Abstract
In a spatial cueing paradigm it was investigated whether endogenous orienting is sensitive to orienting processes in the previous trial. Specifically, the effect of the previous cue direction, the previous trial type (valid, invalid, neutral, catch) and target alternation effects were studied. Strategic effects were shown as attentional costs and benefits were larger after a valid than after an invalid trial. Following catch trials, an overall response slowing was observed, but costs and benefits were unaffected. This was interpreted as a reduction in alertness and as support for the dissociation between spatial and temporal attentional mechanisms. Repetition of target position per se had no effect, but in neutral trials responses were slower to targets appearing at the location that was cued in the previous trial, independent of validity of the preceding trial. This suggests that long-term inhibition-of-return can occur between trials when attention is controlled endogenously.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traditionally, most reaction time (RT) studies report only average results. However, research on sequence effects has indicated that individual trials typically do not contribute to mean RT independently, because their effect is influenced by preceding trials (e.g., Bertelson, 1961, 1963; Bertelson & Renkin, 1966; Hyman, 1953; Kirby, 1976, 1980; McKenna & Sharma, 2004; Soetens, 1998). Therefore, exploration of the effect of preceding events may lead to a better understanding of the way we process signals (Soetens, 1990).
Many studies of visual attention have shown that, on average, subjects respond faster to a target when they are provided with valid advance information about its location by means of a symbolic cue presented at fixation (e.g., Posner, 1980). Yet, the extant literature on this endogenous cueing paradigm does not, to our knowledge, contain a systematic study of sequence effects. The aim of the present study was to examine sequence effects in an endogenous spatial cueing task that was similar to the one used by Posner, Nissen, and Ogden (1978). Cues with a predictive validity of either 50 or 80% were used to generate ‘neutral’, ‘valid’, and ‘invalid’ trials, and catch trials were added to prevent premature responses. According to Kirby (1980), sequence effects in RT tasks may originate from at least two sources. First, there may be automatic facilitation as a result of residual activity in a stimulus or response system or the bypassing of central coding processes. Second, the subject may adopt strategies that are carried out before or after arrival of the stimulus. As shown below, the literature on sequence effects in other RT and attention tasks leads to some interesting hypotheses on both automatic and strategical sequence effects in an endogenous spatial cueing task.
A study by Maylor and Hockey (1987) provides a fine example of the role of automatic processes in sequence effects in a spatial cueing paradigm. In an exogenous cueing paradigm, when the cue–target interval was 500 ms, responses to a target at a given location were slowed when either the cue of the current trial or the target of the previous trial had been presented at that location. In a second and a third experiment where only targets were presented and the preceding four trials were included in the analysis, the results again showed slower responses when the same target location was repeated. This slowing was interpreted as a manifestation of ‘inhibition of return’ (IOR); the presumed tendency of attention to be inhibited to return to a location where it has just been, thus favoring novel locations (e.g., Posner & Cohen, 1984; Posner, Rafal, Chaote, & Vaughan, 1985; Kwak & Egeth, 1992). Effects of IOR are likely automatic (Posner & Cohen, 1984; Jonides, 1981). They have also been demonstrated in endogenous attention tasks, when two peripheral signals were presented in sequence and both asked for a manual response (Pratt & Abrams, 1999; Taylor & Klein, 2000). In our endogenous spatial cueing paradigm (Posner et al., 1978), this is comparable to the case of two target stimuli in subsequent trials. Therefore, automatic effects of peripheral onsets in trial ‘n−1’ might have an inhibitory effect on target detection in trial ‘n’ if the target is presented at the same location and if inhibitory effects survive after the attentional response to the intermediate cue. Relatively fast responses to target alternations would then be predicted.
