Abstract
The vertebrate tooth is covered with enamel in most sarcopterygians or enameloid in chondrichthyans and actinopterygians. The evolutionary relationship among these two tissues, the hardest tissue in the body, and other mineralized tissues has long been controversial. We have recently reported that specific combinations of secretory calcium-binding phosphoprotein (SCPP) genes are involved in the mineralization of bone, dentin, enameloid, and enamel. Thus, the early repertoire of SCPP genes would elucidate the evolutionary relationship across these tissues. However, the diversity of SCPP genes in teleosts and tetrapods and the roles of these genes in distinct tissues have remained unclear, mainly because many SCPP genes are lineage-specific. In this study, I show that the repertoire of SCPP genes in the zebrafish, frog, and humans includes many lineage-specific genes and some widely conserved genes that originated in stem osteichthyans or earlier. Expression analysis demonstrates that some frog and zebrafish SCPP genes are used primarily in bone, but also in dentin, while the reverse is true of other genes, similar to some mammalian SCPP genes. Dentin and enameloid initially use shared genes in the matrix, but enameloid is subsequently hypermineralized. Notably, enameloid and enamel use an orthologous SCPP gene in the hypermineralization process. Thus, the hypermineralization machinery ancestral to both enameloid and enamel arose before the actinopterygian–sarcopterygian divergence. However, enamel employs specialized SCPPs as structuring proteins, not used in enameloid, reflecting the divergence of enamel from enameloid. These results show graded differences in mineralized dental tissues and reinforce the hypothesis that bone–dentin–enameloid–enamel constitutes an evolutionary continuum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vertebrate teeth consist of three primary tissues: basal bone (attachment bone or alveolar bone), body dentin, and a surface tissue. In most sarcopterygians, the surface tissue is enamel, whereas that of chondrichthyans, actinopterygians, and larval urodele is called enameloid (Poole 1967). Enamel and enameloid are both highly mineralized, wear-resistant tissues and thus thought to be functionally equivalent. These mineralized dental tissues form on extracellular matrix (ECM) proteins, deposited by cells that develop through epithelial–mesenchymal interactions (Nanci 2003; Hall 2005). During mammalian tooth development, predentin is initially deposited by odontoblasts of mesenchymal origin (Linde and Goldberg 1993). Then, ameloblasts, differentiated from inner dental epithelial (IDE) cells, deposit mineralizing enamel matrix consisting of specialized enamel-structuring proteins (Fincham et al. 1999; Paine and Snead 2005; Bartlett et al. 2006). These proteins are subsequently degraded by proteinases secreted from ameloblasts and removed from maturing enamel (Simmer and Hu 2002). This process facilitates subsequent growth of enamel crystallites; enamel thus grows into a hypermineralized tissue (Simmer and Fincham 1995; Smith 1998).
In contrast, the initial ECM proteins secreted during teleost tooth development are pre-enameloid, instead of predentin (Sasagawa 1997; Van der heyden et al. 2000). Pre-enameloid primarily consists of type I collagen (COL1), similar to the ECM of bone and dentin, and is deposited by both mesenchyme-derived cells (odontoblasts) and the IDE cells. After pre-enameloid begins mineralization, the IDE cells continue to secrete proteins into enameloid for a short period of time, while odontoblasts deposit ECM proteins for dentin (Shellis and Miles 1974). Enameloid is thus different from the enamel in ECM proteins and early mineralization processes. Yet, enameloid ECM proteins are also degraded by proteinases secreted from the IDE cells, and enameloid matures into a hypermineralized tissue, similar to enamel (Kawasaki et al. 1987). Hence, it has been thought that enamel was derived from enameloid through a shift in the timing of ECM protein secretion (Poole 1971; Shellis and Miles 1974). This view has been, however, challenged by the analysis of tissue distribution in the fossil record; that is, enamel is primitive and enameloid is derived (Smith 1992; Smith 1995). Thus, there has been controversy over the evolutionary relationship among enamel, enameloid, and other mineralized tissues (Kawasaki and Weiss 2008).
Many genes involved in dental tissue mineralization comprise the secretory calcium-binding phosphoprotein (SCPP) gene family (Kawasaki and Weiss 2003; Kawasaki and Weiss 2006; Kawasaki and Weiss 2007). The SCPP genes arose by gene duplication initially from the common ancestor, the secreted protein, acidic, cysteine-rich like 1 (SPARCL1) gene (Kawasaki et al. 2004). The SCPPs are classified into two subclasses: acidic SCPPs containing >25% of Glu, Asp, and phospho-Ser residues and Pro/Gln (P/Q)-rich SCPPs consisting of >20% of Pro and Gln. In mammals, acidic SCPPs are primarily involved in bone and dentin mineralization, whereas P/Q-rich SCPPs enable enamel mineralization (Kawasaki and Weiss 2008). In fugu, on the other hand, both acidic and P/Q-rich SCPP genes are used in dentin, and many distinct P/Q-rich SCPP genes are expressed in enameloid (Kawasaki et al. 2005). However, the diversity of SCPP genes in tetrapods and teleosts and their roles in distinct mineralized tissues have remained unclear. This is mainly because many SCPP genes are lineage-specific and also because only limited species and developmental stages have been studied intensively.
In this study, I report the repertoire of SCPP genes in the frog and zebrafish and investigate their spatiotemporal expression domains. The results reveal genetic similarities between bone and dentin, dentin and enameloid, and enameloid and enamel. In particular, enameloid and enamel both use the odontogenic, ameloblast associate (ODAM) gene for tissue hypermineralization.
Materials and methods
Cloning of SCPP genes by RT-PCR
Mouse, frog (Xenopus tropicalis), and zebrafish SCPP genes were sought from their genome sequence. I designed polymerase chain reaction (PCR) primers for the genomic regions predicted to code SCPP genes and used these primers for screening these genes by reverse transcription PCR (RT-PCR) as previously described (Kawasaki et al. 2005). RNA molecules were purified from the jaws of a 4-day neonate mouse, the upper and lower jaws of an adult frog, and from the pharyngeal jaw of an adult zebrafish using the RNeasy mini kit (Qiagen).
