Abstract
Insect gene function has mainly been studied in the fruit fly Drosophila melanogaster because in this species many techniques and resources are available for gene knock down and the ectopic activation of gene function. However, in order to study biological aspects that are not represented by the Drosophila model, and in order to test to what degree gene functions are conserved within insects and what changes in gene function accompanied the evolution of novel traits, the establishment of respective tools in other insect species is required. While gene knock down can be induced by RNA interference in many insects, methods to misexpress genes are much less developed. In order to allow misexpression of genes in a timely controlled manner in the red flour beetle Tribolium castaneum, we have established a heat shock-mediated misexpression system. We show that endogenous heat shock elements perform better than artificial heat shock elements derived from vertebrates. We carefully determine the optimal conditions for heat shock and define a core promoter for use in future constructs. Finally, using this system, we study the effects of misexpressing the head patterning gene Tc-orthodenticle1 (Tc-otd1), We show that Tc-otd1 suppresses Tc-wingless (Tc-wg) in the trunk and to some degree in the head.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The red flour beetle Tribolium castaneum has developed into an important insect model organism. It has been used as a model system for evolutionary developmental questions and for studying aspects of insect biology and development, which are not well amenable in the Drosophila model. For instance, Tribolium exhibits the short germ mode of embryonic development by adding segments consecutively in an anterior to posterior sequence, which is ancestral within the insects. In contrast, the long germ insect Drosophila builds all its segments almost simultaneously at the blastoderm stage. Furthermore, Drosophila does not develop larval legs and has reduced extraembryonic tissues, while Tribolium represents the insect typical situation. A last example of atypical development in Drosophila is head involution in which the embryonic head is turned outside into the thorax during embryogenesis. Because of the cuticular reduction accompanying the process, embryonic head defects can be interpreted only with difficulty in Drosophila. Tribolium exhibits an typical insect larval head with all typical appendages (Klingler 2004; Posnien et al. 2010; Schröder et al. 2008) and markers for specific head regions (Schinko et al. 2008). In addition, the Tribolium model is a representative of the most species-rich metazoan taxon, the Coleoptera, which represent one fourth of all described animals (Hunt et al. 2007), including many important pest species such as the boll weevil, corn rootworm, Colorado potato beetle and comprising species with intriguing evolutionary adaptations like the horns of horned beetles (Moczek et al. 2006).
The development of resources and techniques has advanced the potential of the Tribolium model system within the last years. The genome was sequenced (Richards et al. 2008) and several techniques were established like efficient transposon mediated germ line transformation (Berghammer et al. 2009; Lorenzen et al. 2003; Pavlopoulos et al. 2004) and robust gene knock down via systemic RNAi (Brown et al. 1999; Bucher et al. 2002; Tomoyasu and Denell 2004). For spatially regulated misexpression of genes, the Gal4/UAS system has recently been developed (Schinko et al. 2010) and a large-scale transposon mutagenesis screen has been performed (Trauner et al. 2009).
Heat shock proteins (hsp) play an important role in the stress response of the cell. Some of these proteins are activated by high temperatures in order to protect the animal by acting as molecular chaperones thus refolding the unfolded proteins and preventing aggregation (Becker and Craig 1994). Importantly, sequences in the 3′UTR confer stability to the hsp mRNAs under heat shock conditions (Moseley et al. 1993). In addition, under these conditions splicing is blocked, which leads to degradation of aberrant transcripts, whereas almost all hsp genes do not contain introns and are therefore expressed normally (Yost et al. 1990). In order to misexpress genes upon heat shock, the coding sequence of the target gene is brought under the control of a heat shock promoter. At normal temperatures, only little or no transcription of the target gene takes place in such transgenic animals, while at critical temperatures transcription is activated. Such systems have been used in vertebrate and invertebrate model organisms for temporally controlled misexpression of genes, but also for gene knock down by expressing hairpin constructs (Adám et al. 2000; Chen et al. 2011; Lis et al. 1983; Shoji et al. 1998; Wheeler et al. 2000). Localized heat shocks were applied by a temperature controlled soldering iron, a hot needle (Hardy et al. 2007; Monsma et al. 1988) or a laser (Halloran et al. 2000; Ramos et al. 2006). Some Tribolium heat shock genes have been studied recently: The upregulation of Tc-hsp83 upon heat shock has been shown (Xu et al. 2010) while Tc-hsp90 appears to be required for survival at several stages of development (Knorr and Vilcinskas 2011) but these insights have not been used for establishing misexpression techniques. In a previous study focused on the Gal4/UAS system, we have used the Tc-hsp68 promoter for misexpressing Gal4 without characterizing the promoter in more detail (Schinko et al. 2010). In this work, we characterize the dynamics of the heat shock system independently of the binary system, by generating lines in which nuclearly localized EGFP is under the control of the Tc-hsp68 promoter. This endogenous promoter works better than a system based on artificial vertebrate heat shock elements. Finally, we apply the system to misexpress the head gap gene Tc-otd1.
Materials and methods
Constructs
All constructs were stably integrated into the genome using the piggyBac vectors pBac[3xP3-DsRedaf] (Horn et al. 2002) or pBac[3xP3-gTc’v] (Lorenzen et al. 2002). Plasmids: pBac[3xP3-DsRed;artHSE-Tc-bhsp68::nlsEGFP] [GenBank acc no JQ417435]; pBac[3xP3-DsRed;endoHSE-Tc-bhsp68::nlsEGFP] [GenBank acc no JQ417434]; pBac[3xP3-DsRed;Tc-bhsp68::nlsEGFP] [GenBank acc no JQ417433]; pBac[3xP3-gTc’v;endoHSE-Tc-bhsp68::Tc-otd1] [GenBank acc no JQ417432]. Sequences of the functional constructs are available at genbank. Detailed maps are available from the authors. Tc: Tribolium castaneum; art: artificial; endo: endogenous; HSE—heat shock elements; bhsp68—core promoter of hsp68.