Several others have invoked the IOR mechanism to explain sequence effects in attention tasks with informative or uninformative central or peripheral events. Although Posner and Cohen (1984) found no evidence for IOR when central predictive cues were used, it was demonstrated in a study by Rafal, Calabresi, Brennan, and Sciolio (1989), but only when eye movements to the target location were made or planned. In a later study, Taylor and Klein (2000) also found IOR when central cues were used and eyes were kept fixated. In this paradigm, two signals were presented on each trial that were peripheral onsets or central arrows. The first was always uninformative with respect to the second. No response, a manual response or a saccadic response was made to the first and the second signal. IOR was shown when the first signal was central and required no response, and the second signal was peripheral and required a manual response. This build-up of a trial has similarities with a standard endogenous cueing task (Posner et al., 1978), but an important difference is that in Taylor and Klein’s study, the first signal was always uninformative with respect to the second. This was done to prevent effects of voluntary attention. As stated by Taylor and Klein, previous reports with central arrow cues may have failed to observe IOR because inhibitory effects of central arrow cues were “overpowered” by facilitatory effects of voluntary orienting. Since our arrow cues are valid 80% of the time, we do not expect an effect of IOR within trials for the same reason. However, studies by Maylor and Hockey (1987) and Pratt and Abrams (1999) have shown that effects of IOR can carry over to the next trial. While a cue will be predictive concerning the target location in that particular trial, it will not be predictive with regard to the next trial. Therefore, central cues that direct attention to the left or to the right in one trial might have an inhibitory effect on attentional orienting to the same location in the next trial.
In summary, as an automatic effect, a target alternation effect may be observed, based on various studies that used peripheral stimuli (Maylor & Hockey, 1987; Pratt & Abrams, 1999; Taylor & Klein, 2000). Furthermore, an analysis of sequence effects is apt to investigate whether central endogenous cues lead to IOR, because the cue on trial ‘n−1’ has lost all its predictive power after the target in that trial has elapsed, possibly leaving traces of IOR that can be measured on trial ‘n’ because they are not overpowered anymore by voluntary orienting in trial ‘n−1’. Add to this other indications that IOR can occur between trials (Maylor & Hockey, 1987; Pratt & Abrams, 1999), and the hypothesis ensues that central cues have an inhibitory effect on attentional orienting to the same location in the next trial. Both effects of orienting in trial ‘n−1’ would be visible in pure form when the cue in trial ‘n’ is neutral, and could simply add up to the effect of the cue in trial ‘n’ if it is directional.
A role for strategic adjustments in sequence effects has been demonstrated in focused attention or conflict tasks. Gratton et al. (1992, exp. 1) used a noise-compatibility paradigm (Eriksen & Eriksen, 1974), and studied changes from trial to trial in the participants’ sensitivity to irrelevant noise letters surrounding a central target. Sequence analyses showed a larger interference effect on trials that followed a trial with compatible in comparison to incompatible noise. This effect was explained by supposing an expectation that was created by the type of noise on the previous trial, effectively an expectation for repetitions. Similarly, in a Simon task, it has repeatedly been shown that if the Simon effect is analyzed as a function of the spatial stimulus-response correspondence in the preceding trial, a sizeable effect is only present after corresponding trials (e.g., Ridderinkhof, 2002; Stürmer, Leuthold, Soetens, Schröter, & Sommer, 2002). In the current study, four types of trials (valid, invalid, neutral, catch) were presented. If momentary strategical adjustments in the endogenous cueing paradigm are similar to those in the Eriksen noise-compatibility and Simon task, a valid trial might enhance the expectation that it is beneficial to direct attention to the cued location whereas an invalid trial might weaken this expectation or even promote orienting to the uncued location. This would lead to an increase of both costs (the difference between invalid and neutral) and benefits (the difference between valid and neutral) of attention after a valid trial in comparison with after an invalid trial.
To summarize, this study aimed at examining sequence effects in an endogenous spatial cueing paradigm to find out if, in addition to the current cue, other recent events affect attentional orienting in a trial. The effects of interest are illustrated in Fig. 1. First, strategical adjustments will be studied by examining the interaction between validity of the preceding trial and validity of the current trial to find out if costs and benefits depend on validity of the preceding trial. In addition, it will be examined if similar to other studies (Alegria, 1978; Correa, Lupiáñez, Milliken, & Tudela, 2004; Los, 2004) an overall delay in RT is present after a catch trial. Second, automatic effects of the previous cue and target will be examined to find out if there is support for (a) an advantage for target alternations in comparison to target repetitions, and (b) effects of IOR after central endogenous cues that “survive” until the target in the next trial. The relation between preceding cue and current target can be described in terms of validity, and because it occurs between trials it will be referred to as ‘inter-trial validity’. The hypothesis then amounts to slower responses if the preceding trial’s cue and current trial’s target are corresponding, or ‘inter-trial valid’, than when they are non-corresponding, or ‘inter-trial invalid’. The same hypotheses were already examined in an unpublished pilot study from our lab. The results showed weak (trend-level) support for strategical adjustments, as costs were smaller after an invalid than after a valid or neutral trial. After catch trials, there was a substantial overall delay in responses, but costs and benefits of attention were unchanged. There was no advantage for target alternations in successive trials, but central endogenous cues had an inhibitory effect on attentional orienting to the same location in the next trial. This effect was remarkably independent of the validity of both the previous and the current trial, and it combined with the effect of the present cue in a purely additive way. In this previous study the order of trial types was truly random with the disadvantage that the number of occurrences of rare sequences was uncontrolled and thus low in some participants. The current experiment aimed at replicating these results, using more trials and a complete balancing of all possible sequences.