For the mouse SCPP-Pro/Gln-rich 1 (SCPP-PQ1) gene, the following PCR primers (primer sequences are all shown in supplementary data) were used for rapid amplification of cDNA ends (RACE): SCPPPQ1dwn1, SCPPPQ1dwn2, and SCPPPQ1up2 (nested PCR primers were not required for all genes).
For frog SCPP genes, amelogenin (AMEL), ameloblastin (AMBN), enamelin (ENAM), and ODAM were cloned from the tooth-bearing upper jaw (with teeth at different developmental stages) and dentin matrix acidic phosphoprotein 1 (DMP1) and integrin-binding sialoprotein (IBSP) from the toothless lower jaw. The following PCR primers were used for RACE: for the SCPP acidic 1 gene (SCPPA1), XSCPP1dwn1, XSCPP1dwn2, XSCPP1up2, and XSCPP1up3; for DMP1, XDMP1dwn2, XDMP1dwn4, XDMP1up2, and XDMP1up5; for the SCPP acidic 2 gene (SCPPA2), XSCPP2dwn1, XSCPP2dwn2, and XSCPP2up1; for IBSP, XIBSPdwn1, XIBSPdwn2, and XIBSPup2; for ODAM, XSCPP3dwn1, XSCPP3dwn2, and XSCPP3up1; for ENAM, XENAMdwn1, XENAMdwn2, XENAMup1, and XENAMup2; for the 3′-end of AMBN, XAMBNup1 and XAMBNup2; and for the 3′-end of AMEL, XAMELup1 and XAMELup2.
The following PCR primers were also used to amplify intermediate regions of frog SCPP1 and DMP1: XSCPP1up1 used with XSCPP1dwn1, XDMP1up1 and XDMP1dwn3, and XDMP1dwn1 used with XDMP1up2.
For zebrafish SCPP genes, the following primers were used for RACE: for SCPP1, dSCPP1dwn1, dSCPP1dwn2, dSCPP1up1, and dSCPP1up2; for SCPP6, dSCPP6dwn1, dSCPP6dwn2, dSCPP6up1, and dSCPP6up2; for SCPP8, dSCPP8dwn1, dSCPP8dwn2, dSCPP8up1, and dSCPP8up2; and for SCPP9, dSCPP9up1, dSCPP9up2, dSCPP9dwn1, and dSCPP9dwn2.
Nucleotide sequences used in this study
GenBank accession numbers obtained in this study are as follows: mouse SCPPPQ1 (EU642618), frog IBSP (EU642607), DMP1 (EU642605), ODAM (EU642609), ENAM (EU642606), SCPPA1 (EU642616), and SCPPA2 (EU642617), and zebrafish ODAM (EU642608), SCPP1 (EU642610), SCPP5 (EU642611), SCPP6 (EU642612), SCPP7 (EU642613), SCPP8 (EU642614), and SCPP9 (EU642615). The 5′-regions of X. tropicalis AMEL and AMBN were reconstructed from the genome sequence using the nucleotide sequences of the Xenopus laevis orthologs (Toyosawa et al. 1998; Shintani et al. 2003).
Histological analysis and in situ hybridization
The adult frog was obtained from Prof. Martin Flajnik and Dr. Yuko Ohta (University of Maryland), and tadpoles (Daudin’s stage 59; Nieuwkoop and Faber 1967) were purchased from Xenopus I Inc. (Dexter, MI, USA). Adult and 14-day post-fertilization (dpf) zebrafish were obtained from Prof. Keith Cheng and Ms. Peggy Hubley (Penn State University). Both frog tadpoles and zebrafish were fixed with Bouin’s solution (Sigma) and used for in situ hybridization (ISH) analysis as previously described (Kawasaki et al. 2005).
For ISH analysis of frog SCPP genes, probes were prepared from plasmid clones containing the entire 3′-RACE products with the exception of SCPPA2, for which the 689-nucleotide (nt) fragment between XSCPP2up1 to the downstream HindIII site was subcloned. For zebrafish gene analysis, plasmid clones [FDR306-P00046_I05 for ODAM, FDR306-P00007_L05 for SCPP5, FDR103-P00052_M23 for SCPP7, FDR103-P00030_A10 for secreted phosphoprotein 1 (SPP1), and FDR306-P00041_P14 for SPARC], constructed at the Genome Institute of Singapore (Ng et al. 2005), were recloned or subcloned and used for probing. The insert of these plasmid clones was amplified by PCR using M13seq and M13revseq primers, digested with BamHI that has restriction sites at both termini of the vector, and recloned into the BamHI site of pBluescript II SK(+). For SPP1, dSPP1dwn1 (containing a BamHI recognition site) primer was used with M13seq for subcloning the 1,121-nt BamHI fragment. Similarly for SPARC, dSPARCdwn1 (containing a BamHI site) and M13seq primers were used for subcloning the 912-nt fragment. Other zebrafish probes were derived from the PCR products obtained using the following primer pairs: dSCPP1up1-dSCPP1dwn1, dSCPP6up1-dSCPP6dwn1, dSCPP8up1-dSCPP8dwn1, dSCPP9up1-dSCPP9dwn1, and dCOL1up1-dCOL1dwn1 for COL1α1.
Results
SCPPPQ1 and the SCPP gene cluster of stem amniotes
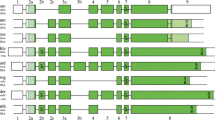
In the current study, I discovered SCPPPQ1 in mammalian and lizard (Anolis carolinensis, version anoCar1) genomes and cloned this gene from the mouse. SCPPPQ1 is clustered in an array with SPARCL1 on the other side of the acidic SCPP genes, hence separated from the other P/Q-rich SCPP genes in the human genome (Fig. 1a). Notably, the last exon of SCPPPQ1 is totally untranslated; that is, the termination codon sits in the penultimate exon. This untranslated last exon is a characteristic shared by all P/Q-rich SCPP genes located between the αS1-casein (CSN1S1) and κ-casein (CSN3) genes (Fig. 1a), indicating their close evolutionary relationship to SCPPPQ1. These findings suggest that the originally contiguous P/Q-rich SCPP gene cluster was split by an intrachromosomal rearrangement before the divergence of mammals and the lizard (Fig. 2).