Tribolium strains and germline transformation
Standard methods were used to perform Tribolium germline transformation (Berghammer et al. 2009). The piggyBac constructs (500 ng/μl in injection buffer, i.e. 5 mM KCl, 0.1 mM KH2PO4, 0.1 mM NaH2PO4 pH 6.8) were injected along with 300 ng/μl helper plasmid phspBac (Handler and Harrell 1999) into embryos of the vermilion white (v w) strain. Pulled and cut borosilicate glass capillaries in combination with a Femto Jet device (Eppendorf, Hamburg, Germany) were used to inject embryos. The embryos were kept humid at 32 °C/90 °F for 2 days and then transferred to lower humidity for hatching. The hatched larvae were transferred to full wheat flour and the adult G0 beetles were crossed to v w wildtype animals. Transgenic offspring was outcrossed with v w before heterozygous animals were pooled to give offspring, which results in a mixture of heterozygous and homozygous animals.
Transformation markers and epifluorescence microscopy
DsRed1 (Horn et al. 2002) and Tc-vermilion (Lorenzen et al. 2002) were used as transformation markers. The 3xP3 driven expression of the marker genes in the eyes were detected with the Leica MZ16FA fluorescence stereomicroscope with a planachromatic 0.8× objective (Leica, Wetzlar, Germany). In the case of DsRed1 a DsRedwide filter (Ext. 546/12; Emm. 605/75) was used whereas dark pigmentation of the eyes could be observed after successful transformation with Tc-vermilion without any filter.
Heat shock conditions
The optimized heat shock conditions are 5–10 min at 48 °C/118 °F for the embryonic and the pupal stages. The heat shocks were performed in a water bath. These conditions do not interfere with viability but induce strong heat shock-mediated misexpression. Both by extending the time of incubation and by raising the temperature, the activity cannot be strongly enhanced without the tradeoff of elevated lethality and potential side effects. We find that giving heat shocks in air incubators is not very reproducible, especially between different incubators. Apparently, the different dynamics of heating and air circulation systems lead to quite different conditions for the incubated animals. Therefore, in order to ensure reproducibility between experiments and labs, and in order to ensure uniform conditions for all treated animals, heat shock should be performed in a PS-container with a flat base, where all individuals touch the bottom, e.g., containers which are used for Drosophila stock keeping (Greiner-bio-one: 53,0/100MM). We consider coned vials (e.g., centrifugation vials) less useful because not all animals touch the surface of the vial producing different conditions within one batch.
Quantification of fluorescence
The software Image-Pro (Media Cybernetics) was used for quantifying fluorescence intensity for Fig. 3a. Images of two to four fluorescent animals were converted into gray scale and the area, length and width of the animals and the signal intensity within this area was determined. These values were normalized to non-heat shocked siblings. Two pupae were measured per treatment. For all other figures, ImageJ was used for quantifying fluorescence intensity. The mean of 13–15 pupae and 10–20 embryos was calculated and normalized to non-heat shocked siblings.
Local heat shock by soldering iron
A 2-cm-long copper wire of 1 mm diameter attached to a voltage-controlled soldering iron (Aldi Nord, Germany) was used to apply local heat shocks in pupae by touching the tissue with the tip of the wire for 3 s.
Results and discussion
Identification of a hsp promoter in Tribolium
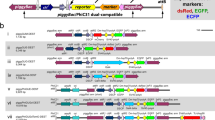
In order to identify putative heat shock promoters, we searched for homologs of the Dm-hsp70 gene in the Tribolium genome and analyzed the respective upstream regions. Based on its sequence and position, we identified a putative HSE from −104 to −91 bp upstream of the initiator site of Tc-hsp68, consisting of three repeats of the 5 bp consensus NGAAN and its complement NTTCN (Cunniff and Morgan 1993). Additional putative heat shock elements were found in the upstream sequence of Tc-hsp68 (up to 450 bps). This region plus the basal hsp68 promoter (bhsp68, 150 bp), including the TATA-box, transcription initiator site and putative motif ten elements (Lim et al. 2004) were cloned to drive expression of nuclearly localized EGFP. In order to ensure stabilization of the mRNA under heat shock conditions, we used the 295 bp Tc-hsp68 3′UTR (Fig. 1a; see Online Resource 1 for sequences). We refer to this construct as endoHSE::nls-EGFP.
Schematic representation of constructs used for generating transgenic lines. a The reporter gene EGFP is fused to a nuclear localization sequence (nls) downstream of a the endogenous regulatory region (endoHSEs) of Tc-hsp68 including its basal promoter (bhsp68), b a repeat of artificial HSEs (artHSEs) followed by bhsp68 and c the bhsp68 only. The 3′UTR of Tc-hsp68 (hsp68 3′UTR) serves as polyadenylation signal in all three constructs
Endogenous heat shock elements are more efficient than vertebrate artificial elements
As an alternative to endogenous HSEs, artificial HSEs have been shown to activate gene expression more efficiently. A repeat of artificially optimized HSEs is more efficient in driving reporter gene expression and moreover exhibits less background activity than an endogenous promoter in the zebrafish (Bajoghli et al. 2004). Therefore, we cloned these artificial vertebrate HSEs (artHSEs) upstream of the basal hsp68 promoter to test them in Tribolium and compare their ability to drive reporter gene expression to the endoHSE. We refer to this construct as artHSEs::nls-EGFP.
In order to confirm that the core promoter bhsp68 itself is not responsive to heat shock we included a bhsp68::nlsEGFP construct in our analysis. Two independent transgenic lines were recovered for endoHSEs (e1 and e2). Of seven artHSE lines, the two with strongest activation were used for comparisons (a1 and a2). Of 15 lines with bhsp68, one showed very weak fluorescence during embryonic stages (b1) while all others were non-fluorescent. We chose b1 and one non-fluorescent line (b2) for further comparison. Since only 1 out of 15 bhsp68-nlsEGFP transgenic lines showed fluorescence, we assume that this exceptional expression in embryos was due to a position effect.