Sequence effects of interest are illustrated in a two-trial sequence (previous trial is indicated as: trial N−1; current trial is indicated as: trial N). First of all, the dependence of attention effects on validity of the preceding trial is illustrated by the interaction between validity of the preceding and current trial. Second, effects of target alternation and repetition will be examined. Finally, inhibitory effects of a cue on the target in the next trial will be examined. The relation between preceding cue and current target can be described in terms of ‘inter-trial validity’
Method
Subjects
Eighteen subjects (4 male), 21–30 years old (mean age 25), participated in the study. All had (corrected to) normal vision and four were left-handed. The experiment received prior approval of the institutional ethics committee. All participants signed an informed consent and were paid for their participation. Data of two subjects were excluded from the analyses, because the number of errors and outliers was very high. Footnote 1 Data of the remaining 16 subjects will be described.
Stimuli, apparatus and procedure
Subjects were comfortably seated in a dimly lit, sound attenuated room. The stimuli were presented on a VGA monitor at 75 cm. To control stimulus presentation, record accuracy and latency, ERTSVIPL V3.32c (Beringer, 1987) was used. Target stimuli consisted of dark gray-and-light gray, vertical square-wave grating stimuli (2.1°, 7 cycles/degree) presented to the left or right (7°) of fixation on a gray, equiluminant background. The task was a detection task where every grating stimulus required a right index finger response, independent of its spatial frequency or location. The response was given on a microswitch which was covered by a round response button (1.5 cm in diameter) that was placed centrally in front of participants. Catch trials were added to prevent premature responses.
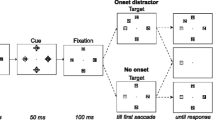
Each trial started with a fixation cross, which stayed on throughout the trial (see Fig. 2). After 100 ms, a cue was presented for 600 ms that consisted of an arrow to the left (<<) or to the right (>>) both with a predictive validity of 80%, or a neutral cue (<>) that had no predictive validity (50%), meaning that the target could appear on either location with equal likelihood. At 900 ms after cue onset, the target was presented for 200 ms. Then there was a 2,000 ms inter-trial interval.
The main task consisted of 2,349 trials. Trials were presented in random order with the restriction that every trial type was immediately preceded by every trial type a fixed number of times in the same proportion as the overall proportion. The proportion of valid:invalid:neutral:catch trials in the experimental block was 8:2:4:3. Since every trial was a combination of the factors cue (3: left, neutral, right) and target (3: left, right, catch), there were 3 × 3 = 9 trial types, and 9 × 9 = 81 different sequences. The number of trials for the rarest sequences (e.g., ‘invalid cue left’ preceded by an ‘invalid cue left’) was 8; for all other sequences it was a multiple of 8.
Subjects were instructed to respond quickly and accurately and to maintain central eye fixation during the trials. Corrective feedback (an error message and short tone) was given on misses and false alarms and on responses that preceded target onset, were too fast (<120 ms) or too slow (>800 ms). After three 12-trial practice blocks, and feedback about their RT after every block, subjects performed the main task. They received feedback about their RT and could pause for a moment every 64 trials. After each pause, the last trial prior to the pause was repeated in order to create the proper “history” for the next trial, and it was later omitted from the analyses so that our sequence effects were not confounded by the pauses, and sequences remained perfectly balanced. The very first trial was deleted for the same reason, leaving 2,312 trials for analyses.