SCPP gene clusters in the human (a), frog (b), zebrafish (c), and fugu (d) genomes. These maps illustrate location and transcriptional orientation of SPARCL1 (open pentagon), acidic SCPP genes (shaded pentagon), P/Q-rich SCPP genes with the termination codon present in the last exon (closed pentagon), and P/Q-rich SCPP genes possessing the totally untranslated last exon (closed box with open tail). a In the human genome, two large SCPP gene clusters are 17 Mb apart. Gene symbols not described in the text were summarized previously (Kawasaki et al. 2007). b Frog SPARCL1 has not been identified in the genome sequence or EST databases. In the human and frog genomes, AMEL and the other SCPP genes reside on different chromosomes. c In the zebrafish genome, SPP1 and SCPP8 reside on a chromosome different from the other SCPP genes. The P/Q-rich SCPP gene (SCPP5–SCPP9) cluster was originally located adjacent to the SPARCL1–SCPP1 cluster as shown here, but an intrachromosomal rearrangement has separated these two clusters 10 Mb away (arrow). d No linkage between SPP1 and the other SCPP genes has been detected in the fugu genome. Note that zebrafish SCPP5, SCPP7, and ODAM have a totally untranslated last exon, whereas no fugu SCPP genes have such an exon. The untranslated last exons were probably lost from fugu SCPP genes during the evolution of the small genome size (Loh et al. 2008). These maps were based on the following versions of the genome sequences: hg18 (human), xenTro2 (frog), fr2 (fugu), and danRer5 (zebrafish)
Reconstruction of ancient SCPP gene clusters. SCPP genes involved in tissue mineralization and SCPPPQ1 are shown. The SCPP gene cluster was rearranged independently in a stem amniote and an ancestral frog (rearrangements shown by arrows). Because enamel structuring genes have been identified only in tetrapods, the presence or absence of enamel SCPP genes in the stem bony fish (osteichthyans) is not clear. See text for SCPP1 or DMP1 in the stem bony fish
Frog SCPP gene repertoire and gene expression in teeth and bone
I identified four P/Q-rich SCPP genes, including all three major enamel-structuring protein genes, AMEL, AMBN, and ENAM, and four acidic SCPP genes in the frog (X. tropicalis) genome (Fig. 1b). Among these, ENAM and ODAM have not been previously identified in the frog, and SCPPA1 and SCPPA2 never been reported in any vertebrates. Expression of these genes was detected by RT-PCR either in the tooth-bearing upper jaw alone (AMEL, AMBN, ENAM, ODAM, and SCPPA1) or in both the upper and the toothless lower jaws (DMP1, IBSP, and SCPPA2) of an adult frog (data not shown). Then, by ISH using tadpoles, I confirmed expression of AMEL and ODAM in ameloblasts and DMP1 in odontoblasts (Fig. 3f–h and Table 1). These expression domains are similar to those of their mammalian orthologs. Expression of SCPPA2 was weak in odontoblasts and strong in both osteoblasts surrounding bone and osteocytes embedded in bone (Fig. 3i, j and Table 1), indicating the use of SCPPA2 primarily in bone but also more weakly in dentin. Expression of SCPPA1 was not confirmed by ISH.
Expression of frog AMEL, ODAM, DMP1, and SCPPA2. The upper jaw of frog tadpoles was sagittally dissected (a) and stained (b, c, e) or used for ISH analysis (f–j). Tooth buds and the maxillary bone are indicated by rectangles (b) and separately shown in (c) and (e). The histological section in c was illustrated in d, showing tall columnar ameloblasts (Am, closed arrowhead; these cells correspond to the IDE cells in zebrafish teeth) lining outside the tooth enamel (E) and odontoblasts (Od, open arrowhead) extending cell processes into dentin (D) from inside. Enamel was dissolved by the acidic fixative and a white gap remains in the enamel space (c, d). Closed and open arrowheads in f–i also indicate ameloblasts and odontoblasts, respectively. The orange and blue areas between ameloblasts and odontoblasts in c and d represent mineralized dentin and not-yet mineralized predentin, respectively, and those between mesenchymal cells in e indicate mineralized bone (B) and unmineralized collagenous ECM, respectively. ISH analysis reveals expression of AMEL (f) and ODAM (g), DMP1 (h), and SCPPA2 (i) in tooth buds, and SCPPA2 (j) in bone at an early developmental stage. Probes used in ISH analysis are shown at the bottom (f–j)
SCPP gene cluster of early frogs and stem tetrapods
In the frog genome, P/Q-rich ODAM is only 33-kb from acidic SCPPA1 (Fig. 1b, not to scale). In contrast, ENAM resides 112-kb away from ODAM in the opposite transcriptional direction. The order and transcriptional directions of AMBN, ENAM, and ODAM in the human and frog genomes suggest that a small inversion has split the originally contiguous ENAM–AMBN–ODAM array in an ancestral frog after the divergence from the amniote lineage (Fig. 2), corroborating our earlier study (Kawasaki et al. 2007). The reconstructed genomic orders of SCPP genes in stem amniotes and ancestral frogs are consistent, which further suggests the order of SCPP genes in stem tetrapods (Fig. 2). The location of AMEL is unclear but, before translocation to a different chromosome, ancient AMEL was most likely located adjacent to a closely related enamel gene, AMBN or ENAM.
Teleost SCPP gene repertoire and gene cluster
In the zebrafish genome, I identified eight SCPP genes. Among these genes, five orthologs were identified in the fugu genome based on amino acid sequence similarity, but three were not found (SCPP6, SCPP8, and SCPP9 in Fig. 1c, d). I have now identified SCPP2 as the teleost ortholog of ODAM, based on their similarities in the coded amino acid sequence, size distribution of exons, totally untranslated last exon, original location within the cluster (Figs. 1 and 2, see below), and common expression pattern in the IDE cells as described below.