For a first assessment of activity, embryos, larvae, pupae, and adults of all lines were subjected to a strong heat shock (1 h at 46 °C/115 °F); 24 h after the heat shock they were qualitatively checked for EGFP fluorescence intensity and compared to transgenic animals without heat shock and heat shocked wildtype animals (Fig. 2). Both e1 and e2 showed strong EGFP fluorescence in all developmental stages after heat shock. No EGFP fluorescence was observed in non-heat shocked controls of the e1 line at all developmental stages (Fig. 2a-d). Interestingly, the e2 line exhibited strong EGFP fluorescence in embryonic stages (Fig. 2e). This putative enhancer trap was active only in the embryo proper and the amnion while additional heat shock-mediated expression was observed in the serosa (compare Fig. 2e, +hs and −hs). Larval, pupal, and adult stages of the e2 line were non-fluorescent without heat shock (Fig. 2f-h).
EGFP fluorescence of different transgenic strains at different developmental stages. Embryos, larvae, pupae and adults of two independent lines for each construct were heat shocked for 1 h at 46 °C (+hs) and checked for EGFP fluorescence 24 h after heat shock and compared to heat shocked wildtype (wt + hs) and non-heat shocked transgenic siblings (−hs). The endoHSE driven EGFP line e1 shows strong fluorescence at all developmental stages upon heat shock (a–d) as does e2 (e–h). In addition e2 embryos exhibited strong ubiquitous fluorescence in the embryo proper and the amnion without heat shock (e, −hs). Line a1 of the artHSEs driven EGFP line shows strong fluorescence of EGFP at all developmental stages (i–l). Line a2 shows only weak heat shock activation at the embryonic, larval, and pupal stages (m–o) and no activation at the adult stage (p). Of 15 transgenic lines that carry the bhsp68 core promoter, line b1 is the only one which shows very faint fluorescence upon heat shock at the embryonic stage (q) whereas no fluorescence is observed at the other stages (r–t). Line b2 does not exhibit any fluorescence (u–x)
The artHSE::nlsEGFP construct was heat shock-responsive in a1 embryos and adults (Fig. 2i,l). However, these stages were non-fluorescent after heat shock in a2 (Fig. 2m,p). Both lines exhibited heat-induced fluorescence during larval and pupal stages (Fig. 2j,k and n,o). No EGFP was observed in these lines without heat shock.
Neither the heat shocked nor the non-heat shocked animals of b2 showed EGFP fluorescence (Fig. 2u–x). No additional EGFP expression upon heat shock was observed in embryos of b1 (Fig. 2q). Thus, all developmental stages of b1 were not heat shock-responsive.
We conclude that the endoHSEs are a robust driver for heat shock-mediated misexpression, while the artHSEs perform less efficient in Tribolium. A similar artificial construct based on Tribolium HSE sequences might enhance performance as it was observed for the artHSEs in vertebrates (Bajoghli et al. 2004). We also confirmed that the bhsp68 core promoter is not active on its own, but that it can be activated by adjacent enhancer elements (e.g. the artHSEs and putative enhancer traps in line b1and e2). Due to these properties, bhsp68 is the first suitable core promoter useful for enhancer trapping or other transgenic constructs in Tribolium similar to the bhsp70 core promoter in Drosophila.
Optimization of heat shock conditions
Based on the performance of the different constructs, we decided to carry on further experiments with both endoHSEs lines to determine optimum conditions for temperature and duration of the heat shock. Experiments with pupae and adults were carried out with the e2 line, experiments with embryos with the e1 line.
Determination of heat shock-dependent lethality
From our previous experiment, we know that a heat shock for 60 min at 46 °C/115 °F induces strong HSE-dependent reporter gene expression. This temperature was chosen since it differs significantly from normal rearing temperatures of Tribolium (22°–35 °C (72°–95 °F) (Sokoloff 1974)).
In order to determine the optimal heat shock conditions we first scored the lethality rate of embryos and pupae for different heat shock durations and temperatures. Pupal survival was scored by counting the proportion of pupae that developed into viable adults. Heat shocks at 46 °C, even for 60 min, caused only a minor decrease in survival rate (73 % compared to 80 % of non-heat shocked; Online Resource 2a). In contrast, heat shocks at 48 °C led to a strong decrease in survival when heat shocks were conducted for more than 15 min (53 % after 20 min and 20 % after 30 min; Online Resource 2a). The embryonic survival rate was scored by the development of a normal larval cuticle. A heat shock of 5 min at 46 °C did not alter the survival rate but prolonging the heat shock decreased survival to 73 % after 10 min and 59 % after 15 min, whereas the survival of non-heat shocked embryos was 90 % (Online Resource 2b). At 48 °C, the decrease in viability was more pronounced and was detectable already after a heat shock of 5 min (70 % survival; Online Resource 2b).
