Abstract
Main conclusion
Global food insecurity concerns due to climate change, emphasizes the need to focus on the sensitivity of sorghum to climate change and potential crop improvement strategies available, which is discussed in the current review to promote climate-smart agriculture.
Abstract
Climate change effects immensely disturb the global agricultural systems by reducing crop production. Changes in extreme weather and climate events such as high-temperature episodes and extreme rainfalls events, droughts, flooding adversely affect the production of staple food crops, posing threat to ecosystem resilience. The resulting crop losses lead to food insecurity and poverty and question the sustainable livelihoods of small farmer communities, particularly in developing countries. In view of this, it is essential to focus and adapt climate-resilient food crops which need lower inputs and produce sustainable yields through various biotic and abiotic stress-tolerant traits. Sorghum, “the camel of cereals”, is one such climate-resilient food crop that is less sensitive to climate change vulnerabilities and also an important staple food in many parts of Asia and Africa. It is a rainfed crop and provides many essential nutrients. Understanding sorghum’s sensitivity to climate change provides scope for improvement of the crop both in terms of quantity and quality and alleviates food and feed security in future climate change scenarios. Thus, the current review focused on understanding the sensitivity of sorghum crop to various stress events due to climate change and throws light on different crop improvement strategies available to pave the way for climate-smart agriculture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change is a serious and growing threat to global food security. The major effects of climate change are increased frequency and magnitude of extreme climate events such as extreme rainfall events, increased dry spells, droughts, water shortages, land degradations, and rise in sea levels. All these effects could negatively impact the global agricultural system which in turn leads to food insecurity in all its dimensions—availability, stability, access, and utilization (Peng et al. 2019). Global atmospheric temperature is predicted to rise by 2–4.5 °C by the end of the twenty-first century with increased concentrations of greenhouse gases (Raza et al. 2019). This global warming leads to increased interannual rainfall variability, reduced precipitation during monsoon season, and an increase in unseasonal rainfall activity which poses a severe threat to agriculture’s ability to deal with the world’s hunger, poverty, and malnutrition. According to IPCC report (Roy et al. 2018), global warming could drive 122 million more people into extreme poverty by 2030. The world population is expected to reach 9.7 billion by 2050 (UN 2019), which furthermore increases pressure on the agriculture sector for growing food requirements. Climate change negatively influences crop yields globally, moreover, extreme temperatures and variable rainfall prevent the growth of crops completely. Especially in tropical regions, extreme weather and droughts are two major hazards for rainfed agriculture (Dilley et al. 2005). In the long term, these extreme events adversely affect the agroecological systems and social resilience (Rosenzweig et al. 2001). Likewise, climate variability has a great impact on global food production in the arid and semi-arid tropical (SAT) regions of the world which account for 30% of the world’s total area and approximately 20% of the world’s population (Lobell et al. 2008). Lobell et al. (2008) reported that increasing temperatures and declining precipitation over semi-arid regions are likely to reduce crop yields due to climate change and variability, particularly rural households which are extremely dependent on agriculture and farming systems are overwhelmed. Thus, it is important to develop and adapt the strategies for changing climate in the SAT regions due to already warmer climates, but also subsistence farmers in the SAT regions will have far fewer options in their agricultural systems to cope with changes in climate.
Cereal grains such as wheat, maize, and paddy are the primary staple food crops across the globe. By 2050, a 70–100% increase in the cereal food supply is desirable for the projected world population (Godfray et al. 2010). But due to a global decrease in fertile and arable lands, it is almost impossible to meet the global food demand with current agricultural practices under climate change scenario. A more hazardous situation could be possible in the SAT regions of the world due to the adverse effects of climate change in these regions. So, it is important to focus on the alternative crops which could adapt to climate change, and could sufficiently fulfill the nutritional needs of the undernourished people across the globe. Sorghum is one such hardy crop that can grow on marginal lands and tolerant to climatic change in different agroecological regions. Sorghum acts as a staple diet for millions of people in the SAT regions of Asia and Africa. It is the major source of food and fodder and is primarily consumed by the producers. Apart from it, sorghum is one of the staple foods for the population in semi-arid and arid regions of the developing countries where malnourishment and poverty are more prevalent. It is a major source of energy and contains many essential nutrients which are necessary to meet the daily nutritional demand of an individual. Thus, it became an important crop for the sustainable livelihood of poor farmers in arid and semi-arid regions. Sorghum can grow in marginal lands with low input and is a predominately rainfed crop in these regions. Although a hardy crop, rainfall variability and heat stress due to changing climate could reduce crop yields substantially in many regions of the world. According to studies, due to climate change, yields of post rainy sorghum likely to reduce 7% by 2020, 11% by 2050 and 32% by 2080 (Srivastava et al. 2010). Climate change variability mostly affects sorghum during reproductive and grain-filling stages and leads to loss of crop. Not only the yield, but the nutritional quality of the crop could also suffer the impacts of climate change by decreasing the major and essential nutritional components in the grain. So, it is essential to improve crop yields without compromising quality. Implementing strategic adaptation approaches like varietal selection and sowing dates could benefit the crop yield to some extent, but the complete loss cannot be prevented if the severity of global warming continues to increase in near future. With the availability of a wide array of new technologies in plant breeding and molecular studies, strategies for climate change adaptation should focus on improving crop yields as well as grain quality. There is no recent assessment of climate variability and change affecting sorghum production at a regional or global scale. The last assessment conducted by the International Crops Research Institute for the Semi-arid Tropics (ICRISAT) and Food and Agriculture Organization (FAO) dated 1996 (ICRISAT and FAO, 1996). There are other studies particularly focused on regional challenges in Sub-Saharan Africa and arid and semi-arid regions of South Asia (Adhikari et al. 2015; Reynolds et al. 2015). Similarly, recent work showed potential impacts of climate change on sorghum crop production (Raymundo et al. 2018). Thus, in the current review, we examine the existing literature to identify the most potential climate change impacts on crop yields and grain quality and adaptation strategies available were discussed with the major emphasis on the sorghum crop.