Among these, all five P/Q-rich SCPP genes (SCPP5, SCPP6, SCPP7, SCPP9, and ODAM) are clustered 10-megabases away from acidic SCPP1 on the same chromosome (Fig. 1c). These P/Q-rich SCPP genes appear to have been recently separated from SCPP1 in the ancestral zebrafish (arrow in Fig. 1c) because all these SCPP genes are clustered in the fugu (Fig. 1d), medaka, and stickleback genomes. In contrast, two other acidic SCPP genes, SPP1 and SCPP8, are clustered together but on a different chromosome from the other SCPP genes in the zebrafish (Fig. 1c) and stickleback. Similarly, no chromosomal linkage between SPARCL1 and SPP1 has been detected in fugu (Fig. 1d) or medaka, suggesting that these two loci separated before the divergence of these teleosts.
Zebrafish SCPP gene, SPARC, and COL1 expression in teeth and the jaw
Expression of all these eight zebrafish SCPP genes, COL1, and SPARC was detected in the tooth-bearing pharyngeal jaw by RT-PCR (data not shown). ISH analysis confirmed expression of five zebrafish SCPP genes (SCPP1, SPP1, SCPP5, ODAM, and SCPP9) in adult dental tissues (Fig. 4f–r), whereas expression of the other three genes (SCPP6, SCPP7, and SCPP8) has not been detected in teeth or other parts of the jaws. Table 1 summarizes the results of ISH analysis.
Expression of zebrafish SCPP, SPARC, and COL1 genes. The pharyngeal jaw was coronally dissected (a) and stained (b–e) or used for ISH analysis (f–t). A tooth bud and the attachment bone (fused with the jaw bone) of an erupted tooth are indicated by rectangles in the left half of the pharyngeal jaw (b). Adult zebrafish have teeth at various developmental stages in the pharyngeal jaw (Van der heyden and Huysseune 2000; Stock 2007). Three representative developmental stages of tooth buds are shown: the secretory stage of enameloid (c) and early (d) and late (e) secretory stages of dentin (maturation stages of enameloid). Closed and open arrowheads indicate the IDE cells (IDE) and odontoblasts (Od), respectively (c–r). See Fig. 3d for distributions of the IDE cells and odontoblasts. The blue area between the IDE cells and odontoblasts in c represents not-yet mineralized pre-enameloid. The white gap (Ed) in d and e represents the space of enameloid, dissolved by the acidic fixative. The orange areas (D) underlying the white gap represent mineralized dentin (d, e). ISH analysis reveals expression of SCPP1 (f, g), SCPP9 (h, i), ODAM (j, k), SCPP5 (l, m), SPP1 (n), SPARC (o, p), and COL1 (q, r) in tooth buds, and SCPP1 (s) and SPP1 (t) in the attachment bone and the jawbone. Probes and developmental stages of tooth buds (c, d, or e) in each ISH analysis (f–t) are shown at the bottom
Initially weak expression of SCPP1 by odontoblasts at the secretory stage of enameloid is upregulated during dentin formation (Fig. 4f, g), which is consistent with our previous study in fugu (Kawasaki et al. 2005). In this study, weak expression of SCPP1 was also detected in the IDE cells and osteocytes located within the jaw bone and the attachment bone of an adult (Fig. 4g, s). These observations suggest the use of SCPP1 primarily in dentin but also weakly in bone and probably in enameloid as well.
Strong expression of SPP1 was detected in cells present on the surface of the attachment bone and the jaw bone and weak expression was also detected in odontoblasts (Fig. 4n, t). Because the distribution of SPP1- and COL1-expressing cells on these bones are well correlated, this SPP1-expressing cell population appears to contain osteoblasts (see also below). This expression pattern demonstrates the use of SPP1 primarily in bone and slightly in dentin, similar to the mammalian ortholog (Fujisawa et al. 1993; Qin et al. 2001).
Expression of both SCPP1 and SPP1 was further analyzed in 14-dpf larvae. At this stage, three functional teeth and two successive teeth are supported by each pharyngeal jaw (fifth ceratobranchial; CBV) via the attachment bone (Fig. 5a; Van der heyden and Huysseune 2000). Expression of SCPP1 was detected in the dental pulp, where odontoblasts reside (Fig. 5b). However, significant expression was not observed within or on the surface of the attachment bone, although some cells are located within bone matrix. These results in both adults and larvae suggest that SCPP1 is important for later stages of bone formation, probably for homeostasis of bone, which is reminiscent of the function of tetrapod DMP1 in bone (Toyosawa et al. 2001).
Expression of zebrafish SCPP1 and SPP1 in 14-dpf larvae. The whole body was sagittally sectioned (a, 1) and used for ISH (b–e; upper half) and histological (b–e; lower half) analyses. At 14 dpf, three functional teeth (FT) and two successive teeth (ST) are attached to CBV (a, 3, 5), located posterior to ceratohyal (CH) and the first four ceratobranchials (CBI-IV in a, 2), via the attachment bone. For these teeth, the border between dentin (D) and bone (B) matrices is indistinguishable (a, 4). In addition to these five teeth, tooth matrix is deposited in four tooth buds (TB in b). Strong hybridization signals are shown by an open arrowhead (b–e). Probes used in this analysis are indicated at the bottom of the upper half (b–e). Note that bone matrix in c–e is largely blue, demonstrating that these portions are not yet mineralized or the degree of mineralization is considerably low. T tooth, MB membrane bone
In 14-dpf larvae, remarkably strong expression of SPP1 was detected in teeth and the jaw, the bottom of the attachment bone (Fig. 5c), a perichondrial region of the CBV (Fig. 5d), and the membrane bone growing from the CBV (Fig. 5e). The SPP1-expressing cells located on the newly deposited mineralizing membrane bone are probably largely osteoblasts. This observation is consistent with a recent study showing that SPP1 expression is strong in active osteoblasts at early developmental stages in the zebrafish (Laue et al. 2008). In contrast, the bottom of the attachment bone, where a functional tooth and its successive tooth are associated (Fig. 5a, c), is under remodeling by both osteoblasts and osteoclasts (Huysseune et al. 1998). Expression of SPP1 in osteoclasts is known in mammals (Sodek et al. 2000) but remains to be confirmed in teleosts.