Optimization of temperature and duration of heat shock
In order to determine the optimal temperature for heat shock-mediated misexpression in pupae, heat shocks were performed at 38, 42, 46, or 50 °C (100, 108, 115, 122 °F). Transgenic pupae of the same line without heat shock were used for normalization. We measured the relative EGFP fluorescence intensity over a time course of 32 h after heat shock. For each temperature, we plotted the mean fluorescence intensity of two pupae normalized to the mean fluorescence intensity of two non-heat shocked transgenic pupae against time (Fig. 3a). This experiment confirmed that 46 °C/115 °F is a suitable temperature to induce heat shock-mediated misexpression by the Tribolium endogenous HSEs. We detected faint fluorescence already 3 h after heat shock. The maximum was reached 8 h after heat shock and from 12 h on the fluorescence level began to decrease again. Lower temperatures did not cause much activation of the endogenous HSEs, whereas a higher temperature (50 °C/122 °F) led to quite strong but delayed EGFP fluorescence (starting between 12 and 24 h after heat shock). This delay might be due to a complete block of biosynthetic activity after extreme heat shock with subsequent recovery and onset of transcription and/or translation. Such an effect is also known from Drosophila (Margulis et al. 1991). To further narrow down the optimal temperature conditions, we repeated the experiment with 13–15 pupae at 46 and 48 °C (115 and 118 °F). We also performed the heat shocks for different durations (5, 10, 15, 20, 30, and 60 min; Fig. 3b,c). This experiment showed that the response depends on both temperature and duration within this range. At 48 °C/118 °F, more than double the intensity was induced compared to 46 °C/115 °F. However, the onset of fluorescence was delayed if long heat shocks were performed at 48 °C/118 °F, which indicates that the pupae started to need some additional recovery time before they started expression, similar to what was observed at 50 °C/122 °F (Fig. 3a). Heat shocks for a short time (5–10 min) at 48 °C caused fast and very strong EGFP fluorescence in pupae (Fig. 3c).
Influence of heat shock temperature and duration on fluorescence intensity. The fluorescence intensity of animals heat shocked at different temperatures and for different durations were measured. The mean fluorescence was calculated from 2 to 4 (a) or 13 to 15 (b,c) pupae of the e2 line, respectively, while 10–20 embryos of the e1 line were measured. All values were normalized by subtracting the mean fluorescence of non-heat shocked animals of the same line. Relative fluorescence was calculated by setting the maximal/minimal mean of measured fluorescence intensity to 100 %/0 %. This was done separately for data in a and b, c and d, e. The relative fluorescence was plotted over time. a Up to a heat shock temperature of 42 °C/108 °F hardly any EGFP fluorescence was observed in pupae. At a heat shock temperature of 46 °C/115 °F a strong increase of relative fluorescence was found. Heat shock with 50 °C/122 °F caused a strong heat shock response but the observed delay indicates that the heat shocked pupae need to recover before they can initiate the production of novel protein. b More detailed analysis of heat shock temperature and duration revealed that heat shocks at 46 °C/115 °F caused only weak heat shock response. c Short heat shocks at 48 °C/118 °F led to very strong response, which was more than double compared to pupae shocked at 46 °C/115 °F. Heat shocks longer than 15 min caused a delayed response. d Embryos heat shocked at 46 °C/115 °F exhibited increasing heat shock response by prolonging the heat shock duration. e At 48 °C/118 °F, already short heat shocks caused a much stronger response. We conclude that a short heat shock (5 min for embryos and 5 to 10 min for pupae) at 48 °C/118 °F is the optimum with respect to the tradeoff between activation and survival
We performed the equivalent experiment to determine the optimal heat shock conditions for embryos. 10 to 20 embryos of the e1 line were heat shocked at 46 °C/115 °F and 48 °C/118 °F for 2.5, 5, 10, 15, and 20 min (Fig. 3d,e). Heat shocks at 48 °C/118 °F (Fig. 3e) induced more EGFP fluorescence compared to 46 °C/115 °F (Fig. 3d). Prolonging the heat shock up to 10 min increased the fluorescence intensity.
Our results suggest that for optimal gene activation without strong increase of lethality the heat shock should be 5 to 10 min at 48 °C/118 °F at the pupal stage and 5 min at 48 °C/118 °F at the embryonic stage. Raising time or temperature further results in an increase of heat shock promoter activity but at the expense of viability and at the risk of non-specific effects.
Tribolium endogenous HSEs are inducible in many tissues
In order to confirm activity of the endoHSE in different tissues, we dissected several organs from male and female Tribolium adults to check for EGFP fluorescence 24 h after heat shock. Tissues scrutinized are the gut, the brain, the testis, and the ovaries. All these tissues exhibited EGFP fluorescence upon heat shock (Fig. 4a–d), whereas untreated transgenic tissues (Fig. 4e–h) and heat shocked v w wildtype tissues (Fig. 4i-l) did not display EGFP fluorescence.
Tissues responsive to heat shock. Different tissues were dissected from adult beetles of the e2 line 24 h after heat shock, checked for fluorescence and compared to non-heat shocked tissues of the same line and to heat shocked v w wildtype tissues. The heat shocked tissue of the e2 line showed EGFP fluorescence in most parts of the gut (a), the midgut (a′) and very strong in the hindgut (a″) whereas the foregut did not exhibit EGFP fluorescence (arrowhead in a). The brain showed weak EGFP fluorescence (b) whereas the testis (c) and especially the ovaries (d) exhibited very strong EGFP fluorescence. No EGFP fluorescence was observed in the controls (e–h and i–l)
The different cell types of the Tribolium ovaries have been described previously (Bäumer et al. 2011; Trauner and Büning 2007). Detailed examination of heat shocked ovaries using confocal microscopy revealed strong signal in the somatic tissue including follicle cells, somatic plug cells, and inner sheath cells of the tropharium. However, some follicle cells remained negative for fluorescence (see patches in Fig. 5f). The reason could be that these cells undergo endocycles during which they might not be responsive to heat shock. We did not detect signal in developing oocytes (Fig. 5b). However, we noted that some nurse cell clusters became EGFP positive, albeit later than the somatic cells (Fig. 5f). The lack of detectable signal in the oocytes could be due to inefficient transport of the nuclear localized EGFP from the nurse cells to the oocyte. We conclude that heat shock-mediated misexpression is targeting somatic cells of the ovary, whereas only some germline cells can be targeted. Loading of oocytes with protein remains to be shown and may require signals for efficient transport of the produced protein.
