Abstract
Main conclusion
Peptide-receptor complexes activate distinct downstream regulatory networks to mediate plant adaptions to abiotic environmental stress.
Abstract
Plants are constantly exposed to various adverse environmental factors; thus they must adjust their growth accordingly. Plants recruit small secretory peptides to adapt to these detrimental environments. These small peptides, which are perceived by their corresponding receptors and/or co-receptors, act as local- or long-distance mobile signaling molecules to establish cell-to-cell regulatory networks, resulting in optimal cellular and physiological outputs. In this review, we highlight recent advances on the regulatory role of small peptides in plant abiotic responses and nutrients signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants are frequently exposed to adverse environments such as multiple abiotic stresses and nutrients deficiency conditions. Abiotic stresses severely harm plant growth and reduce crop yield (Zulfiqar et al. 2019; van Zelm et al. 2020; Chen et al. 2021). The nutrient shortages, for instance, nitrate (N) or phosphate (P) deficiency influences plant architecture and growth (Motte et al. 2019; Huang and Zhang 2020; Luo et al. 2020). To better optimize plant development under fickle conditions, plants have evolved a plethora of mechanisms to integrate various environmental cues into coordination of cellular behaviors and overall growth. Activation or de-activation of plant phytohormone signaling pathway is one of the adaptive strategies for plants to modulate their growth under abiotic stress conditions (Skalak et al. 2021; Salvi et al. 2021). Nutrient deficiencies interfere with phytohormones biosynthesis, signaling and distribution to shape plant architecture (Motte et al. 2019; Luo et al. 2020). Notably, small peptides act as local or long-distance signals to coordinate plant adaptations to abiotic stress and nutrients availability (Lay and Takahashi 2018; Takahashi et al. 2019; Gautrat et al. 2021).
Many different approaches have been carried out to identify the existence of small peptide, and mass spectrometry has been used to dissect the small peptide structure (Matsubayashi 2014, 2018). Based on the peptide structure, bioinformatic approach has been implemented to uncover gene members of distinct peptide family from genome sequences. More than 7000 small peptide encoding genes have been identified in the Arabidopsis thaliana genome, and most of them are likely to encode hormone-like peptides (Takahashi et al. 2019). In general, plant peptides are derived from unfunctional precursor proteins, functional proteins, or directly translated from a short open reading frame (Tavormina et al. 2015). Peptides are usually less than 120 amino acids, and the bioactive form is generally shorter than 20 amino acids in length (Murphy et al. 2012; Tavormina et al. 2015). Based on structural differences, plant secretory peptides are classified into two main groups as follows: (1) post-translationally modified peptides (PMTs) and (2) cysteine-rich peptides (CRPs) (Olsson et al. 2019). Generally, PMTs and CRPs contain an N-terminal secretory sequence, a central variable region and a conserved motif or cysteine-rich domain at or near C-terminus. Mature PMTs and CRPs are generated by enzyme-mediated processing or modifications from their precursors-prepropeptides (Matsubayashi 2014, 2018). To date, numerous enzymes involved in peptide processing and post-translational modifications have been identified, although their impacts on peptides bioactivity and signal transduction are not fully understood (Matsubayashi 2014, 2018; Stührwohldt and Schaller 2019).
Receptor-like kinases (RLKs) and co-receptor proteins perceive the corresponding small peptides to integrate both external and internal signals into complex regulatory networks to achieve optimal responses and growth. Although some of the small peptides also play essential roles in biotic stress via distinct mechanisms (Segonzac and Monaghan 2019), it is out of the scope of this review. This review mainly aims to provide an overview of recent advances on small peptide-mediated plant adaptions to abiotic stresses. And their roles in plant response to nutrients are also discussed.
Small secretory peptides mediate plant drought stress
CLE peptide
The CLAVATA3(CLV)/EMBRYO-SURROUNDING REGION-RELATED (CLE) peptide family is well known for its role in regulation of meristem differentiation and proliferation as well as other developmental processes (Hirakawa and Sawa 2019; Fletcher 2020; Willoughby and Nimchuk 2021; Song et al. 2022). Typically, the length of bioactive CLE peptide is 12–14 amino acids generated with post-translational modifications (Fig. 1; Olsson et al. 2019; Stührwohldt et al. 2020). Recently, CLE9 and CLE25 have been reported to play roles in dehydration stress, which is dependent on ABA signaling (Table 1; Figs. 2, 3a, b; Takahashi et al. 2018; Zhang et al. 2019).
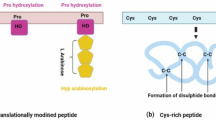
Biogenesis of post-translationally modified small peptides. a Peptide encoding genes are translated into prepropeptides. The signal peptide is cleaved to ensure it enters into the secretory pathway. Propeptides undergo at least one type of post-translational modifications including tyrosine sulfation, proline hydroxylation, arabinosylation and glycosylation to generate mature peptide. b WebLogo alignment showing the sequences of representative motifs of CLE, CEP, PSK RALF, CAPE and PEP peptide family
Summary of small peptides in plant abiotic stress response. a Dehydration stress triggers accumulation of CLE25 peptide in roots, CLE25 is then transported to leaves, and is perceived by BAMs receptors to induce ABA biosynthesis via promoting NCED3 expression, resulting in stomatal closure and drought resistance. b Drought stress triggers accumulation of CLE9 peptide, CLE9 is perceived by unknown receptors to simultaneously active MPK3/MPK6 and OST1-ROS-SLAC1 signaling cascade to close stomatal, resulting in drought resistance. c CEP5 expression is induced by osmotic stress to confer plants drought/osmotic tolerance via two distinct mechanisms. On the one hand, CPE5 is recognized by CEPR1/CEPR2 to stabilize AUX/IAA transcriptional repressors, which suppresses transcriptional auxin response. On the other hand, CEP5 alters the expression of stress-related genes via undefined receptors. d Osmotic stress elicits expression of proPSKs and SBT3.8, and the SBT3.8 then cleaves proPSK1 to generate mature PSK1 peptide. PSK1 binds to unidentified receptors to confer plants the drought tolerance. e Salinity downregulates RALF1 expression in roots. Bioactive RALF1 peptide is perceived by FER receptor to regulate AHA2 and N+/K+ antiporter activity, resulting in accumulation of Na+ and enhanced salt toxicity. On the other hand, salinity triggers accumulation of RALF22/23 peptide, which interacts with LRX protein. Salinity causes dissociation of mature RALF22/23 peptides from LRX proteins, thereby triggering FER internalization, and resulting in the change of cell wall integrity, ABA and ROS signaling and salt tolerance. Salinity also regulates FER-LLG1-Ca2+ signaling cascade to maintain cell wall integrity during salt stress response. f Under salt conditions, CAPE1 level is downregulated, and undefined receptors perceive CAPE1 peptide signal to downregulate salt-related genes, thus negatively regulating plant salt tolerance. Salt stress also induces PEP3 expression, PEP3 then binds to PEPR1 receptor to improve salt tolerance via an unknown mechanism. g High temperature induces CLE45 expression, and CLE45 binds to SKM receptor to protect pollen tube growth under high temperature
Root specifically expressed CLE25 peptide acts as a long-distance mobile signal during drought response. When roots sense lower water potential, the transcriptional level of CLE25 is elevated, subsequently, CLE25 peptide is transported from roots to leaves. In leaves, plasma membrane-localized BARELY ANY MERISTEM (BAM) receptors bind to CLE25 peptide and transmit the drought signal to accelerate expression of NINE‐CIS‐EPOXY-CAROTENOID DIOXYGENASE 3 (NCED3), an ABA biosynthesis enzyme. Consequently, the leaf produces more ABA to close stomata, thus enabling plants adapt to the drought stress (Fig. 3a; Takahashi et al. 2018). CLE25 peptide also binds to CLE‐RESISTANT RECEPTOR KINASE (CLERK) and CLAVATA2 (CLV2), but their role in CLE25-mediated stomatal closure and drought response is elusive (Ren et al. 2019). On the other hand, other signaling components downstream of the CLE25-BAM signaling pathway in the regulation of plant drought stress require further clarification.