The expression pattern of SCPP5 (Fig. 4l, m) and SPARC (Fig. 4o, p) is similar, strong in both the IDE cells and odontoblasts during deposition of enameloid and dentin matrix. These results are consistent with our previous study using fugu and suggest that SCPP5 and SPARC are included in both the enameloid and dentin matrix.
Strong expression of COL1 was detected in both the IDE cells and odontoblasts during deposition of enameloid matrix (Fig. 4q) and only in odontoblasts during dentin formation (Fig. 4r). The results of this analysis are consistent with our previous study but reveal the expression pattern in more detail. The strong expression of COL1 in the IDE cells is immediately downregulated at the maturation stage of cap enameloid covering the apex of teeth. At this stage, weak COL1 expression is detected in the IDE cells adjacent to the nascent matrix for the tooth body (Fig. 4r). A distinct thin hypermineralized tissue, called collar enamel or enameloid, is known to surround the teleost tooth body (Sasagawa and Ishiyama 1988; Smith 1995). These results suggest that collar enameloid is also produced by both the IDE cells and odontoblasts, similar to cap enameloid.
Strong expression of SCPP9 and ODAM was detected only in the IDE cells at the maturation stage of enameloid after the underlying enameloid is fully mineralized (Fig. 4h, j). This late expression was not identified previously due to the limited developmental stages studied (Kawasaki et al. 2005), but now, this result suggests that neither SCPP9 nor ODAM is included in enameloid matrix, but instead, these proteins are both involved in the hypermineralization process, which I will discuss below. Subsequently, the IDE cells overlying the fully mineralized tooth body also express SCPP9 and ODAM (Fig. 4i, k), suggesting that SCPP9 and ODAM are also involved in hypermineralization of collar enameloid, in addition to cap enameloid.
Discussion
Major bone SCPP genes in tetrapods and teleosts
SPP1 is the major bone SCPP in mammals, strongly expressed in both osteoblasts and osteocytes (Sodek et al. 2000). SPP1 has also been detected in dentin extracts at a level less than 1/10 or 1/70 of bone extracts (Fujisawa et al. 1993; Qin et al. 2001). Similarly, expression of zebrafish SPP1 is strong in osteoblasts and weak in odontoblasts as described above.
In the frog, expression of SCPPA2 is strong in both osteoblasts and osteocytes, similar to mammalian SPP1. However, SCPPA2 does not show any significant sequence homology to SPP1 even in the region around their integrin-binding Arg-Gly-Asp sequence that is well conserved in SPP1 from mammals to teleosts (Bobe and Goetz 2001). Instead, SCPPA2 shows the highest amino acid sequence identity to mammalian DMP1 in the N-terminal region coded by exon 2. Moreover, the location of SCPPA2 is not equivalent to that of SPP1 within the gene cluster (Fig. 2). To date, no SPP1 ortholog has been identified in the frog genome sequence or expressed sequence tag (EST; X. tropicalis and X. laevis) databases, even though SPP1 is abundant in EST databases of other vertebrates, including zebrafish and stickleback. These results suggest that frog SCPPA2 is not orthologous to mammalian or teleost SPP1, although these genes may have adopted similar functions in bone mineralization.
Major dentin SCPP genes in tetrapods and teleosts
In mammals, expression of DSPP is strong in dentin and weak in bone (Qin et al. 2002). This gene has not been identified in the frog, however. Although SCPPA1 is located upstream of DMP1 at the position equivalent to mammalian DSPP (Fig. 1a, b), expression of SCPPA1 was not detected in dentin or bone in the tadpole. In mammals, DMP1 is expressed in both odontoblasts and osteocytes (Toyosawa et al. 2001; Qin et al. 2007). In this study, RT-PCR demonstrated expression of frog DMP1 in the toothless lower jaw, possibly in bone; yet, ISH analysis failed to detect expression of DMP1 in osteocytes or osteoblasts. These results show that expression of frog DMP1 in bone is, if any, considerably weaker than that in dentin. Thus, DMP1 is the major dentin SCPP gene in the frog.
Expression of zebrafish SCPP1 is strong in odontoblasts and weak in osteocytes (Fig. 4g, s). The strong expression of teleost SCPP1 in odontoblasts is similar to that of both mammalian DSPP and frog DMP1, and the expression in both odontoblasts and osteocytes is common to that of mammalian DMP1. In addition, the location of SCPP1 within the cluster is similar to that of both DSPP and DMP1, upstream of the major bone SCPP genes, SPP1 and SCPPA2 (Fig. 2). Despite these similarities, SCPP1 does not show any significant amino acid sequence similarity to DSPP or DMP1 (with the exception of the signal peptide relatively well conserved across distinct SCPPs). Although orthology between SCPP1 and DMP1 or DSPP remains to be confirmed, the similar expression profiles and phylogenetic distributions of these genes suggest that either SCPP1 or DMP1, or perhaps a closely related acidic SCPP gene primarily used in dentin, was already present in stem osteichthyans (Fig. 2).
Common and different SCPP genes used in teleost enameloid
ODAM was initially purified as a short C-terminal product, coded by exons 6–11, from amyloid associated with human calcified epithelial odontogenic tumor (Solomon et al. 2003). The N-terminal of a longer product was subsequently identified from the rat enamel organ (Moffatt et al. 2006a). Because SCPP2 (teleost ODAM) shows highest sequence similarity to mammalian and frog ODAM at the N-terminal region coded by exons 2–4, fugu SCPP2 was not identified as the ortholog of ODAM in our earlier study (Kawasaki et al. 2005).
Strong expression of zebrafish ODAM and SCPP9 was detected only in the IDE cells, which is similar to ODAM and SCPP4 in fugu (Kawasaki et al. 2005). This similar expression pattern suggests a close relationship between zebrafish SCPP9 and fugu SCPP4. However, while zebrafish, medaka, and stickleback SCPP9 all show high sequence similarity to one another, none of these SCPP9 sequences has significant similarity to puffer fish (fugu and Tetraodon) SCPP4. Furthermore, the location of zebrafish SCPP9 and fugu SCPP4 is not equivalent within the gene cluster (Fig. 1c, d). Thus, SCPP9 does not appear to be orthologous to SCPP4, although these two genes may have common functions.