Detailed analysis of EGFP fluorescence in ovarioles. a Projection of a 3D stack of ovaries 6 h after heat shock. Strong EGFP fluorescence was detected in the vitellarium, whereas the tropharium showed weak or no fluorescence. b Merge of two layers of a 3D stack which section an oocyte 24 h after heat shock. The surrounding follicle cells were strongly stained while there was no signal in the oocyte. Heat shocked v w ovaries (c) and non-heat shocked ovaries of the e2 line (d) exhibit no and very weak EGFP fluorescence, respectively. e Six hours after heat shock, only cells of the vitellarium displayed EGFP fluorescence but the signal extended into the tropharium 12 h after heat shock (f). Also some nurse cell clusters became EGFP positive, indicating activity in the germline. g Twenty-four hours after heat shock the complete ovariole (except for the oocyte), including the tropharium, exhibited EGFP fluorescence
An unexpected dynamic was found with respect to EGFP signal in the tropharium. While the somatic follicle cells were marked shortly after heat shock, fluorescence was weaker in the tropharium during the first 6 hours (Fig. 5e). After 12 hours, EGFP signal appeared in the proximal parts of the tropharium (Fig. 5f), while after 24 h the entire tropharium was marked (Fig. 5g). This dynamic may be due to delayed expression and/or delayed translation in the distal parts of the tropharium. An intriguing alternative is that the maturation of EGFP is delayed: Maturation requires an oxidative step, which is rate limiting (Tsien 1998) and antioxidants reduce the aging of germ line stem cells in Drosophila (Pan et al. 2007). Hence, an antioxidant environment in the tropharium would be advantageous for keeping the germ line intact, but would at the same time reduce maturation of EGFP.
Local heat shock by hot needle
Drosophila has an atypical mode of metamorphosis, in that all larval epidermal cells are replaced by imaginal cells during metamorphosis. This is different in Tribolium and most other insects, where most larval epidermal cells become part of the adult epidermis. In order to study this process, local activation of genes active during metamorphic patterning would be useful. As previously described by Hardy et al. 2007 for zebrafish, we performed spatial heat shock experiments at the pupal stage using a voltage controlled soldering iron. The pupae were touched for 3 s with an approximately 2-cm-long copper wire fixed in the soldering iron, at three different spots, the head, the wing, and the abdomen; 24 h later, the pupae were checked for EGFP fluorescence. The touched areas showed strong fluorescence, although in some cases fluorescence was seen in a circle around the contact point between the copper wire and the pupa (Fig. 6). This is probably due to overheating of the central tissue, as an exact temperature control was difficult with this setup. Hence, the system can be used to locally misexpress genes during metamorphosis.
Heat shock-mediated transcription starts directly after heat shock
Knowing the lag between heat shock and onset of heat shock-mediated transcription is required for scheduled misexpression of genes. To this end, embryos of different stages were fixed directly (5 min delay due to fixation protocol) after a 10 min heat shock at 46 °C/115 °F. Subsequently, these embryos were stained for EGFP transcripts by whole mount in situ hybridization. Heat shocked v w wildtype and non-heat shocked transgenic embryos were hybridized in parallel. The heat shocked transgenic embryos exhibited ubiquitous and strong staining (Fig. 7c,d), which also included the serosal cells (Fig. 7d). wildtype embryos did not show any staining (Fig. 7a). In non-heat shocked transgenic embryos very faint staining after in situ hybridization was observed (Fig. 7b). This was probably due to a position effect of this specific line, because such staining was never observed in three lines containing Tc-otd1 (Online Resource 3) or Tc-ems (not shown) under control of the hsp68 promoter. The earliest stage responsive to heat shock expression appeared to be the differentiated blastoderm stage, where a patchy expression was found indicating that some cells were already responsive while others were not. We did not detect staining in earlier blastoderm stages (not shown).
EGFP mRNA expression induced by heat shock. EGFP expression was visualized by in situ hybridization with an EGFP-RNA antisense probe. a No signal was detected in heat shocked wildtype embryos. c–d Embryos were fixed 5 min after a 10 min heat shock at 46 °C. Germ band embryos responded very fast and strongly to heat shock. d Also, the cells of the serosa are heat shock responsive. b Non-heat shocked embryos of the same line showed slightly increased staining compared to wildtype embryos (a). f Staining was still present if embryos were fixed 2 h after heat shock, whereas the staining decreased significantly 3 h after heat shock (f); and 4 h after heat shock no signal was detected any more (g)
EGFP mRNA levels decrease within hours after heat shock-mediated expression
In order to determine how long the heat shock induced transcripts remain stable, we performed whole mount in situ hybridization on e1 embryos 2, 3, and 4 h after heat shock. We observed a strong decrease in EGFP mRNA levels 3 to 4 h after heat shock (Fig. 7f,g) compared to the levels after 2 h (Fig. 7e). As the stability of mRNA is highly dependent on its 3′UTR (Cybulsky et al. 2007), it is likely that other genes will show a similar turnover, as long as the same 3′UTR is used.
Misexpression of Tc-otd1 via the heat shock promoter causes reduction of Tc-wg expression
Tc-otd1 has an important function in early axis formation (Kotkamp et al. 2010; Schröder 2003), but also a later function in head patterning (Schinko et al. 2008). The ocular Tc-wg domain lies within the Tc-otd1 domain but the entire anterior head is absent in Tc-otd1 RNAi, making it difficult to test whether Tc-otd1 is required for the expression of the ocular Tc-wg domain. To test if Tc-otd1 overexpression is sufficient to induce the ocular Tc-wg, we generated three transgenic lines with Tc-otd1 under the control of the heat shock promoter and confirmed strong ubiquitous Tc-otd1 expression upon heat shock for two of them (shown for the subsequently used line M3 in Online Resource 3).