CLE9 is highly expressed in leaf guard cells, and its expression is prominently induced by abiotic stresses such as NaCl and mannitol. Exogenous application of synthetic CLE9 peptide or overexpression CLE9 (CLE9OE), resulted in leaf stomatal closure. Accordingly, the CLE9OE transgenic plant shows much stronger resistance to drought stress (Zhang et al. 2019). Further study indicated that the CLE9 peptide-mediated stomatal closure involves a signaling cascade including MITOGEN-ACTIVATED PROTEIN KINASE 3/6 (MPK3/6), reactive oxygen species (ROS), and ABA signaling-related components, OPEN STOMATA 1 (OST1) and SLOW ANION CHANNEL-ASSOCIATED 1 (SLAC1). However, MPK3/MPK6 mediated stomatal closure is independent of ABA signaling (Montillet et al. 2013; Su et al. 2017). Hence, CLE9 induced stomatal is mediated parallelly by MPK3/MPK6 and ABA signaling (Fig. 3b; Zhang et al. 2019). In the future, the identification of receptors and co-factors involved in CLE9 peptide-induced stomatal closure would further clarify the CLE9 role in drought resistance.
CEP peptide
The C-TERMINALLY ENCODED PEPTIDE (CEP) genes encode proteins which contain an N-terminal signaling peptide, a variable central region, a 15 amino acids of CEP motif with post-translational modifications at C-terminus (Fig. 1; Delay et al. 2013; Roberts et al. 2013; Taleski et al. 2018). The bioactive CEP peptides are perceived by their receptors CEP RECEPTOR 1/2 (CEPR1/CEPR2) to regulate a variety of plant developmental processes (Djordjevic et al. 2015; Okamoto et al. 2016; Taleski et al. 2018; Jeon et al. 2021). Among the CEP peptides, CEP5 plays an essential role in conferring plant stress resistance (Table 1; Figs. 2; 3c; Smith et al. 2020). CEP5 transcription is induced by osmotic stress, and CEP5OE overexpression plant shows a tolerance to both drought and osmotic stresses. Seedlings treated with synthetic CEP5 peptide yield a similar tolerance. However, the function of CEP5 in stress tolerance does not fully depend on its well-known receptors, CEPR1 or CEPR2, because no obvious phenotype was observed in cepr1 cepr2 double mutant under osmotic conditions. In contrast, CEP5 peptide promotes the stability of AUX/IAA, a key regulatory component of SCFTIR1/AFB nuclear auxin signaling pathway, partially via the CEPR receptors, resulting in repression of auxin-mediated gene expression, which in turn may confer plants resistance to stress (Shani et al. 2017; Sadok and Schoppach 2019; Smith et al. 2020). Thus, CEP5 peptide regulates plants stress responses through two mechanisms, dependent or independent of the CEPR receptors (Fig. 3c). Whether CEP5 peptide can be transported to leaves to regulate ABA signaling and stomatal closure during drought stress remains an open question, as CEP5 is also expressed in root phloem (Roberts et al. 2013, 2016; Takahashi et al. 2018).
PSK peptide
PHYTOSULFOKINE (PSK), a group of sulfated peptides (Fig. 1), are perceived by plasma membrane localized PSK RECEPTOR 1 (PSKR1) and PSKR2, together with SOMATIC EMBRYOGENESIS RECEPTOR KINASE 3 (SERK3) coreceptors to modulate multiple physiological processes including stress response (Table 1; Fig. 2; Sauter 2015; Ladwig et al. 2015; Wang et al. 2015; Kaufmann et al. 2021). Osmotic stress prominently induces the expression of PSK precursor genes and PSK cleavage genes the SUBTILISIN (SBT). The overexpression transgenic plant proPSK1OE or SBT3.8OE displayed significantly improved osmotic stress tolerance, which is evident by its enhanced root and shoot growth under osmotic stress. Additionally, PSK1 peptide treatment recovers the osmotic stress-induced sensitive phenotypes in sbt3.8 mutant. Thus, SBT3.8 cleaves the proPSK1 protein to generate biologically activated PSK1 peptide, which positively regulates plant resistance to drought stress. However, the involvement of PSKRs or SERK3 in PSK1-mediated drought/osmotic tolerance needs to be defined in future (Fig. 3d; Stührwohldt et al. 2021).
Small peptide-mediated response to salinity stress
RALF peptide
The Rapid Alkalinization Factor (RALF) peptides belong to the CRP family with post-translational modifications (Fig. 1) that are perceived by the receptors FERONIA (FER) and THESEUS1 to regulate various plant developmental processes including salt stress (Table 1; Stegmann et al. 2017; Yu and Assmann 2018; Gonneau et al. 2018; Blackburn et al. 2020; Gjetting et al. 2020). The ralf1 mutant showed a comparable growth inhibition to wild-type upon NaCl treatment; however, the overexpression RALF1OE transgenic line exhibits relative resistance to salt treatment, implying a positive role of RALF1 peptide in salt stress (Feng et al. 2018). On the other hand, RALF1 expression in root is downregulated by salinity, and exogenous application of active RALF1 peptide triggers an accumulation of Na+ via inhibiting the ARABIDOPSIS H+-ATPASE 2 (AHA2) and Na+/K+ transporters activity, leading to enhanced salt toxicity. RALF1 induced salt toxicity requires its receptor FERONIA (FER) (Yu and Assmann 2018). Interestingly, fer mutant is insensitive to RALF1 peptide treatment, but displays a hypersensitivity to salinity (Feng et al. 2018; Yu and Assmann 2018). It has been suggested that fer mutant rapidly loses cell integrity under salt stress and this defect depends on the Ca2+ signaling and its co-receptor LORELEI-like GPI-AP1 (LLG1) (Feng et al. 2018). RALF1 peptide treatment also induces an increase of cytoplasmic Ca2+ (Haruta et al. 2008), indicating a potential linkage between RALF1 peptide and salt tolerance. Notably, salinity also induces accumulation of RALF22/23 peptides which interact with LEUCINE-RICH REPEAT EXTENSINS (LRX) proteins to regulate FER-mediated cell wall integrity, ABA signaling, and Reactive Oxygen Species (ROS), resulting in salt tolerance (Zhao et al. 2018, 2020; Feng et al. 2018). RALF1-FER module is also involved in regulating ABA signaling (Yu et al. 2012; Chen et al. 2016) and GLYCINE-RICH RNA BINDING PROTEIN 7 (GRP7) splicing dynamics (Wang et al. 2020), which in turn, ensures plant responses to stress. Overall, RALF peptide signaling regulates salinity response via distinct mechanisms (Fig. 3e). However, through what precise mechanisms plants integrate these complex stress-related signaling networks mediated by RALF peptides are beyond our understanding.