During the formation of fugu enameloid and dentin, strong expression of two closely related SCPP3 genes (SCPP3A and SCPP3B) was detected in the inner pharyngeal epithelium overlying tooth buds (Kawasaki et al. 2005). Similar SCPP3 genes are also present in the medaka and stickleback genomes but have not been identified in the zebrafish genome. Thus, among SCPP genes used in mineralization, only four SCPP genes, SCPP5, ODAM, SCPP1, and SPP1, have been confirmed in all these teleosts (Fig. 2).
Common hypermineralization machinery in enamel and enameloid
At the onset of the enamel maturation stage, a specialized basal lamina is formed between ameloblasts and the enamel surface (Takano 1979; Nanci et al. 1993; Al Kawas and Warshawsky 2008). During the maturation stage, ameloblasts actively transport substantial calcium ions to enamel, while water and enzymatically degraded ECM proteins are removed from enamel (Smith 1998; Simmer and Hu 2002). In rodents, expression of both AMTN and ODAM is detected in ameloblasts at this stage (Iwasaki et al. 2005; Park et al. 2007). While AMTN is localized precisely to the basal lamina, ODAM is distributed more diffusely along apical surfaces of ameloblasts abutting the basal lamina (Moffatt et al. 2006b; Moffatt et al. 2008).
In contrast to enamel formation, the basal lamina is absent at the maturation stage of enameloid (Sasagawa 1997), implying that AMTN-like basal lamina proteins are not used in enameloid. However, the expression of zebrafish ODAM is detected in the IDE cells at this stage. The similar expression pattern of ODAM in both tetrapods and teleosts strongly suggests that ancient hypermineralization machinery, which arose at least before the divergence of actinopterygians and sarcopterygians, has descended to both enamel and enameloid. On the other hand, specialized scaffold proteins are not necessarily required for hypermineralization as shown by teleost enameloid. Thus, I hypothesize that ODAM or its closely related P/Q-rich SCPP genes evolved early and were used for tissue hypermineralization, and one of these genes was later co-opted for use in more specialized enamel-structuring proteins.
Phylogenetic distributions of the P/Q-rich SCPP genes possessing the totally untranslated last exon (Fig. 1) suggest that ODAM is probably the last common ancestor of the casein genes, whose products make milk supersaturated with calcium phosphate and prevent its precipitation in the mammary glands (Little and Holt 2004; Smyth et al. 2004). In fact, expression of ODAM itself has been detected in lactating mammary glands (Rijnkels et al. 2003). Thus, I suggest that, during enamel hypermineralization, ODAM creates a specialized microenvironment preventing spontaneous calcium phosphate precipitation and facilitating its transport between the hypermineralizing tooth surface and the overlying dental epithelium. This function appears to be cardinal to the hypermineralization process in general and is not opposed to but rather complements the previous proposition that ODAM mediates adhesion of dental epithelium to the tooth surface (Moffatt et al. 2008).
Genetic similarities and differences among bone, dentin, enameloid, and enamel
I have shown that in both the frog and zebrafish, some acidic SCPP genes are strongly expressed in dentin and weakly expressed in bone or vice versa (although expression of frog DMP1 has not been detected in bone). These findings are consistent with the idea that dentin and bone use common SCPPs, and their essential differences lie in their composition and post-translational modifications, as suggested by studies of mammalian acidic SCPPs (Qin et al. 2001; Butler et al. 2003; Qin et al. 2004). The use of common SCPPs with different compositions in bone and dentin, in both tetrapods and teleosts, suggests a close evolutionary relationship between these two tissues.
ODAM and SCPP9 are not included in the enameloid ECM. Therefore, the shared expression of COL1, SPARC, SCPP5, and SCPP1 in odontoblasts and the IDE cells indicates the use of common ECM proteins in dentin and enameloid, although their composition and post-translational modifications may be different. Pre-enameloid is thus similar to predentin, but enameloid is hypermineralized after initial mineralization.
Both teleost enameloid and tetrapod enamel use similar ODAM-based hypermineralization machinery descended from their common ancestral tissue. Thus, the significant difference between these two tissues lies in the use of specialized P/Q-rich SCPPs as the mineralization scaffold in enamel (Fincham et al. 1999), demonstrating the divergence of enamel from enameloid, dentin, and bone.
It has been proposed that the enamel–enameloid–dentin system forms a continuum of tissues that have diverged from one another (Meinke 1982; Meinke and Thomson 1983). Recent biomechanical, paleontological, and genetic studies integrate bone (Currey 2002; Kawasaki and Weiss 2008) and also cartilage in the continuum (Donoghue et al. 2006). The results of this study illustrate graded differences among the four principal dental tissues, which reinforces the hypothesis that enamel–enameloid–dentin–bone constitutes an evolutionary continuum. It is possible that distinct SCPP genes were independently co-opted for different mineralized tissues. However, it is more likely that an early SCPP gene was employed by the initial mineralized tissue, and new duplicated SCPP genes were used for subsequently diversified mineralized tissues. The reconstructed genomic array of SCPP genes in the stem osteichthyan genome is arranged as ENAM/AMBN–ODAM–(SCPP1 or DMP1)–SPP1, although the enamel structuring genes may have arisen later in sarcopterygians (Fig. 2). Interestingly, this arrangement is reminiscent of the tissue grades in the mineralization continuum. Thus, I assume that early tandem duplications of SCPP genes were correlated with incremental diversification of mineralized tissues. This hypothesis could be tested by studying the distribution of mineralized tissues early in vertebrate phylogeny and SCPP genes in various additional vertebrates, especially chondrichthyans.