Data analyses
Trials that contained an error or that followed an error were deleted from the analyses. Possible errors were false alarms, misses, responses on the cue, and responses on the target that were either faster than 120 ms or slower than 800 ms. Main analyses of validity and sequence analyses were done. It was examined (a) if costs and benefits of spatial attention in trial ‘n’ were modified by validity of trial ‘n−1’, and if a catch trial ‘n−1’ caused an overall slowing of RT in trial ‘n’, and (b) if cue direction and target position of trial ‘n−1’ had an influence on attention in trial ‘n’. The latter effects were first examined when trial ‘n’ was neutral and then when trial ‘n’ had a directional cue.
Results
An alpha level of 0.05 was maintained for all statistical tests.
Errors and outliers
The average percentage of RT-outliers ranged from 0 to 2.6 (average 0.79). The percentage of false alarms ranged from 0.25 to 7.35 (average 3.6), of misses from 0 to 0.79 (average 0.22), and of responses on the cue from 0.09 to 1.17 (average 0.49). After exclusion of errors and outliers, 98.0% of the data remained for analyses.
Validity
A 3 (validity: valid, neutral, invalid) × 2 (target position: left, right) ANOVA was used to analyze effects of validity disregarding sequence effects. Figure 3 displays RTs as a function of the relevant task variables. Attentional orienting effects were reflected by a main effect of validity, F(2, 14) = 48.4, P < 0.0005. Post hoc analyses showed both significant costs (invalid vs. neutral), F(1, 15) = 58.8, P < 0.0005, and benefits (valid vs. neutral), F(1, 15) = 85.0, P < 0.0005. An interaction between validity and target position, F(2, 14) = 5.2, P = 0.02, indicated larger benefits for target left than for target right, F(1, 15) = 11.0, P = 0.005. Taken together, these results confirmed that participants successfully used the arrow cues to orient their attention.
Sequence effects
Costs and benefits: validity of the preceding trial
A 4 (validity of the preceding trial (n−1): valid, invalid, neutral, catch) × 3 (validity of the current trial (n): valid, invalid, neutral) ANOVA was performed to examine the influence of validity of the previous trial on costs and benefits of the current trial. There were main effects of validity of the current trial, F(2, 14) = 50.9, P < 0.0005, and preceding trial, F(3, 13) = 6.4, P = 0.007, and there was an interaction between these factors, F(6, 10) = 3.8, P = 0.031. Results are displayed in Fig. 4a, b. They suggest the hypothesized delay after a catch trial and larger costs and benefits after a valid than after an invalid trial.
To verify if after a catch trial RTs were delayed, a reverse Helmert contrast was used to compare catch trials with the average of the other three preceding trial types in an analysis with the same factors as before. It confirmed that after a catch trial RTs were slower than after the average of the other three trial types, F(1, 15) = 17.9, p = 0.001. Post hoc analyses showed that in comparison with the average of the other three preceding trial types there was no difference in costs or benefits [both F(1, 15) < 1] after a catch trial. Figure 4b shows that as hypothesized costs and benefits were smaller after an invalid trial than after a valid trial. To verify this, a 3 [validity of the preceding trial (n−1): valid, invalid, neutral] × 3 [validity of the current trial (n): valid, invalid, neutral] ANOVA was performed. In addition to the main effects, there was an interaction between validity of the preceding and current trial, F(4, 12) = 5.7, p = 0.008. Post hoc analyses showed both smaller benefits [F(1, 15) = 6.7, P = 0.02] and costs [F(1, 15) = 23.1, P < 0.0005] when the preceding trial was an invalid trial than a valid trial. Costs after an invalid trial also were significantly smaller than after a neutral [F(1, 15) = 19.1, P = 0.001] trial.
To summarize, there was an overall slowing of responses after a catch trial but no change in costs or benefits. In addition, costs and benefits were larger after a valid than after an invalid trial, matching our hypotheses on strategic effects.