Early blastoderm-stage embryos (8–16 h at 25 °C) of wildtype (v w) and transgenic lines were collected and were heat shocked for 10 min at 46 °C/115 °F. After an additional 7 h of development at 25 °C/77 °F, they reached germ rudiment to elongation stages. These embryos were stained for Tc-wg expression. We did not detect aberrations in the v w embryos (Fig. 8a,b). In the transgenic embryos, the growth zone expression of Tc-wg was always present serving as an internal control for successful staining. In germ rudiments, the ocular Tc-wg staining was frequently absent or strongly reduced or showed an aberrant pattern (Fig. 8c,d). The domain was at least partially present at elongating germband stages (Fig. 8e′). Unexpectedly, the trunk Tc-wg stripes were strongly suppressed by Tc-otd1 misexpression (Fig. 8e). The same experiment was conducted with late blastoderm embryos (12–20 h at 25 °C), which were allowed to develop for another 5 h after heat shock. In these embryos, the ocular domain was usually present, but sometimes showed a slightly aberrant pattern (not shown). Again, the trunk stripes were strongly reduced while the growth zone expression domain was always present (not shown). Apparently, misexpression of Tc-otd1 interferes with ocular Tc-wg expression in early blastoderm embryos, but late blastoderm stages are less sensitive. These data indicate that Tc-otd1 alone is not sufficient for activating Tc-wg in the ocular domain. This is not unexpected because the Tc-otd1 domain is larger than the Tc-wg domain (see Online Resource 3). Hence, an additional activator or repressor would be required to shape the nested Tc-wg domain. Because of the permanent co-expression within the ocular domain, the repressive effect of Tc-otd1 on Tc-wg is likely to be indirect. Tc-otd1 could for instance be required for repressing the posterior adjacent ocular Tc-hh (or Tc-gsc or Tc-lim) expression domains (Posnien et al. 2011), which in turn could be required for the activation or maintenance of the ocular Tc-wg domain. A positive regulatory loop between Wnt and Hedgehog pathways at the trunk parasegment boundaries is known from Drosophila but this interaction does not occur at the ocular segment of Drosophila (Gallitano-Mendel and Finkelstein 1997).
Misexpression of Tc-otd1 affects ocular and trunk Tc-wg expression. Tc-wg expression was visualized by in situ hybridization with a Tc-wg RNA antisense probe. Close-ups of the heads are oriented anterior to the top. Heat shocked v w wildtype embryos displayed the normal Tc-wg expression in an ocular domain (a, arrowhead in a′ and b′) and segmentally in the trunk (b). In heat shocked transgenic germ rudiments the ocular Tc-wg domain was strongly reduced (c, arrowhead c′) or misshaped (d, arrowhead d′) but was at least partially present in elongating germband embryos (arrowhead e′). The trunk Tc-wg expression was suppressed by ubiquitous over expression of Tc-otd1 (e)
The different location of the Tribolium and Drosophila head anlagen within the blastoderm make different interactions of head patterning genes likely (Posnien et al. 2010) and this has been shown for several genes (e.g. Tc-ems, Tc-btd, Tc-knirps and Tc-mex3 (Cerny et al. 2008; Schinko et al. 2008; Schoppmeier et al. 2009)). In line with this, similar otd-misexpression experiments in Drosophila did not lead to reduction of the ocular wg domain but rather to its enhanced expression (Gallitano-Mendel and Finkelstein 1998). Apparently, the regulation of the ocular wg stripe is different in these two species with more direct positive input of otd in Drosophila. Interestingly, the suppression of antennal and trunk wg stripes and the maintenance of the posterior wg domain after otd misexpression is observed in both Tribolium and Drosophila (Gallitano-Mendel and Finkelstein 1998) indicating that the evolutionary change is rather specific to the anteriormost i.e. the ocular wg domain.
Larval cuticles of the heat shocked embryos were analyzed for phenotypes. Unexpectedly and in contrast to findings in Drosophila, no abnormalities are observed despite the abnormal Tc-wg expression during early embryonic development. This result is in line with the recovery of the ocular domain in later stages—possibly by the positive feedback loop of the segment polarity gene network. In addition, the embryos were kept at a low temperature (25 °C/77 °F), leaving a lot of time for repair mechanisms to take place.
Conclusion
With this work, we establish a tool for heat shock-mediated misexpression in Tribolium. As long as an extensive Gal4 driver collection is not available, this method is an adequate tool to study the ubiquitous misexpression of genes. Further, we characterize a core promoter, which does not drive expression on its own, but is activated by enhancers. Given that our previous research showed that endogenous core promoters are essential for efficient activity of transgenic constructs (Schinko et al. 2010) in Tribolium, this will be a valuable element for the design of transgenic constructs in the future.