CEP3 peptides
The CEP peptide family has also been implicated in salt response. The expression of CEP genes is differentially regulated by environmental clues such as salinity (Delay et al. 2013; Aggarwal et al. 2020). In Arabidopsis, CEP3 is strongly upregulated by NaCl treatment. In addition, the Arabidopsis cep3 mutant partially exhibits resistance to NaCl, indicated by a longer primary root of the mutant upon NaCl treatment (Delay et al. 2013). However, the molecular mechanism of CEP3-mediated salt stress response is still elusive.
CAPE1 peptide
CAP-DERIVED PEPTIDE 1 (CAPE1) is a member of the CRP family (Fig. 1; Chen et al. 2014). Nine CAPEs have been identified as precursor Arabidopsis thaliana CAPEs (PROAtCAPEs), and PROAtCAPE1 specifically is downregulated under salt stress (Chien et al. 2015). The proatcape1 knock-out mutant exhibits resistance to high salinity; in contrast, overexpression of PROAtCAPE1 or exogenous application of synthetic AtCAPE1 peptide restores the salinity response in proatcape1 mutant. Transcriptome analysis further shows that AtCAPE1 negatively regulates the expression of salt stress response genes (Table 1; Figs. 2, 3f; Chien et al. 2015). Hence, AtCAPE1 plays a negative role in plant salt stress response via undetermined mechanism.
PEP3 peptide
PLANT ELICITOR PEPTIDEs (PEPs) are endogenous elicitors of plant immunity (Fig. 1); however, it has been shown that PEPs are involved in plant salt stress (Fig. 2; Table 1). Among the eight members of Arabidopsis precursor PEP (AtPROPEP), AtPROPEP3 is prominently induced by high salt concentration (Nakaminami et al. 2018). Knock down the AtPROPEP3 expression results in the hypersensitivity to salt stress. In contrast, overexpression of AtPROPEP3 or application of the synthetic AtPEP3 peptide induces plant resistance to the salinity. Further analysis shows that AtPEP3 binds to the receptor PEP RECEPTOR 1 (PEPR1) to confer plant tolerance to salinity, but the precise mechanisms require more investigations (Fig. 3f; Nakaminami et al. 2018).
CLE45 peptide mediates high-temperature response
CLE45 is expressed in stigma, and its expression is induced by high temperature. CLE45 peptide is capable of mediating pollen tube growth in response to high temperature (Table 1; Fig. 2; Endo et al. 2013). CLE45 peptide treatment prolongs pollen tube growth without affecting pollen germination at high temperature. By screening the T-DNA insertion lines, two candidate proteins, STERILITY-REGULATING KINASE MEMBER1 (SKM1) and SKM2, are recognized as the CLE45 receptors. Biochemical experiments demonstrated that the CLE45 physically binds to SKM1. SKM1 and SKM2 are expressed in pollen tube and their transcription is also induced by high temperature. Additionally, the skm mutants are insensitive to CLE45 peptide treatment in pollen tube growth. Collectively, these findings suggest CLE45-SKM pathway involves in a successful seed production under high temperature (Fig. 3g; Endo et al. 2013).
CLE peptides mediate nitrogen, phosphate and sulfur signaling
The availability of nitrogen (N) affects various plant developmental processes such as shoot branching, flowering and root development (Luo et al. 2020; Jia and von Wirén 2020). Nitrogen consists of two forms: inorganic (nitrate [NO3-], and ammonium [NH4+]) and organic (amino acids and urea). The discovery of N transport and key genes in N uptake and signaling using biochemical and genetic studies in the past decades provided fundamental insights into the nitrogen use efficiency in plants (Vidal et al. 2020). A regulatory network between N and phytohormones has also been established (Ristova et al. 2016). Interestingly, the CLE peptides also participate in N-mediated root growth (Araya et al. 2014).
CLEs gene expression is enhanced by N signaling (Patterson et al. 2010; Ruffel et al. 2011). Indeed, CLE1/3/4/7 transcription levels are elevated in roots under low N conditions. The phloem-localized receptor kinase CLAVATA1 (CLV1) perceives the bioactive CLE3 peptide to inhibit lateral root development (Table 1; Figs. 2, 4a; Araya et al. 2014). In turn, a yet unknown signal activated by CLV1-mediated N-demanding signal represses CLE3 expression. Therefore, CLV1-CLE signaling forms a feedback loop to balance the CLE transcription in response to N availability (Fig. 4a). Many other receptor-like kinases (RLKs) are also suggested to transmit CLE signal (Fletcher 2020); however, it is unknown whether these RLKs also participate in CLE-mediated N signaling during plant development.
Summary of small peptides that mediate plant nutrient signaling and nodulation. a Under low nitrate environment, CLE3 expression is increased, and CLV1 recognizes CLE3 peptide to repress lateral root development via unknown downstream players. A feedback signal is activated by the CLV1-mediated N-demanding signal to repress CLE3 expression. Hence a feedback loop is formed to control CLE-CLV1 cascade during lateral root development under a nitrate starvation environment. b Under low phosphate conditions, CLE14 peptide binds to CLV2 and PEPR2 receptors to attenuate POL and PLL1 function, which in turn affects the function of transcription factor SHR, SCR, WOX5, and auxin signaling, leading to root apical meristem (RAM) cell differentiation. c Under sulfur starvation, CLE2/3 expression is suppressed, and CLV1 functions as CLE2/3 receptor to repress lateral root development. d In roots, CEP expression is promoted under N deficiency condition. CEP is then transported to shoots and is recognized by CEPRs receptors. CEPDs act as downstream players of CEP-CEPR signaling to accelerate N transporter NRT2.1 expression, thus activating N acquisition. While in shoots, N deficiency enables CEPDL2 to promote NRT2.1 or NRT1.5 expression, which activates N acquisition. e Sucrose treatment increases CEP expression, and CEP binds to CEPR1 and unknown receptors to simultaneously repress lateral root development. f N/rhizobia infection promotes the expression of transcription factor NLP or NIN, which then binds to LjCLE and MtCLE promoters to control the expression of LjCLE and MtCLE, respectively. LjCLE and MtCLE are then separately perceived by LjHAR1 and MtSUNN to inhibit miR211 expression, thus promoting TML1/2 expression, resulting in a reduction of nodule numbers. Under low N conditions, MtCEP expression is increased, and MtCRA2 perceives MtCEP peptide to promote miR211 expression. miR211 then represses TML1/2 expression, resulting in an increased number of nodules. Hence, CLE and CEP peptides play antagonistic roles in nodulation
To adapt to low-phosphate (P) environments, plants develop intricate and adaptive mechanisms to maintain P homeostasis, which are regulated by intricate gene networks involving the plasma membrane-localized P transporters and P starvation-induced genes (Wang et al. 2018). Plants modify their root system architecture by an increase of lateral root number, promotion of root hair growth and termination of cell differentiation in root meristem to enlarge the contact surface with the soil for P uptake (Liu 2021). Recently, CLE14 peptide has been reported to be downstream of P signaling to regulate root apical meristem (RAM) cell differentiation (Gutiérrez-Alanís et al. 2017). Under P starvation, CLE14 transcription is upregulated. CLAVATA2 (CLV2) and PEPR2 perceive CLE14 peptide, to inhibit POLTERGEIST (POLL) and POLTERGEIST-LIKE 1 (PLL1) function, which in turn affects the major players involved in root meristem differentiation including the transcription factors SHORT ROOT (SHR), SCARECROW (SCR) and WUSCHEL-RELATED HOMEOBOX 5 (WOX5), and plant hormone auxin, leading to root meristem exhaustion (Table 1; Figs. 2, 4b; Gutiérrez-Alanís et al. 2017). Nevertheless, the mechanism regarding how the CLE14-CLV2/PEPR2 signaling regulates downstream players during P starvation is still not clear.