Effects of the preceding cue and target
The influence of preceding cue direction and target position were first examined in circumstances where the present cue did not direct attention: neutral trials. A 3 (cue direction of the preceding trial: left, neutral, right) × 2 (target position of the preceding trial: left, right) × 2 (target position of the current trial: left, right) ANOVA was carried out on the mean RTs of neutral trials. There were main effects of the previous [F(1, 15) = 9.1, P = 0.01], and the current [F(1, 15) = 6.4, P = 0.02] target. Subjects were faster when the previous target was presented to the left or the current target was presented to the right. The interaction between preceding and current target that reflected the effect of target alternation was not significant, F < 1. Instead, there was an interaction between previous cue direction and current target position, F(2, 14) = 5.7, P = 0.02. If the data are reordered, this interaction can be described in terms of inter-trial validity (Fig. 1). Inter-trial valid and inter-trial invalid trials can be compared to test our hypothesis that responses are faster on inter-trial invalid trials, and inter-trial neutral trials can serve as a baseline to find out if any differences between inter-trial valid and inter-trial invalid trials are due to inhibition or facilitation. The results are displayed in Fig. 5. A 3 (inter-trial validity: valid, neutral, invalid) × 2 (target position of the preceding trial: left, right) × 2 (target position in the current trial: left, right) ANOVA showed a main effect of inter-trial validity, F(2, 14) = 6.5, P = 0.01. Post hoc analyses showed slower responses to inter-trial valid than to inter-trial invalid trials, F(1, 15) = 11.4, P = 0.004, supporting our hypothesis. Responses to inter-trial valid responses were also slower than to inter-trial neutral sequences, F(1, 15) = 9.6, P = 0.007, but inter-trial invalid and inter-trial neutral responses did not differ, F < 1. In sum, the results showed an inhibitory effect for the position where attention was directed to by the cue in the preceding trial and this effect was independent of the target position and cue validity in the preceding trial. There was no evidence for an effect of target alternation.
Sequence effects of previous cue and target on mean RTs in neutral trials. Inter-trial validity refers to the relation between previous cue direction and current target position and is neutral if the previous cue was neutral, is valid if previous cue direction and current target position are the same, and is invalid if previous cue direction and current target position differ
Second, the effect of preceding cue direction and target position was examined for left and right-cued trials. A 3 (cue direction of the preceding trial: left, neutral, right) × 2 (target position of the preceding trial: left, right) × 2 (cue direction of the current trial: left, right) × 2 (target position of the current trial: left, right) ANOVA was conducted on the mean RTs. There was no effect of target alternation as shown by an absence of interaction between previous and current target, F = 1. In addition there was no effect of the previous cue on the current target, F < 1. There was an interaction between previous cue and previous target, F(2, 14) = 8.7, P = 0.003, and between current cue and current target, F(1, 15) = 92.2, P < 0.0005. These are effectively validity effects of the previous and current trial and have been described above. In addition, the four-way interaction was significant, F(2, 14) = 10.8, P = 0.001. This interaction is part of the interaction that was already described as the effect of validity of the previous trial on validity of the current trial (cf. Fig. 4). In sum, in trials where attention was driven by a directional cue there was no evidence for inhibitory effects of the previous cue that were shown in neutral trials. Similar to neutral trials there was no effect of target alternation. Instead, strategic effects were shown.
Discussion
Sequence effects in a spatial endogenous cueing paradigm were explored with separate analyses of the effect of cue validity on costs and benefits in the next trial and of effects of the previous cue and target.
Both costs and benefits of attentional orienting were smaller after an invalid than after a valid trial. The typical explanation is that subjects have some degree of strategic control and adapt their utilization of the cue depending on if it correctly or wrongly directed their attention to a location on the previous trial. Costs and benefits have been described in terms of mental processes involving engagement, disengagement and movement of attention. As they are both affected after an invalid trial, it could either mean that attention is not oriented on a number of trials, or is not fully engaged to the indicated location. Comparable strategic effects have been demonstrated in focused attention and conflict paradigms (Gratton et al., 1992; Ridderinkhof, 2002; Stürmer et al., 2002). Although these effects have typically been attributed to strategic control, Hommel and colleagues proposed a “feature integration account” that does not require voluntary control (Hommel, Proctor, & Vu, 2004; Hommel, 2004). It assumes that co-occurrence of a cue and target in a trial leads to a representation of the relation in which their features are integrated. This relation would be reactivated in the next trial, and would influence performance in such a way that good performance is predicted if validity is repeated but interference would occur if it alternates. This boils down to the same prediction of smaller benefits and costs after an invalid trial than after a valid trial.