References
Adám A, Bártfai R, Lele Z et al (2000) Heat-inducible expression of a reporter gene detected by transient assay in zebrafish. Exp Cell Res 256:282–290. doi:10.1006/excr.2000.4805
Bajoghli B, Aghaallaei N, Heimbucher T, Czerny T (2004) An artificial promoter construct for heat-inducible misexpression during fish embryogenesis. Dev Biol 271:416–430. doi:10.1016/j.ydbio.2004.04.006
Bäumer D, Trauner J, Hollfelder D et al (2011) JAK-STAT signalling is required throughout telotrophic oogenesis and short-germ embryogenesis of the beetle Tribolium. Dev Biol 350:169–182. doi:10.1016/j.ydbio.2010.10.020
Becker J, Craig EA (1994) Heat-shock proteins as molecular chaperones. FEBS 219:11–23
Berghammer AJ, Weber M, Trauner J, Klingler M (2009) Red flour beetle (Tribolium) germline transformation and insertional mutagenesis. CSHP 2009:pdb.prot5259. doi: 10.1101/pdb.prot5259
Brown SJ, Mahaffey JP, Lorenzen MD et al (1999) Using RNAi to investigate orthologous homeotic gene function during development of distantly related insects. Evol Dev 1:11–15
Bucher G, Scholten J, Klingler M (2002) Parental RNAi in Tribolium (Coleoptera). Curr Biol 12:R85–R86. doi:10.1016/S0960-9822(02)00666-8
Cerny AC, Grossmann D, Bucher G, Klingler M (2008) The Tribolium ortholog of knirps and knirps-related is crucial for head segmentation but plays a minor role during abdominal patterning. Dev Biol 321:284–294. doi:10.1016/j.ydbio.2008.05.527
Chen B, Hrycaj S, Schinko JB et al (2011) Pogostick: a new versatile piggyBAC vector for inducible gene over-expression and down-regulation in emerging model systems. PLoS One 6:e18659. doi:10.1371/journal.pone.0018659
Cunniff N, Morgan WD (1993) Analysis of heat shock element recognition by saturation mutagenesis of the human HSP70. 1 gene promoter. J Biol Chem 268:8317
Cybulsky AV, Takano T, Papillon J et al (2007) The 3’-untranslated region of the Ste20-like kinase SLK regulates SLK expression. Am J Physiol Renal Physiol 292:F845–F852. doi:10.1152/ajprenal.00234.2006
Gallitano-Mendel A, Finkelstein R (1997) Novel segment polarity gene interactions during embryonic head development in Drosophila. Dev Biol 192:599–613. doi:10.1006/dbio.1997.8753
Gallitano-Mendel A, Finkelstein R (1998) Ectopic orthodenticle expression alters segment polarity gene expression but not head segment identity in the Drosophila embryo. Dev Biol 199:125–137. doi:10.1006/dbio.1998.8917
Halloran MC, Sato-Maeda M, Warren JT et al (2000) Laser-induced gene expression in specific cells of transgenic zebrafish. Dev (Camb Engl) 127:1953–1960
Handler AM, Harrell RA (1999) Germline transformation of Drosophila melanogaster with the piggyBac transposon vector. Insect mol biol 8:449–457
Hardy ME, Ross LV, Chien C-B (2007) Focal gene misexpression in zebrafish embryos induced by local heat shock using a modified soldering iron. Dev Dyn 236:3071–3076. doi:10.1002/dvdy.21318
Horn C, Schmid BGM, Pogoda FS, Wimmer EA (2002) Fluorescent transformation markers for insect transgenesis. Insect Biochem Mol Biol 32:1221–1235
Hunt T, Bergsten J, Levkanicova Z et al (2007) A comprehensive phylogeny of beetles reveals the evolutionary origins of a superradiation. Science (New York, NY) 318:1913–1916
Klingler M (2004) Tribolium. Curr Biol 14:639–640
Knorr E, Vilcinskas A (2011) Post-embryonic functions of HSP90 in Tribolium castaneum include the regulation of compound eye development. Dev Genes Evol. doi:10.1007/s00427-011-0379-z
Kotkamp K, Klingler M, Schoppmeier M (2010) Apparent role of Tribolium orthodenticle in anteroposterior blastoderm patterning largely reflects novel functions in dorsoventral axis formation and cell survival. Dev (Camb Engl) 137:1853–1862. doi:10.1242/dev.047043
Lim CY, Santoso B, Boulay T et al (2004) The MTE, a new core promoter element for transcription by RNA polymerase II. Genes Dev 18:1606–1617. doi:10.1101/gad.1193404
Lis JT, Simon JA, Sutton CA (1983) New heat shock puffs and beta-galactosidase activity resulting from transformation of Drosophila with an hsp70-lacZ hybrid gene. Cell 35:403–410
Lorenzen MD, Brown SJ, Denell RE, Beeman RW (2002) Cloning and characterization of the Tribolium castaneum eye-color genes encoding tryptophan oxygenase and kynurenine 3-monooxygenase. Genetics 160:225–234
Lorenzen MD, Berghammer AJ, Brown SJ et al (2003) piggyBac-mediated germline transformation in the beetle Tribolium castaneum. Insect Mol Biol 12:433–440
Margulis BA, Zhivotovski BD, Pospelova TV, Smagina LV (1991) Patterns of protein synthesis in various cells after extreme heat shock. Exp Cell Res 193:219–222. doi:10.1016/0014-4827(91)90559-D
Moczek AP, Cruickshank TE, Shelby A (2006) When ontogeny reveals what phylogeny hides: gain and loss of horns during development and evolution of horned beetles. Evolution 60:2329–2341
Monsma SA, Ard R, Lis JT, Wolfner MF (1988) Localized heat-shock induction in Drosophila melanogaster. J Exp Zool 247:279–284. doi:10.1002/jez.1402470312
Moseley PL, Wallen ES, McCafferty JD et al (1993) Heat stress regulates the human 70-kDa heat-shock gene through the 3’-untranslated region. Am J Physiol 264:L533–L537
Pan L, Chen S, Weng C et al (2007) Stem cell aging is controlled both intrinsically and extrinsically in the Drosophila ovary. Cell Stem Cell 1:458–469. doi:10.1016/j.stem.2007.09.010
Pavlopoulos A, Berghammer AJ, Averof M, Klingler M (2004) Efficient transformation of the beetle Tribolium castaneum using the Minos transposable element: quantitative and qualitative analysis of genomic integration events. Genetics 167:737–746. doi:10.1534/genetics.103.023085
Posnien N, Schinko JB, Kittelmann S, Bucher G (2010) Genetics, development and composition of the insect head—a beetle’s view. Arthropod Struct Dev 39:399–410. doi:10.1016/j.asd.2010.08.002