The macronutrient sulfur (S) has various effects on plant growth (Li et al. 2020; Aarabi et al. 2020). Despite the involvement of phytohormones in the regulation of S nutrient (Li et al. 2020), additional evidence supports the function of CLE peptide in regulation of root development in response to S (Table 1; Dong et al. 2019). CLE expression is controlled by the availability of S (Czyzewicz et al. 2015; Dong et al. 2019). S deprivation represses CLE2 and CLE3 expression levels, resulting in a reduction of lateral root density. However, this repression of CLE expression and lateral root density is diminished in clv1 mutant. Hence, CLE-CLV1 module controls lateral root development under S deprivation (Table 1; Figs. 2, 4c; Dong et al. 2019), but the precise mechanism requires further investigations.
CEP peptides mediate nitrogen acquisition and sucrose signaling
N distribution is often heterogenous in soil; CEP peptides function as systemic long-distance signaling to ensure plants’ efficient N uptake (Tabata et al. 2014; Ohkubo et al. 2017). The expression of seven CEP genes is prominently upregulated in roots under N-starvation condition, then the synthesized CEP peptides are transported to leaves. The leaf expressed CEPR1/2 receptors sense the root-derived CEP peptides to regulate the transcription of nitrate transporters NRT2.1, resulting in the promotion of N acquisition (Table 1; Figs. 2, 4d; Tabata et al. 2014). The polypeptides CEP DOWNSTREAM1 (CEPD1) and CEPD2, belonging to the glutaredoxin family, act as a second signal in shoot-to-root N signaling downstream of the CEPR-CEP signaling pathway (Ohkubo et al. 2017). Perception of CEP peptide by CEPR on the leaf phloem cells surface leads to the production of CEPDs polypeptides in shoots. CEPDs then act as phloem-mobile descending signals directed to roots exposed to sufficient N, where they accelerate NRT2.1 expression and N uptake (Fig. 4d; Ohkubo et al. 2017). CEPD-LIKE2 (CEPDL2) is a leaf-derived signal to regulate root N uptake and transport. When roots are unable to absorb enough N to meet shoots N demand, CEPDL2 expression is significantly upregulated, which helps roots to take up and transport N via upregulating the transcription of NRT1.5 and NRT2.1 (Ota et al. 2020). Taken together, CEP-CEPR-CEPD-CEPDL module forms a root-to-shoot-to-root regulatory network to control N uptake and transport dependent on the environmental N availability (Fig. 4d).
Sucrose is the product of photosynthesis, it breaks down into several forms of sugars such as glucose, fructose and trehalose 6-phosphate (T6P), and it is transiently stored in compartments for further use. Sucrose is transported from the source tissues to sink tissues via the phloem, which is mediated by multiple sucrose transporters (Fichtner et al. 2021). Sucrose is involved in various plant developmental processes including lateral root development. High sucrose concentration dramatically represses lateral root initiation, whereas low sucrose promotes lateral root development (Malamy and Ryan 2001). Sucrose upregulates multiple CEP genes expression; then CEP binds to the CEPR1 receptor and subsequently inhibits lateral root development. Notably, the sucrose-mediated upregulation of CEP gene expression is independent of CEPR1 receptor, implying that some unknown mechanisms also exist to mediate sucrose-CEP signaling involved in lateral root development. Additionally, RNA-seq analysis showed that many genes respond differently to sucrose in the cepr1 mutant as compared to wild type, which suggests an alternative sucrose response dependent on the CEP-CEPR signaling pathway (Table 1; Figs. 2, 4e; Chapman et al. 2019).
Antagonistic roles of CLE and CEP peptide in Nodulation
Legumes fix atmospheric N2 through a specific root organ, the nodule. As fixing N2 is an energy-consuming biological process, the nodule number is tightly regulated via the autoregulation of nodulation (AON) and nitrate-dependent signaling pathway (Ferguson et al. 2019; Gautrat et al. 2021). The AON signaling initiates the synthesis of CLE peptides in Medicago truncatula (MtCLE12, MtCLE13 and MtCLE35) (Mortier et al. 2010; Mens et al. 2021; Moreau et al. 2021), and in Lotus japonicus (LjCLE-RS1, LjCLE-RS2 and LjCLE-RS3) (Okamoto et al. 2009, 2013; Nishida et al. 2018). The induction of CLE expression by N or rhizobia relies on the transcription factor NODULE INCEPTION (NIN) and NIN-LIKE PROTEIN (NLP) family. NIN or NLP binds to the CLE gene promoter to upregulate CLEs expression (Mortier et al. 2010; Nishida et al. 2018; Lin et al. 2018; Laffont et al. 2020; Moreau et al. 2021); then CLE peptides are transported to shoots and sensed by Medicago truncatula SUPER NUMERIC NODULES (MtSUNN) and Lotus japonicus HYPERNODULATON AND ABERRANT ROOT 1(LjHAR1) receptors (Mortier et al. 2010; Mens et al. 2021; Okamoto et al. 2013). The MtCLE-MtSUNN and LjCLE-RS-LjHAR1 modules repress the key shoot-to-root microRNA, miR2111 expression (Fig. 4f; Tsikou et al. 2018; Gautrat et al. 2020). TOO MUCH LOVE 1/2 (TML1/2) are targets of miR2111 (Magori et al. 2009; Takahara et al. 2013). The low expression level of miR2111 leads to an elevated TML1/2 expression and thus reduces nodule numbers (Magori et al. 2009; Takahara et al. 2013; Tsikou et al. 2018; Gautrat et al. 2020).
In contrast to the negative impact of CLE peptides on nodulation, CEP peptides positively regulate the nodulation formation. Under N starvation, the CEP expression is elevated and then binds to the COMPACT ROOT ARCHITECTURE 2 (CRA2) receptor to stimulate nodulation (Imin et al. 2013; Huault et al. 2014; Mohd-Radzman et al. 2016). Under low N condition, miR2111 expression is upregulated, whereas the TML1/2 transcription is decreased, which results in an increase of the nodule number (Fig. 4f; Gautrat et al. 2020). This transcriptional regulation of miR211 and TML1/2 expression depends on the CEP-CRA2 pathway (Gautrat et al. 2020).
In short, plants activate either CLE or CEP signaling to antagonistically regulate miR2111 and TML1/2 expression (Laffont et al. 2020; Gautrat et al. 2020). As a result, the nodule numbers are tightly controlled depending on the local environmental N status (Table 1; Figs. 2, 4f).
