022516199
Silva EM et al (2014) microRNA156-targeted SPL/SBP box transcription factors regulate tomato ovary and fruit development. Plant J 78:604–618
Sun X, Xue B, Jones WT, Rikkerink E, Dunker AK, Uversky VN (2011) A functionally required unfoldome from the plant kingdom: intrinsically disordered N-terminal domains of GRAS proteins are involved in molecular recognition during plant development. Plant Mol Biol 77:205–223
Tian C, Wan P, Sun S, Li J, Chen M (2004) Genome-wide analysis of the GRAS gene family in rice and Arabidopsis. Plant Mol Biol 54:519–532
Torres-Galea P, Huang L-F, Chua N-H, Bolle C (2006) The GRAS protein SCL13 is a positive regulator of phytochrome-dependent red light signaling, but can also modulate phytochrome A responses. Mol Genet Genomics 276:13–30
Waseem M, Rong X, Li Z (2019) Dissecting the role of a basic helix-loop-helix transcription factor, SlbHLH22, under salt and drought stresses in transgenic Solanum lycopersicum L. Front Plant Sci 10
Xie Q, Hu Z, Zhu Z, Dong T, Zhao Z, Cui B, Chen G (2014) Overexpression of a novel MADS-box gene SlFYFL delays senescence, fruit ripening and abscission in tomato. Sci Rep 4:4367
Yamaguchi S (2008) Gibberellin metabolism and its regulation. Annu Rev Plant Biol 59:225–251. https://doi.org/10.1146/annurev.arplant.59.032607.092804
Zhang L, Zhu M, Ren L, Li A, Chen G, Hu Z (2018) The SlFSR gene controls fruit shelf-life in tomato. J Exp Bot 69:2897–2909
Zhou X, Hua D, Chen Z, Zhou Z, Gong Z (2009) Elongator mediates ABA responses, oxidative stress resistance and anthocyanin biosynthesis in Arabidopsis the. Plant J 60:79–90
Zhou S, Hu Z, Li F, Yu X, Naeem M, Zhang Y, Chen G (2018) Manipulation of plant architecture and flowering time by down-regulation of the GRAS transcription factor SlGRAS26 in Solanum lycopersicum. Plant Sci 271:81–93
Acknowledgments
We are grateful to Professor Guoping Chen and Zongli Hu for conceiving this work. We especially thank Rafia Muqarab for her technical assistance throughout the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
M.N designed and managed the research; M.N., M.W., Z.Z., and L.Z performed research and revised the manuscript; M.N. wrote the paper. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Angelika Stollewerk
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Naeem, M., Waseem, M., Zhu, Z. et al. Downregulation of SlGRAS15 manipulates plant architecture in tomato (Solanum lycopersicum). Dev Genes Evol 230, 1–12 (2020). https://doi.org/10.1007/s00427-019-00643-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-019-00643-7