References
Al Kawas S, Warshawsky H (2008) Ultrastructure and composition of basement membrane separating mature ameloblasts from enamel. Arch Oral Biol 53:310–317
Bartlett JD, Ganss B, Goldberg M, Moradian-Oldak J, Paine ML, Snead ML, Wen X, White SN, Zhou YL (2006) Protein–protein interactions of the developing enamel matrix. Curr Top Dev Biol 74:57–115
Bobe J, Goetz FW (2001) A novel osteopontin-like protein is expressed in the trout ovary during ovulation. FEBS Lett 489:119–124
Butler WT, Brunn JC, Qin C (2003) Dentin extracellular matrix (ECM) proteins: comparison to bone ECM and contribution to dynamics of dentinogenesis. Connect Tissue Res 44(Suppl 1):171–178
Currey JD (2002) Bones: structure and mechanics. Princeton University Press, Princeton
Donoghue PC, Sansom IJ, Downs JP (2006) Early evolution of vertebrate skeletal tissues and cellular interactions, and the canalization of skeletal development. J Exp Zoolog B Mol Dev Evol 306B:278–294
Fincham AG, Moradian-Oldak J, Simmer JP (1999) The structural biology of the developing dental enamel matrix. J Struct Biol 126:270–299
Fujisawa R, Butler WT, Brunn JC, Zhou HY, Kuboki Y (1993) Differences in composition of cell-attachment sialoproteins between dentin and bone. J Dent Res 72:1222–1226
Hall BK (2005) Bones and cartilage: developmental and evolutionary skeletal biology. Elsevier, San Diego
Huysseune A, Van der heyden C, Sire JY (1998) Early development of the zebrafish (Danio rerio) pharyngeal dentition (Teleostei, Cyprinidae). Anat Embryol (Berl) 198:289–305
Iwasaki K, Bajenova E, Somogyi-Ganss E, Miller M, Nguyen V, Nourkeyhani H, Gao Y, Wendel M, Ganss B (2005) Amelotin—a novel secreted, ameloblast-specific protein. J Dent Res 84:1127–1132
Kawasaki K, Weiss KM (2003) Mineralized tissue and vertebrate evolution: the secretory calcium-binding phosphoprotein gene cluster. Proc Natl Acad Sci U S A 100:4060–4065
Kawasaki K, Weiss KM (2006) Evolutionary genetics of vertebrate tissue mineralization: the origin and evolution of the secretory calcium-binding phosphoprotein family. J Exp Zoolog B Mol Dev Evol 306B:295–316
Kawasaki K, Weiss KM (2007) Genetic basis for the evolution of vertebrate mineralized tissue. In: Bäuerlein E (ed) Handbook of biomineralization. Wiley-VCH, Weinheim, pp 331–347
Kawasaki K, Weiss KM (2008) SCPP gene evolution and the dental mineralization continuum. J Dent Res 87:520–531
Kawasaki K, Shimoda S, Fukae M (1987) Histological and biochemical observations of developing enameloid of the sea bream. Adv Dent Res 1:191–195
Kawasaki K, Suzuki T, Weiss KM (2004) Genetic basis for the evolution of vertebrate mineralized tissue. Proc Natl Acad Sci U S A 101:11356–11361
Kawasaki K, Suzuki T, Weiss KM (2005) Phenogenetic drift in evolution: the changing genetic basis of vertebrate teeth. Proc Natl Acad Sci U S A 102:18063–18068
Kawasaki K, Buchanan AV, Weiss KM (2007) Gene duplication and the evolution of vertebrate skeletal mineralization. Cells Tissues Organs 186:7–24
Laue K, Jänicke M, Plaster N, Sonntag C, Hammerschmidt M (2008) Restriction of retinoic acid activity by Cyp26b1 is required for proper timing and patterning of osteogenesis during zebrafish development. Development 135:3775–3787
Linde A, Goldberg M (1993) Dentinogenesis. Crit Rev Oral Biol Med 4:679–728
Little EM, Holt C (2004) An equilibrium thermodynamic model of the sequestration of calcium phosphate by casein phosphopeptides. Eur Biophys J 33:435–447
Loh YH, Brenner S, Venkatesh B (2008) Investigation of loss and gain of introns in the compact genomes sof pufferfishes (fugu and tetraodon). Mol Biol Evol 25:526–535
Meinke DK (1982) A histological and histochemical study of developing teeth in Polypterus (Pisces, Actinopterygii). Arch Oral Biol 27:197–206
Meinke DK, Thomson KS (1983) The distribution and significance of enamel and enameloid in the dermal skeleton of osteolepiform. Paleobiology 9:138–149
Moffatt P, Smith CE, Sooknanan R, St-Arnaud R, Nanci A (2006a) Identification of secreted and membrane proteins in the rat incisor enamel organ using a signal-trap screening approach. Eur J Oral Sci 114(Suppl 1):139–146
Moffatt P, Smith CE, St-Arnaud R, Simmons D, Wright JT, Nanci A (2006b) Cloning of rat amelotin and localization of the protein to the basal lamina of maturation stage ameloblasts and junctional epithelium. Biochem J 399:37–46
Moffatt P, Smith CE, St-Arnaud R, Nanci A (2008) Characterization of Apin, a secreted protein highly expressed in tooth-associated epithelia. J Cell Biochem 103:941–956
Nanci A, Zalzal S, Kogaya Y (1993) Cytochemical characterization of basement membranes in the enamel organ of the rat incisor. Histochemistry 99:321–331
Nanci A (2003) Ten Cate’s oral histology, 6th edn. Mosby, St. Louis
Ng P, Wei CL, Sung WK, Chiu KP, Lipovich L, Ang CC, Gupta S, Shahab A, Ridwan A, Wong CH, Liu ET, Ruan Y (2005) Gene identification signature (GIS) analysis for transcriptome characterization and genome annotation. Nat Methods 2:105–111
Nieuwkoop PD, Faber J (1967) Normal table of Xenopus laevis (Daudin), 2nd edn. North-Holland, Amsterdam
Paine ML, Snead ML (2005) Tooth developmental biology: disruptions to enamel–matrix assembly and its impact on biomineralization. Orthod Craniofac Res 8:239–251
Park JC, Park JT, Son HH, Kim HJ, Jeong MJ, Lee CS, Dey R, Cho MI (2007) The amyloid protein APin is highly expressed during enamel mineralization and maturation in rat incisors. Eur J Oral Sci 115:153–160