Conclusions and further perspectives
Plants have developed a plethora of pathways, notably including small peptides to enable optimal cellular and physiological outputs under constantly changing environments. The RLKs and co-receptor proteins perceive small peptides and translate the environmental signals to activate the complex downstream regulatory networks and thus modulate plant growth accordingly. Although many small peptides have been identified in many crops, the physiological roles of these peptides, particularly their roles in abiotic stress and nutrient signaling-related developmental processes are still untapped.
Arabidopsis genome contains more than 7000 small peptide encoding genes and 600 RLKs; the corresponding ligands for most of RLKs have not been identified yet (Takahashi et al. 2019). This suggests that various potential peptide-receptor signaling cascades enable plants to transmit a wide range of environmental signals under diverse conditions. Due to the redundancy among large family members, only a few members of identified peptides or RLKs have been characterized with a clear biological function. The application of novel genome editing technology to obtain multiple gain-of-function and loss-of-function mutants will enable us to understand the critical roles of small peptides and their interacting receptors in various aspects of plant development and stress responses (Wu et al. 2016; Yamaguchi et al. 2017). In addition, photoaffinity labeling peptides can be used to screen RLK expression library, which will enable to identify peptide-receptor pairs and to elucidate their biological functions (Tabata et al. 2014; Shinohara et al. 2016; Nakayama et al. 2017).
Recently, the emerging roles of small peptides in abiotic stress have been uncovered (Kim et al. 2021), but the mechanistic basis is still largely unknown. Despite their participations in plant response to drought, salinity, and nutrient starvation outlined above, they are also differentially induced by other environmental stimuli (Delay et al. 2013; Czyzewicz et al. 2015; Wang et al. 2016), whereas the undefined roles of small peptides in these unclarified environmental stimuli remain uncharted, which will be of great interest in future. In addition, to discover the downstream key players involved in these small peptide-mediated various abiotic stress will provide novel strategies to genetically engineer crops and hence improve their growth under adverse environments.
Moreover, when and how plants acquired these peptides signaling networks during evolution is currently unsolved; therefore, the study of peptides in early land plants (Delay et al. 2013; Roberts et al. 2013; Goad et al. 2017; Campbell and Turner 2017) will greatly help to elucidate the evolutionary mechanisms of small peptides in plant abiotic stress and nutrients signaling. Additionally, dissection of the crosstalk between phytohormones and small peptides would provide us novel strategies to improve plant tolerance to adverse environments.
The small peptides play essential roles in response to nutrients such as N and P; however, the mechanisms are still far from fully understood. On the other hand, antagonistic and synergistic interactions also exist among different nutrients (Kumar et al. 2021), while how the signaling of different nutrients is integrated by the peptide-receptor system remains obscure. Other phytohormones also take part in the regulation of plants’ reactions to nutrients starvation; thus mechanistic basis of crosstalk between the phytohormone and peptide signaling are also attractive subjects for further investigation. Additionally, how small peptides shape plant architecture in response to other micronutrients, for example, iron (Fe) and zinc (Zn), is also untapped.
In the future, a comprehensive understanding of small peptides-mediated plant abiotic and nutrient responses will require the knowledge regarding the role of small peptides in environmental sensing and the subsequent signal transduction. The basic knowledge obtained from Arabidopsis will provide a blueprint to engineer crops for better growth and yield under unfavorable environmental conditions.
Author contribution statement
HPX, WZ and WLL wrote the draft manuscript. HBH, YZZ, and JH designed the figures, wrote and edited the manuscript. All authors contributed to this article and approved the submitted version.
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Aarabi F, Naake T, Fernie AR, Hoefgen R (2020) Coordinating sulfur pools under sulfate deprivation. Trends Plant Sci 25:1227–1239
Aggarwal S, Kumar A, Jain M, Sudan J, Singh K, Kumari S, Mustafiz A (2020) C-terminally encoded peptides (CEPs) are potential mediators of abiotic stress response in plants. Physiol Mol Biol Plants 26:2019–2033
Araya T, Miyamoto M, Wibowo J, Suzuki A, Kojima S, Tsuchiya YN, Sawa S, Fukuda H, von Wirén N, Takahashi H (2014) CLE-CLAVATA1 peptide-receptor signaling module regulates the expansion of plant root systems in a nitrogen-dependent manner. Proc Natl Acad Sci USA 111:2029–2034
Blackburn MR, Haruta M, Moura DS (2020) Twenty years of progress in physiological and biochemical investigation of RALF peptides. Plant Physiol 182:1657–1666
Campbell L, Turner SR (2017) A comprehensive analysis of RALF proteins in green plants suggests there are two distinct functional groups. Front Plant Sci 8:37
Chapman K, Taleski M, Ogilvie HA, Imin N, Djordjevic MA (2019) CEPCEPR1 signalling inhibits the sucrose-dependent enhancement of lateral root growth. J Exp Bot 70:3955–3967
Chen YL, Lee CY, Cheng KT, Chang WH, Huang RN, Nam HG et al (2014) Quantitative peptidomics study reveals that a wound-induced peptide from PR-1 regulates immune signaling in tomato. Plant Cell 26:4135–4148
Chen J, Yu F, Liu Y, Du C, Li X, Zhu S, Wang X, Lan W, Rodriguez PL, Liu X, Li D, Chen L, Luan S (2016) FERONIA interacts with ABI2-type phosphatases to facilitate signaling cross-talk between abscisic acid and RALF peptide in Arabidopsis. Proc Natl Acad Sci USA 113:E5519–E5527
Chen X, Ding Y, Yang Y, Song C, Wang B, Yang S, Guo Y, Gong Z (2021) Protein kinases in plant responses to drought, salt, and cold stress. J Integr Plant Biol 63:53–78
Chien PS, Nam HG, Chen YR (2015) A salt-regulated peptide derived from the CAP superfamily protein negatively regulates salt-stress tolerance in Arabidopsis. J Exp Bot 66:5301–5313