Posnien N, Koniszewski NDB, Hein HJ, Bucher G (2011) Candidate gene screen in the red flour beetle Tribolium reveals six3 as ancient regulator of anterior median head and central complex development. PLoS Genetics 7:e1002416. doi:10.1371/journal.pgen.1002416
Ramos DM, Kamal F, Wimmer EA et al (2006) Temporal and spatial control of transgene expression using laser induction of the hsp70 promoter. BMC Dev Biol 6:55. doi:10.1186/1471-213X-6-55
Richards S, Gibbs RA, Weinstock GM et al (2008) The genome of the model beetle and pest Tribolium castaneum. Nature 452:949–955. doi:10.1038/nature06784
Schinko JB, Kreuzer N, Offen N et al (2008) Divergent functions of orthodenticle, empty spiracles and buttonhead in early head patterning of the beetle Tribolium castaneum (Coleoptera). Dev Biol 317:600–613. doi:10.1016/j.ydbio.2008.03.005
Schinko JB, Weber M, Viktorinova I et al (2010) Functionality of the GAL4/UAS system in Tribolium requires the use of endogenous core promoters. BMC Dev Biol 10:53. doi:10.1186/1471-213X-10-53
Schoppmeier M, Fischer S, Schmitt-Engel C et al (2009) An ancient anterior patterning system promotes caudal repression and head formation in ecdysozoa. Current Biology: CB 19:1811–1815. doi:10.1016/j.cub.2009.09.026
Schröder R (2003) The genes orthodenticle and hunchback substitute for bicoid in the beetle Tribolium. Nature 422:621–625. doi:10.1038/nature01518.1
Schröder R, Beermann A, Wittkopp N, Lutz R (2008) From development to biodiversity—Tribolium castaneum, an insect model organism for short germband development. Dev Genes Evol 218:119–126. doi:10.1007/s00427-008-0214-3
Shoji W, Yee CS, Kuwada JY (1998) Zebrafish semaphorin Z1a collapses specific growth cones and alters their pathway in vivo. Dev (Camb Engl) 125:1275–1283
Sokoloff A (1974) The biology of Tribolium. Oxford University Press, Oxford
Tomoyasu Y, Denell RE (2004) Larval RNAi in Tribolium (Coleoptera) for analyzing adult development. Dev Genes Evol 214:575–578. doi:10.1007/s00427-004-0434-0
Trauner J, Büning J (2007) Germ-cell cluster formation in the telotrophic meroistic ovary of Tribolium castaneum (Coleoptera, Polyphaga, Tenebrionidae) and its implication on insect phylogeny. Dev Genes Evol 217:13–27. doi:10.1007/s00427-006-0114-3
Trauner J, Schinko J, Lorenzen MD et al (2009) Large-scale insertional mutagenesis of a coleopteran stored grain pest, the red flour beetle Tribolium castaneum, identifies embryonic lethal mutations and enhancer traps. BMC Biol 7:73. doi:10.1186/1741-7007-7-73
Tsien RY (1998) The green fluorescent protein. Annu Rev Biochem 67:509–544. doi:10.1146/annurev.biochem.67.1.509
Wheeler GN, Hamilton FS, Hoppler S (2000) Inducible gene expression in transgenic Xenopus embryos. Curr Biol 10:849–852
Xu J, Shu J, Zhang Q (2010) Expression of the Tribolium castaneum (Coleoptera: Tenebrionidae) hsp83 gene and its relation to oogenesis during ovarian maturation. J Genet Genom 37:513–522. doi:10.1016/S1673-8527(09)60071-0
Yost HJ, Petersen RB, Lindquist S (1990) RNA metabolism: strategies for regulation in the heat shock response. Trends Genet 6:223–227
Acknowledgments
We thank Dr. Thomas Czerny for the artificial heat shock elements, Dr. Fakrudin Bashasab for identifying the hsp68 promoter. Dr. Michael Schoppmeier and Dr. Jochen Trauner for help with the interpretation of the signal in the ovaries. We also thank Anna Gilles for help with in situ hybridizations and helpful discussions and Dr. Andrew D. Peel for language corrections.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Roth
Johannes Benno Schinko and Kathrin Hillebrand equal contributors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Sequences of the constructs shown in Fig. 1 are provided in gb format in this file. (PDF 84 kb)
ESM 2
Heat shock-mediated lethality of pupae (a) and embryos (b). Pupae and embryos were heat shocked at 46°C/115°F and 48°C/118°F for 5, 10, 15, 20, 30, and 60 min. Pupal survival was scored by development into viable adult beetles whereas embryonic development was scored by development of a wildtype larva cuticle. At both developmental stages lethality was increased at the higher temperature. Whereas pupae tolerated a 60-min heat shock at 46°C/115°F with only slight decrease in viability (73% compared to 80% of unshocked), embryos were more sensitive (37% compared to 90% of unshocked). At 48°C/118°F pupal survival was strongly reduced if heat shocks were given for 20 min (53% survival). A heat shock for 60 min at this temperature caused death of all shocked pupae. At the embryonic stage a heat shock of 5 min at 48°C/118°F already decreased the survival by 20%. A 10-min heat shock reduced viability to 65% and longer heat shocks led to death of more than 50% of the embryos. (PDF 80 kb)
ESM 3
Heat shock induced misexpression of Tc-otd1. Heat shocked v w wildtype embryos, as well as heat shocked embryos of the transgenic line M3 (endoHSE::Tc-otd1) were fixed two hours after heat shock. As a control, non-heat shocked transgenic embryos of the same line and same stage were also fixed. Double whole mount in situ hybridization was performed with Tc-wg (blue) and Tc-otd1 (orange) antisense RNA probes. The v w embryos exhibited normal Tc-wg and Tc-otd1 expression (a–b′), as did the non-heat shocked transgenic embryos (c–d′). Two hours after heat shock Tc-otd1 was strongly expressed ubiquitously in transgenic embryos at a level similar to the endogenous expression (e–f). Expression of Tc-wg was still present in the trunk (e,f) 2 h after onset of misexpression of Tc-otd1. (PDF 170 kb)
Rights and permissions
About this article
Cite this article
Schinko, J.B., Hillebrand, K. & Bucher, G. Heat shock-mediated misexpression of genes in the beetle Tribolium castaneum . Dev Genes Evol 222, 287–298 (2012). https://doi.org/10.1007/s00427-012-0412-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-012-0412-x