There was an overall delay after a catch trial. It has been reported that a decrease of the probability of stimulus occurrence causes an increase of RTs (Gordon, 1967; Näätänen, 1972). Snodgrass (1969) attributed this to a decrease in the frequency of anticipations, but Alegria (1978) analyzed trial sequences and showed that independent of the overall catch-trial probability, after an uninterrupted sequence of targets, RT became as fast as when catch-trial probability was zero. From this, he concluded that event probability effects boiled down to sequence effects. In two recent cueing studies, sequence analyses also showed an increase in RTs in trials that followed on catch trials (Correa et al., 2004; Los, 2004). In agreement with Alegria (1978), Correa et al. suggested that the increase in RT was related to a decrease in preparation after a catch trial. Alternatively, Los proposed that it is caused by processes of inhibition that follow the presentation of a cue to prevent premature processing.
The combination of a decrease in speed but unchanged costs and benefits following a catch trial supports the distinction between orienting and alerting processes of attention. Similarly, Fernandez-Duque and Posner (1997) showed a decrease in speed but no change of the validity effect in an exogenous cueing paradigm when an auditory alerting cue was presented. Separate anatomical networks have been proposed for these processes of attention (Posner & Peterson, 1990). Coull and Nobre (1998) showed that, analogous to spatial orienting, attention can be “temporally oriented” to a point in time and together, temporal orienting and alerting processes were suggested to be temporal processes of attention. Correa et al. (2004) demonstrated that the sequence effect of catch trials on RTs was independent of the validity effect of temporal orienting. Likewise, our results showed that the effect of catch trials did not interact with the effects of spatial orienting. All together, these results therefore support the notion of different spatial and temporal processes of attention that can act independently (Coull & Nobre, 1998; Griffin, Miniussi, & Nobre, 2002; Los, 2004; Milliken, Lupiáñez, Roberts, & Stevanovski, 2003). Catch trials mainly appear to affect processes of alertness, independently from other mechanisms of attention.
Another effect that was examined was an advantage for target alternations that has been attributed to exogenous IOR (Pratt & Abrams, 1999; Maylor & Hockey, 1987; Taylor & Klein, 2000). At least for neutral–neutral sequences, feature integration (Hommel et al., 2004) might have predicted the opposite (an advantage for target repetition), because that would represent repetition of all features. However, no advantage was found for target alternations or repetitions in successive trials. A sufficient explanation is probably that the time between targets in consecutive trials was rather long (about four seconds), thereby reducing short-lasting exogenous IOR effects, although Tipper, Grison, and Kessler (2003), using a different paradigm, found effects of IOR that lasted for even 13 min. Another reason may be that we used a simple detection task, whereas in other studies responses were based on location (Taylor & Klein, 2000) or identity (Pratt & Abrams, 1999) of the stimulus, possibly enhancing exogenous orienting effects because the target and its location are more salient in these paradigms. In Maylor and Hockey (1987), no predictive central cues were used that could interfere with traces of exogenous orienting.
In addition to target–target effects we examined effects of the endogenous cue that may carry over to the next trial, when it has lost its predictive power. To describe the relation between previous cue and current target the term “inter-trial validity” was introduced. In inter-trial valid trials the previous cue direction and current target location were similar, whereas they were different in inter-trial invalid trials. In inter-trial neutral trials the previous cue was neutral and these trials served as a baseline. Endogenous IOR would be manifested by faster responses on inter-trial invalid than inter-trial valid trials. This effect was demonstrated in “current” neutral trials. A comparison with inter-trial neutral trials and an analysis of inter-trial costs and benefits showed that the effect was due to inhibition of the position that was cued in the previous trial. This effect was independent of validity in the previous trial. In the pilot study that we mentioned in the introduction the inhibitory effect of the previous cue was present also in trials with directional cues where it combined with the validity effect of the present trial in an additive way. However, in the current study strategic effects in trials with directional cues were stronger than in the pilot experiment maybe as a consequence of the substantial increase in the number of trials and task duration that was needed to achieve balancing of the sequences. These strategies may have had an overpowering effect on the automatic inhibitory effects of the previous cue. A comparison of the two experiments showed that the “current” effect of validity was larger now. Therefore, inhibitory effects of the previous cue might also have been overpowered by facilitatory effects of voluntary orienting in the current trial.