Czyzewicz N, Shi CL, Vu LD, Van De Cotte B, Hodgman C, Butenko MA, De Smet I (2015) Modulation of Arabidopsis and monocot root architecture by CLAVATA3/EMBRYO SURROUNDING REGION 26 peptide. J Exp Bot 66:5229–5243
Delay C, Imin N, Djordjevic MA (2013) CEP genes regulate root and shoot development in response to environmental cues and are specific to seed plants. J Exp Bot 64:5383–5394
Djordjevic MA, Mohd-Radzman NA, Imin N (2015) Small-peptide signals that control root nodule number, development, and symbiosis. J Exp Bot 66:5171–5181
Dong W, Wang Y, Takahashi H (2019) CLE-CLAVATA1 signaling pathway modulates lateral root development under sulfur deficiency. Plants (basel) 8:103
Endo S, Shinohara H, Matsubayashi Y, Fukuda H (2013) A novel pollen pistil interaction conferring high-temperature tolerance during reproduction via CLE45 signaling. Curr Biol 23:1670–1676
Feng W, Kita D, Peaucelle A, Cartwright HN, Doan V, Duan Q, Liu MC, Maman J, Steinhorst L, Schmitz-Thom I, Yvon R, Kudla J, Wu HM, Cheung AY, Dinneny JR (2018) The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Curr Biol 28:666–675
Ferguson BJ, Mens C, Hastwell AH, Zhang M, Su H, Jones CH, Chu X, Gresshoff PM (2019) Legume nodulation: the host controls the party. Plant Cell Environ 42:41–51
Fichtner F, Dissanayake IM, Lacombe B, Barbier F (2021) Sugar and nitrate sensing: a multi-billion-year story. Trends Plant Sci 26:352–374
Fletcher JC (2020) Recent advances in Arabidopsis CLE peptide signaling. Trends Plant Sci 25:1005–1016
Gautrat P, Laffont C, Frugier F (2020) Compact root architecture 2 promotes root competence for nodulation through the miR2111 systemic effector. Curr Biol 30:1339–1345
Gautrat P, Laffont C, Frugier F, Ruffel S (2021) Nitrogen systemic signaling: from symbiotic nodulation to root acquisition. Trends Plant Sci 26:392–406
Gjetting SK, Mahmood K, Shabala L, Kristensen A, Shabala S, Palmgren M, Fuglsang AT (2020) Evidence for multiple receptors mediating RALF-triggered Ca2+ signaling and proton pump inhibition. Plant J 104:433–446
Goad DM, Zhu C, Kellogg EA (2017) Comprehensive identification and clustering of CLV3/ESR-related (CLE) genes in plants finds groups with potentially shared function. New Phytol 216:605–616
Gonneau M, Desprez T, Martin M, Doblas VG, Bacete L, Miart F, Sormani R, Hématy K, Renou J, Landrein B, Murphy E, Van De Cotte B, Vernhettes S, De Smet I, Höfte H (2018) Receptor kinase THESEUS1 is a rapid alkalinization factor 34 receptor in Arabidopsis. Curr Biol 28:2452–2458
Gutiérrez-Alanís D, Yong-Villalobos L, Jiménez-Sandoval P, Alatorre-Cobos F, Oropeza-Aburto A, Mora-Macías J, Sánchez-Rodríguez F, Cruz-Ramírez A, Herrera-Estrella L (2017) Phosphate starvation-dependent iron mobilization I-induces CLE14 expression to trigger root meristem differentiation through CLV2/PEPR2 signaling. Dev Cell 41:555–570
Haruta M, Monshausen G, Gilroy S, Sussman MR (2008) A cytoplasmic Ca2+ functional assay for identifying and purifying endogenous cell signaling peptides in Arabidopsis seedlings: identification of AtRALF1 peptide. Biochemistry 47:6311–6321
Hirakawa Y, Sawa S (2019) Diverse function of plant peptide hormones in local signaling and development. Curr Opin Plant Biol 51:81–87
Huang G, Zhang D (2020) The plasticity of root systems in response to external phosphate. Int J Mol Sci 21:5955
Huault E, Laffont C, Wen J, Mysore KS, Ratet P, Duc G, Frugier F (2014) Local and systemic regulation of plant root system architecture and symbiotic nodulation by a receptor-like kinase. PLoS Genet 10:e1004891
Imin N, Mohd-Radzman NA, Ogilvie HA, Djordjevic MA (2013) The peptide-encoding CEP1 gene modulates lateral root and nodule numbers in Medicago truncatula. J Exp Bot 64:5395–5409
Jeon BW, Kim MJ, Pandey SK, Oh E, Seo PJ, Kim J (2021) Recent advances in peptide signaling during Arabidopsis root development. J Exp Bot 72:2889–2902
Jia Z, von Wirén N (2020) Signaling pathways underlying nitrogen dependent changes in root system architecture: from model to crop species. J Exp Bot 71:4393–4404
Kaufmann C, Stührwohldt N, Sauter M (2021) Tyrosylprotein sulfotransferase-dependent and -independent regulation of root development and signaling by PSK LRR receptor kinases in Arabidopsis. J Exp Bot 72:5508–5521
Kim JS, Jeon BW, Kim J (2021) Signaling peptides regulating abiotic stress responses in plants. Front Plant Sci 12:704490
Kumar S, Kumar S, Mohapatra T (2021) Interaction between macro- and micro-nutrients in plants. Front Plant Sci 12:665583
Ladwig F, Dahlke RI, Stührwohldt N, Hartmann J, Harter K, Sauter M (2015) Phytosulfokine regulates growth in Arabidopsis through a response module at the plasma membrane that includes CYCLIC NUCLEOTIDEGATED CHANNEL17, H+-ATPase, and BAK1. Plant Cell 27:1718–1729
Laffont C, Ivanovici A, Gautrat P, Brault M, Djordjevic MA, Frugier F (2020) The NIN transcription factor coordinates CEP and CLE signaling peptides that regulate nodulation antagonistically. Nat Commun 11:3167
Lay KS, Takahashi H (2018) Nutrient-responsive small signaling peptides and their influence on the root system architecture. Int J Mol Sci 19:3927
Li Q, Gao Y, Yang A (2020) Sulfur homeostasis in plants. Int J Mol Sci 21:8926
Lin JS, Li X, Luo Z, Mysore KS, Wen J, Xie F (2018) NIN interacts with NLPs to mediate nitrate inhibition of nodulation in Medicago truncatula. Nat Plants 4:942–952
Liu D (2021) Root developmental responses to phosphorus nutrition. J Integr Plant Biol 63:1065–1090
Luo L, Zhang Y, Xu G (2020) How does nitrogen shape plant architecture? J Exp Bot 71:4415–4427
Magori S, Oka-Kira E, Shibata S, Umehara Y, Kouchi H, Hase Y, Tanaka A, Sato S, Tabata S, Kawaguchi M (2009) Too much love, a root regulator associated with the long-distance control of nodulation in Lotus japonicus. Mol Plant Microbe Interact 22:259–268
Malamy JE, Ryan KS (2001) Environmental regulation of lateral root initiation in Arabidopsis. Plant Physiol 127:899–909
Matsubayashi Y (2014) Posttranslationally modified small-peptide signals in plants. Annu Rev Plant Biol 65:385–413
Matsubayashi Y (2018) Exploring peptide hormones in plants: identification of four peptide hormone-receptor pairs and two post-translational modification enzymes. Proc Jpn Acad Ser B Phys Bio Sci 94:59–74