Poole DFG (1967) Phylogeny of tooth tissues: Enameloid and enamel in recent vertebrates with a note in the history of cementum. In: Miles AEW (ed) Structural and chemical organization of teeth. Academic, New York, pp 111–149
Poole DFG (1971) An introduction to the phylogeny of calcified tissues. In: Dahlberg AA (ed) Dental morphology and evolution. University of Chicago Press, Chicago, pp 65–79
Qin C, Brunn JC, Jones J, George A, Ramachandran A, Gorski JP, Butler WT (2001) A comparative study of sialic acid-rich proteins in rat bone and dentin. Eur J Oral Sci 109:133–141
Qin C, Brunn JC, Cadena E, Ridall A, Tsujigiwa H, Nagatsuka H, Nagai N, Butler WT (2002) The expression of dentin sialophosphoprotein gene in bone. J Dent Res 81:392–394
Qin C, Baba O, Butler WT (2004) Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med 15:126–136
Qin C, D’Souza R, Feng JQ (2007) Dentin matrix protein 1 (DMP1): new and important roles for biomineralization and phosphate homeostasis. J Dent Res 86:1134–1141
Rijnkels M, Elnitski L, Miller W, Rosen JM (2003) Multispecies comparative analysis of a mammalian-specific genomic domain encoding secretory proteins. Genomics 82:417–432
Sasagawa I (1997) Fine structure of the cap enameloid and of the dental epithelial cells during enameloid mineralisation and early maturation stages in the tilapia, a teleost. J Anat 190(Pt 4):589–600
Sasagawa I, Ishiyama M (1988) The structure and development of the collar enameloid in two teleost fishes, Halichoeres poecilopterus and Pagrus major. Anat Embryol (Berl) 178:499–511
Shellis RP, Miles AEW (1974) Autoradiographic study of the formation of enameloid and dentine matrices in teleost fishes using tritiated amino acids. Proc R Soc Lond B 185:51–72
Shintani S, Kobata M, Toyosawa S, Ooshima T (2003) Identification and characterization of ameloblastin gene in an amphibian, Xenopus laevis. Gene 318:125–136
Simmer JP, Fincham AG (1995) Molecular mechanisms of dental enamel formation. Crit Rev Oral Biol Med 6:84–108
Simmer JP, Hu JC (2002) Expression, structure, and function of enamel proteinases. Connect Tissue Res 43:441–449
Smith CE (1998) Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med 9:128–161
Smith MM (1992) Microstructure and evolution of enamel amongst osteichthyan and early tetrapods. In: Smith P, Tchernov E (eds) Structure, function and evolution of teeth. Freund, Tel Aviv, pp 73–101
Smith MM (1995) Heterochrony in the evolution of enamel in vertebrates. In: McNamara KJ (ed) Evolutionary change and heterochrony. Wiley, Chichester, pp 125–150
Smyth E, Clegg RA, Holt C (2004) A biological perspective on the structure and function of caseins and casein micelles. Int J Dairy Technol 57:121–126
Sodek J, Ganss B, McKee MD (2000) Osteopontin. Crit Rev Oral Biol Med 11:279–303
Solomon A, Murphy CL, Weaver K, Weiss DT, Hrncic R, Eulitz M, Donnell RL, Sletten K, Westermark G, Westermark P (2003) Calcifying epithelial odontogenic (Pindborg) tumor-associated amyloid consists of a novel human protein. J Lab Clin Med 142:348–355
Stock DW (2007) Zebrafish dentition in comparative context. J Exp Zoolog B Mol Dev Evol 308:523–549
Takano Y (1979) Cytochemical studies of ameloblasts and the surface layer of enamel of the rat incisor at the maturation stage. Arch Histol Jpn 42:11–32
Toyosawa S, O’hUigin C, Figueroa F, Tichy H, Klein J (1998) Identification and characterization of amelogenin genes in monotremes, reptiles, and amphibians. Proc Natl Acad Sci U S A 95:13056–13061
Toyosawa S, Shintani S, Fujiwara T, Ooshima T, Sato A, Ijuhin N, Komori T (2001) Dentin matrix protein 1 is predominantly expressed in chicken and rat osteocytes but not in osteoblasts. J Bone Miner Res 16:2017–2026
Van der heyden C, Huysseune A (2000) Dynamics of tooth formation and replacement in the zebrafish (Danio rerio) (Teleostei, Cyprinidae). Dev Dyn 219:486–496
Van der heyden C, Huysseune A, Sire JY (2000) Development and fine structure of pharyngeal replacement teeth in juvenile zebrafish (Danio rerio) (Teleostei, Cyprinidae). Cell Tissue Res 302:205–219
Acknowledgments
I thank Prof. Keith C. Cheng and Ms. Peggy Hubley at Penn State University for providing me with zebrafish, Prof. Martin Flajnik and Dr. Yuko Ohta at the University of Maryland for the frog, and Dr. Chia-Lin Wei and Mr. Yow Jit Sin at the Genome Institute of Singapore for zebrafish clones. I am grateful to Prof. Kenneth M. Weiss and Dr. Anne V. Buchanan at Penn State University and Dr. Samuel Sholtis at Yale University for critical discussion and generous comments. This work was made possible by the financial support from awards SBR9804907 and BCS0343442 from the US National Science Foundation, and by research funds from Penn State University to Prof. Kenneth M. Weiss; and by NIH grant 5R24RR017441, and research grants from the Jake Gittlen Cancer Research Foundation and the Pennsylvania Tobacco Funds to Prof. Keith C. Cheng.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Hammerschmidt
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 31.5 KB)
Rights and permissions
About this article
Cite this article
Kawasaki, K. The SCPP gene repertoire in bony vertebrates and graded differences in mineralized tissues. Dev Genes Evol 219, 147–157 (2009). https://doi.org/10.1007/s00427-009-0276-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-009-0276-x