In sum, the results show that analyses of sequence effects can enhance our insights in processes that influence task performance. First, after a catch trial overall slower responses but intact costs and benefits were shown, supporting the independence of attentional mechanisms of orienting and alertness. Second, strong strategic effects were demonstrated in trials with directional cues where costs and benefits depended on validity of the previous trial. Third, in neutral trials orienting was influenced by the direction of the cue in the previous trial. More specifically, the position that was cued in the previous trials was inhibited. We therefore propose that endogenous shifts of attention result in long-term inhibitory processes that are independent of exogenous effects and validity of the previous trial. This independence is remarkable because the attentional response to the previous target is closer in time than the response to the previous cue. It would be interesting to study similar effects in a task that requires closer examination of the target. These inhibitory processes might also play a role in the ‘preference of attention to switch’ that was suggested by Posner (1980) when no benefits of attention were found in a cueing paradigm where attention was cued for a whole block instead of on each trial (Posner, Snyder, & Davidson, 1980).
Notes
The pattern of results was the same when these subjects were included, but effects were stronger without them.
References
Alegria, J. (1978). Sequential effects of catch-trials on choice reaction time. Acta Psychologica, 42, 1–6.
Beringer, J. (1987). Experimental Run Time System (Version 3.32c). Frankfurt, Germany: Berisoft Cooperation.
Bertelson, P. (1961). Sequential redundancy and speed in a serial two-choice responding task. Quarterly Journal of Experimental Psychology, 13, 90–102.
Bertelson, P. (1963). S–R relationships and reaction times to new versus repeated signals in a serial task. Journal of Experimental Psychology, 65, 478–484.
Bertelson, P., & Renkin, A. (1966). Reaction times to new versus repeated signals in a serial task as a function of response-signal time interval. Acta Psychologica, 25, 132–136.
Correa, A., Lupiáñez, J., Milliken, B., & Tudela, P. (2004). Endogenous temporal orienting of attention in detection and discrimination tasks. Perception and Psychophysics, 66, 264–278.
Coull, J. T., & Nobre, A. C. (1998). Where and when to pay attention: The neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. Journal of Neuroscience, 18, 7426–7435.
Eriksen, B. A., & Eriksen, C. W. (1974). Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception and Psychophysics, 16, 143–149.
Fernandez-Duque, D., & Posner, M.I. (1997). Relating the mechanisms of orienting and alerting. Neuropsychologia, 35, 477–486.
Gordon, I. E. (1967). Stimulus probability and simple reaction time. Nature, 215, 895–896.
Gratton, G., Coles, M. G., & Donchin, E. (1992). Optimizing the use of information: Strategic control of activation of responses. Journal of Experimental Psychology: General, 121, 480–506.
Griffin, I. C., Miniussi, C., & Nobre, A. C. (2002). Multiple mechanisms of selective attention: Differential modulation of stimulus processing by attention to space or time. Neuropsychologia, 40, 2325–2340.
Hommel, B. (2004). Event files: feature binding in and across perception and action. Trends in Cognitive Sciences, 8, 494–500.
Hommel, B., Proctor, R.W., & Vu, K.L. (2004). A feature-integration account of sequential effects in the Simon task. Psychological Research, 68, 1–17.
Hyman, R. (1953). Stimulus information as a determinant of reaction time. Journal of Experimental Psychology, 45, 188–196.
Jonides, J. (1981). Voluntary versus automatic control over the mind’s eye’s movement. In J. Long (Ed.), Attention and performance IX: 9th international symposium, Cambridge, July (Vol. IX, pp. 187–203). Hillsdale: Erlbaum.
Kirby, N. H. (1976). Sequential effects in two-choice reaction time: Automatic facilitation or subjective expectancy? Journal of Experimental Psychology: Human Perception and Performance, 2, 567–577.
Kirby, N. H. (1980). Sequential effects in choice reaction time. In A. T. Welford (Ed.), Reaction times (pp. 129–172). London: Academic Press.