Mens C, Hastwell AH, Su H, Gresshoff P, Mathesius U, Ferguson BJ (2021) Characterisation of Medicago truncatula CLE34 and CLE35 in nitrate and rhizobia regulation of nodulation. New Phytol 229:2525–2534
Mohd-Radzman NA, Laffont C, Ivanovici A, Patel N, Reid D, Stougaard J, Frugier F, Imin N, Djordjevic MA (2016) Different pathways act downstream of the peptide receptor CRA2 to regulate lateral root and nodule development. Plant Physiol 171:2536–2548
Montillet JL, Leonhardt N, Mondy S, Tranchimand S, Rumeau D, Boudsocq M, Garcia AV, Douki T, Bigeard J, Laurière C, Chevalier A, Castresana C, Hirt H (2013) An abscisic acid-independent oxylipin pathway controls stomatal closure and immune defense in Arabidopsis. PLoS Biol 11:e1001513
Moreau C, Gautrat P, Frugier F (2021) Nitrate-induced CLE35 signaling peptides inhibit nodulation through the SUNN receptor and miR2111 repression. Plant Physiol 185:1216–1228
Mortier V, den Herder G, Whitford R, van de Velde W, Rombauts S, D’Haeseleer K, Holsters M, Goormachtig S (2010) CLE peptides control Medicago truncatula nodulation locally and systemically. Plant Physiol 153:222237
Motte H, Vanneste S, Beeckman T (2019) Molecular and environmental regulation of root development. Annu Rev Plant Biol 70:465–488
Murphy E, Smith S, De Smet I (2012) Small signaling peptides in Arabidopsis development: how cells communicate over a short distance. Plant Cell 24:3198–3217
Nakaminami K, Okamoto M, Higuchi-Takeuchi M, Yoshizumi T, Yamaguchi Y, Fukao Y et al (2018) AtPep3 is a hormone-like peptide that plays a role in the salinity stress tolerance of plants. Proc Natl Acad Sci USA 115:5810–5815
Nakayama T, Shinohara H, Tanaka M, Baba K, Ogawa-Ohnishi M, Matsubayashi Y (2017) A peptide hormone required for Casparian strip diffusion barrier formation in Arabidopsis roots. Science 355:284–286
Nishida H, Tanaka S, Handa Y, Ito M, Sakamoto Y, Matsunaga S, Betsuyaku S, Miura K, Soyano T, Kawaguchi M, Suzaki T (2018) A NIN-LIKE PROTEIN mediates nitrate induced control of root nodule symbiosis in Lotus japonicus. Nat Commun 9:499
Ohkubo Y, Tanaka M, Tabata R, Ogawa-Ohnishi M, Matsubayashi Y (2017) Shoot-to-root mobile polypeptides involved in systemic regulation of nitrogen acquisition. Nat Plants 3:17029
Okamoto S, Ohnishi E, Sato S, Takahashi H, Nakazono M, Tabata S, Kawaguchi M (2009) Nod factor/nitrate-induced CLE genes that drive HAR1-mediated systemic regulation of nodulation. Plant Cell Physiol 50:67–77
Okamoto S, Shinohara H, Mori T, Matsubayashi Y, Kawaguchi M (2013) Root-derived CLE glycopeptides control nodulation by direct binding to HAR1 receptor kinase. Nat Commun 4:2191
Okamoto S, Tabata R, Matsubayashi Y (2016) Long-distance peptide signaling essential for nutrient homeostasis in plants. Curr Opin Plant Biol 34:35–40
Olsson V, Joos L, Zhu S, Gevaert K, Butenko MA, De Smet I (2019) Look closely, the beautiful may be small: precursor-derived peptides in plants. Annu Rev Plant Biol 70:153–186
Ota R, Ohkubo Y, Yamashita Y, Ogawa-Ohnishi M, Matsubayashi Y (2020) Shoot-to-root mobile CEPD-like 2 integrates shoot nitrogen status to systemically regulate nitrate uptake in Arabidopsis. Nat Commun 11:641
Patterson K, Cakmak T, Cooper A, Lager I, Rasmusson AG, Escobar MA (2010) Distinct signalling pathways and transcriptome response signatures differentiate ammonium- and nitrate-supplied plants. Plant Cell Environ 33:1486–1501
Ren SC, Song XF, Chen WQ, Lu R, Lucas WJ, Liu CM (2019) CLE25 peptide regulates phloem initiation in Arabidopsis through a CLERK-CLV2 receptor complex. J Integr Plant Biol 61:1043–1061
Ristova D, Carré C, Pervent M, Medici A, Kim GJ, Scalia D, Ruffel S, Birnbaum KD, Lacombe B, Busch W, Coruzzi GM, Krouk G (2016) Combinatorial interaction network of transcriptomic and phenotypic responses to nitrogen and hormones in the Arabidopsis thaliana root. Sci Signal 9:rs13
Roberts I, Smith S, De Rybel B, Van Den Broeke J, Smet W, De Cokere S, Mispelaere M, De Smet I, Beeckman T (2013) The CEP family in land plants: evolutionary analyses, expression studies, and role in Arabidopsis shoot development. J Exp Bot 64:5371–5381
Roberts I, Smith S, Stes E, De Rybel B, Staes A, van de Cotte B, Njo MF, Dedeyne L, Demol H, Lavenus J, Audenaert D, Gevaert K, Beeckman T, De Smet I (2016) CEP5 and XIP1/CEPR1 regulate lateral root initiation in Arabidopsis. J Exp Bot 67:4889–4899
Ruffel S, Krouk G, Ristova D, Shasha D, Birnbaum K, Coruzzi GM (2011) Nitrogen economics of root foraging: transitive closure of the nitrate-cytokinin relay and distinct systemic signaling for N supply versus demand. Proc Natl Acad Sci USA 108:18524–18529
Sadok W, Schoppach R (2019) Potential involvement of root auxins in drought tolerance by modulating nocturnal and daytime water use in wheat. Ann Bot 124:969–978
Salvi P, Manna M, Kaur H, Thakur T, Gandass N, Bhatt D, Muthamilarasan M (2021) Phytohormone signaling and crosstalk in regulating drought stress response in plants. Plant Cell Rep 40:1305–1329
Sauter M (2015) Phytosulfokine peptide signalling. J Exp Bot 66:5161–5169
Segonzac C, Monaghan J (2019) Modulation of plant innate immune signaling by small peptides. Curr Opin Plant Biol 51:22–28
Shani E, Salehin M, Zhang Y, Sanchez SE, Doherty C, Wang R, Mangado CC, Song L, Tal I, Pisanty O, Ecker JR, Kay SA, Pruneda-Paz J, Estelle M (2017) Plant stress tolerance requires auxin-sensitive Aux/IAA transcriptional repressors. Curr Biol 27:437–444
Shinohara H, Mori A, Yasue N, Sumida K, Matsubayashi Y (2016) Identification of three LRR-RKs involved in perception of root meristem growth factor in Arabidopsis. Proc Natl Acad Sci USA 113:3897–3902
Skalak J, Nicolas KL, Vankova R, Hejatko (2021) Signal integration in plant abiotic stress responses via multistep phosphorelay signaling. Front Plant Sci 12:644823
Smith S, Zhu S, Joos L, Roberts I, Nikonorova N, Vu LD, Stes E, Cho H, Larrieu A, Xuan W, Goodall B, van de Cotte B, Waite JM, Rigal A, Ramans Harborough S, Persiau G, Vanneste S, Kirschner GK, Vandermarliere E, Martens L, Stahl Y, Audenaert D, Friml J, Felix G, Simon R, Bennett MJ, Bishopp A, De Jaeger G, Ljung K, Kepinski S, Robert S, Nemhauser J, Hwang I, Gevaert K, Beeckman T, De Smet I (2020) The CEP5 peptide promotes abiotic stress tolerance, as revealed by quantitative proteomics, and attenuates the AUX/IAA equilibrium in Arabidopsis. Mol Cell Proteom 19:1248–1262