Kwak, H., & Egeth, H. (1992). Consequences of allocating attention to locations and to other attributes. Perception and Psychophysics, 51, 455–464.
Los, S. (2004). Inhibition of return and non-specific preparation: separable inhibitory control mechanisms in space and time. Perception and Psychophysics, 66, 119–130.
Maylor, E. A., & Hockey, R. (1987). Effects of repetition on the facilitatory and inhibitory components of orienting in visual space. Neuropsychologia, 25, 41–54.
McKenna, F.P., & Sharma, D. (2004). Reversing the emotional Stroop effect reveals that it is not what it seems: the role of fast and slow components. Journal of Experimental Psychology, Learning, Memory, and Cognition, 30, 382–392.
Milliken, B., Lupiáñez, J., Roberts, M., & Stevanovski, B. (2003). Orienting in space and time: Joint contributions to exogenous spatial cuing effects. Psychonomic Bulletin and Review, 10, 877–883.
Näätänen, R. (1972). Time uncertainty and occurrence uncertainty of the stimulus in a simple reaction time task. Acta Psychologica, 36, 492–503.
Posner, M. I. (1980). Orienting of attention. Quarterly Journal of Experimental Psychology, 32, 3–25.
Posner, M. I., & Cohen, Y. (1984). Components of visual orienting. In H. Bouma (Ed.), Attention and Performance X: control of language processes (pp. 531–556). London: Erlbaum.
Posner, M. I., & Petersen, S. E. (1990). The attention system of the human brain. Annual Review of Neuroscience, 13, 25–42.
Posner, M. I., Nissen, M. J., & Ogden, W. C. (1978). Attended and unattended processing modes: the role of set for spatial location. In H. L. Pick & E. Saltzman (Eds.), Modes of perceiving and processing information (pp. 137–157). Hillsdale: Lawrence Erlbaum Associates.
Posner, M. I., Snyder, C.R.R., Davidson, B.J. (1980). Attention and the detection of signals. Journal of Experimental Psychology, 109, 160–174.
Posner, M.I., Rafal, R.D., Chaote, L.S., Vaughan, J. (1985). Inhibition of return: Neural basis and function. Cognitive Neuropsychology, 2, 211–228.
Pratt, J., & Abrams, R.A. (1999). Inhibition of return in discrimination tasks. Journal of Experimental Psychology: Human Perception and Performance, 25, 229–242.
Rafal, R. D., Calabresi, P. A., Brennan, C. W., & Sciolto, T. K. (1989). Saccade preparation inhibits reorienting to recently attended locations. Journal of Experimental Psychology: Human Perception and Performance, 15, 673–685.
Ridderinkhof, K. R. (2002). Micro- and macro-adjustments of task set: activation and suppression in conflict tasks. Psychological Research, 66, 312–323.
Snodgrass, J. G. (1969). Foreperiod effects in simple reaction time: Anticipation or expectancy? Journal of Experimental Psychology, 79, 1–19.
Soetens, E. (1990). Sequential effects in two-choice reaction time. Unpublished doctoral dissertation, University of Leiden, Leiden, The Netherlands.
Soetens, E. (1998). Localizing sequential effects in serial choice reaction time with the information reduction procedure. Journal of Experimental Psychology: Human Perception and Performance, 24, 547–568.
Stürmer, B., Leuthold, H., Soetens, E., Schröter, H., & Sommer, W. (2002). Control over location-based response activation in the Simon task: behavioral and electrophysiological evidence. Journal of Experimental Psychology: Human Perception and Performance, 28, 1345–1363.
Taylor, T.L., & Klein, R.M. (2000). Visual and motor effects in inhibition of return. Journal of Experimental Psychology, 26, 1639–1656.
Tipper, S.P., Grison, S., & Kessler, K. (2003). Long-term inhibition of return of attention. Psychological Science, 14, 19–25.
Acknowledgements
We thank Sander Los, Juan Lupiáñez, Jay Pratt, and two anonymous reviewers for their comments on earlier versions of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jongen, E.M.M., Smulders, F.T.Y. Sequence effects in a spatial cueing task: Endogenous orienting is sensitive to orienting in the preceding trial. Psychological Research 71, 516–523 (2007). https://doi.org/10.1007/s00426-006-0065-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00426-006-0065-3