Song XF, Hou XL, Liu CM (2022) CLE peptides: critical regulators for stem cell maintenance in plants. Planta 255:5
Stegmann M, Monaghan J, Smakowska-Luzan E, Rovenich H, Lehner A, Holton N, Belkhadir Y, Zipfel C (2017) The receptor kinase FER is a RALF-regulated scaffold controlling plant immune signaling. Science 355:287–289
Stührwohldt N, Schaller A (2019) Regulation of plant peptide hormones and growth factors by post-translational modification. Plant Biol (stuttg) 1:49–63
Stührwohldt N, Ehinger A, Thellmann K, Schaller A (2020) Processing and formation of bioactive CLE40 peptide are controlled by posttranslational proline hydroxylation. Plant Physiol 184:1573–1584
Stührwohldt N, Bühler E, Sauter M, Schaller A (2021) Phytosulfokine (PSK) precursor processing by subtilase SBT3.8 and PSK signaling improve drought stress tolerance in Arabidopsis. J Exp Bot 72:3427–3440
Su J, Zhang M, Zhang L, Sun T, Liu Y, Lukowitz W, Xu J, Zhang S (2017) Regulation of stomatal immunity by interdependent functions of a pathogen-responsive MPK3/MPK6 cascade and abscisic acid. Plant Cell 29:526–542
Tabata R, Sumida K, Yoshii T, Ohyama K, Shinohara H, Matsubayashi Y (2014) Perception of root-derived peptides by shoot LRR-RKs mediates systemic N-demand signaling. Science 346:343–346
Takahara M, Magori S, Soyano T, Okamoto S, Yoshida C, Yano K, Sato S, Tabata S, Yamaguchi K, Shigenobu S, Takeda N, Suzaki T, Kawaguchi M (2013) Too much love, a novel Kelch repeat-containing F-box protein, functions in the long distance regulation of the legume-Rhizobium symbiosis. Plant Cell Physiol 54:433–447
Takahashi F, Suzuki T, Osakabe Y, Betsuyaku S, Kondo Y, Dohmae N, Fukuda H, Yamaguchi-Shinozaki K, Shinozaki K (2018) A small peptide modulates stomatal control via abscisic acid in long-distance signalling. Nature 556:235–238
Takahashi F, Hanada K, Kondo T, Shinozaki K (2019) Hormone-like peptides and small coding genes in plant stress signaling and development. Curr Opin Plant Biol 51:88–95
Taleski M, Imin N, Djordjevic MA (2018) CEP peptide hormones: key players in orchestrating nitrogen-demand signalling, root nodulation, and lateral root development. J Exp Bot 69:1829–1836
Tavormina P, De Coninck B, Nikonorova N, De Smet I, Cammue BP (2015) The plant peptidome: an expanding repertoire of structural features and biological functions. Plant Cell 27:2095–2118
Tsikou D, Yan Z, Holt DB, Abel NB, Reid DE, Madsen LH, Bhasin H, Sexauer M, Stougaard J, Markmann K (2018) Systemic control of legume susceptibility to rhizobial infection by a mobile microRNA. Science 362:233–236
van Zelm E, Zhang Y, Testerink C (2020) Salt tolerance mechanisms of plants. Annu Rev Plant Biol 71:403–433
Vidal EA, Alvarez JM, Araus V, Riveras E, Brooks MD, Krouk G, Ruffel S, Lejay L, Crawford NM, Coruzzi GM, Gutiérrez RA (2020) Nitrate in 2020: thirty years from transport to signaling networks. Plant Cell 32:2094–2119
Wang J, Li H, Han Z, Zhang H, Wang T, Lin G et al (2015) Allosteric receptor activation by the plant peptide hormone phytosulfokine. Nature 525:265–268
Wang G, Zhang G, Wu M (2016) CLE peptide signaling and crosstalk with phytohormones and environmental stimuli. Front Plant Sci 6:1211
Wang F, Deng M, Xu J, Zhu X, Mao C (2018) Molecular mechanisms of phosphate transport and signaling in higher plants. Semin Cell Dev Biol 74:114–122
Wang L, Yang T, Wang B, Lin Q, Zhu S, Li C, Ma Y, Tang J, Xing J, Li X, Liao H, Staiger D, Hu Z, Yu F (2020) RALF1-FERONIA complex affects splicing dynamics to modulate stress responses and growth in plants. Sci Adv 6:eaaz1622
Willoughby AC, Nimchuk ZL (2021) WOX going on: CLE peptides in plant development. Curr Opin Plant Biol 63:102056
Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ (2007) An “electronic fluorescent pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2:e718
Wu Y, Xun Q, Guo Y, Zhang J, Cheng K, Shi T, He K, Hou S, Gou X, Li J (2016) Genome-wide expression pattern analyses of the arabidopsis leucine-rich repeat receptor-like kinases. Mol Plant 9:289–300
Yamaguchi YL, Ishida T, Yoshimura M, Imamura Y, Shimaoka C, Sawa S (2017) A collection of mutants for CLE-peptide-encoding genes in Arabidopsis generated by CRISPR/Cas9-mediated gene targeting. Plant Cell Physiol 58:1848–1856
Yu Y, Assmann SM (2018) Inter-relationships between the heterotrimeric Gbeta subunit AGB1, the receptor-like kinase FERONIA, and RALF1 in salinity response. Plant Cell Environ 41:2475–2489
Yu F, Qian L, Nibau C, Duan Q, Kita D, Levasseur K, Li X, Lu C, Li H, Hou C, Li L, Buchanan BB, Chen L, Cheung AY, Li D, Luan S (2012) FERONIA receptor kinase pathway suppresses abscisic acid signaling in Arabidopsis by activating ABI2 phosphatase. Proc Natl Acad Sci USA 109:14693–146938
Zhang L, Shi X, Zhang Y, Wang J, Yang J, Ishida T, Jiang W, Han X, Kang J, Wang X, Pan L, Lv S, Cao B, Zhang Y, Wu J, Han H, Hu Z, Cui L, Sawa S, He J, Wang G (2019) CLE9 peptide-induced stomatal closure is mediated by abscisic acid, hydrogen peroxide, and nitric oxide in Arabidopsis thaliana. Plant Cell Environ 42:1033–1044
Zhao C, Zayed O, Yu Z, Jiang W, Zhu P, Hsu CC, Zhang L, Tao WA, Lozano-Durán R, Zhu JK (2018) Leucine-rich repeat extensin proteins regulate plant salt tolerance in Arabidopsis. Proc Natl Acad Sci USA 115:13123–13128
Zhao C, Jiang W, Zayed O, Liu X, Tang K, Nie W, Li Y, Xie S, Li Y, Long T, Liu L, Zhu Y, Zhao Y, Zhu JK (2020) The LRXs-RALFs-FER module controls plant growth and salt stress responses by modulating multiple plant hormones. Natl Sci Rev 8:nwaa149
Zulfiqar F, Akram NA, Ashraf M (2019) Osmoprotection in plants under abiotic stresses: new insights into a classical phenomenon. Planta 251:3
Acknowledgements
We do apologize to those whose great work was not cited. We would like to thank other lab members for their critical reading and comments on the manuscript. This work is supported by a starting fund from Jiangxi Agricultural University (9232308314) to HBH. We also acknowledge the EMBO for supporting JH with a long-term fellowship (ALTF 217-2021).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by Gerhard Leubner.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xie, H., Zhao, W., Li, W. et al. Small signaling peptides mediate plant adaptions to abiotic environmental stress. Planta 255, 72 (2022). https://doi.org/10.1007/s00425-022-03859-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-022-03859-6