Crop response to climate change
Crop yields
During the last several years, global warming has a serious impact on cereal cropping regions in many parts of the world. Rapid changes in climatic conditions resulted in increased incidences of various abiotic stresses, thus causing an adverse effect on plant productivity. With a temperature increase of 3–4 °C, 15–35% loss in crop yields in Africa and West Asia and 25–35% yield loss in the Middle East could be expected (FAO 2008). There is a risk of losing around 280 million tons of cereal production potentially among Asian and African countries (FAO 2005). It is projected that agricultural production could decline by 4–10% in developing countries of Asia due to climate change (Fischer et al. 2005).
Climate change effects are generally assessed by the number of stress events and their effect on day-to-day life and loss of agricultural productivity. Climate change severely disrupts plant development by causing several morphological, physiological, biochemical, and molecular changes which ultimately lead to yield loss (Raza et al. 2019). Predominant yield losses and resulting food insecurity in developing countries show the impact of climate change in these regions.
Water stress and extreme temperature are two major forces that impact the reproductive phase in plants. In cereals, water stress shows a negative effect on flower initiation and inflorescence, which leads to a decrease in grain set and thus reducing the harvest index by 60% (Garrity and O’Toole 1994). At the same time, high-temperature episodes, above 30 °C during the flowering stage lead to sterility in cereals, affecting the grain yields. Also, several crop models predicted high rates of evapotranspiration and less soil moisture in drier regions due to high temperatures. It leads to a loss in crop growing area in these regions (IPCC 2007). Various other stresses such as salinity, drought, and chemical effluence damage plant tissues and organs which results in the production of stress responsive proteins, solutes, and elevated antioxidant ratios. They in turn lead to oxidative and osmotic stress in plants.
Crops, in general, adapt to higher temperatures by reducing the crop cycle, which affects yields substantially. This reduced crop yield is due to a decrease in the rate of photosynthesis, respiration, and grain filling. Although C4 crops have a better photosynthetic capacity, higher temperatures cause a decline in photosynthesis rate, which in turn affects crop yields (Crafts-Brandner and Salvucci 2002). Warming causes an increase in vapour-pressure deficit (VPD) which results in reduced water use efficiency of plants due to loss of more water per unit of carbon gain (Ray et al. 2002). Temperature instability will also provide more favourable environmental conditions for insects and pests of crops to boost their capacity to stay alive in cold temperatures and emerge during critical crop stages. An increase in temperature leads to a reduced grain-filling stage which is the primary cause of reduced crop productivity during climate change scenario (Challinor et al. 2007). Heat stress normally is a function of the intensity of temperature, duration, and rate of increase. When it occurs before anthesis, it causes sterility of florets (Prasad et al. 2000, 2008). If exposed to long-term heat stress, reproductive processes impair significantly which was noticed in rice (Baker et al. 1995), soybean (Boote et al. 2005), peanut (Prasad et al. 2003) and sorghum (Prasad et al. 2006). Heat stress accelerates the overall female development which reduces the duration of their receptiveness to pollen and pollen tubules. When exposed to high temperatures during seed filling, it reduces the seed set and seed weight and decreases the overall yield by reducing the seed filling rate and duration (Siddique et al. 1999). This process is similar to drought stress, however, in heat stress, seed filling duration decreases severely compare to seed filling rate. Thus, heat stress along with drought is a major constraint during grain filling for many cereal crops.
Climate change increases the frequency and magnitude of droughts, thus intensifying the crop water stress. In general, crops can tolerate water stress to some extent by closing stomates. However, an increase in potential heat related impact results in more pronounced water stress which could lead to loss of crops. Particularly, in the tropics, the chances of experiencing drought are high during the start and end of the season, resulting in significant crop losses (Krupa et al. 2017). In general, pre-anthesis water stress affects stand count, tillering capacity, number of panicles and seeds per panicle while post-anthesis water stress affects transpiration efficiency, CO2 fixation, and carbohydrate translocation. These changes ultimately lead to premature plant senescence and yield losses (Thomas and Howarth 2000; Xin et al. 2008). In cereals, water stress during the reproductive phase (Stone et al. 2001; Hatfield et al. 2011) is especially harmful and reduces the yields substantially. Not only the droughts, more intense rainfall in some regions lead to flooding and waterlogged soils that could damage the crop yields. Waterlogging due to floods/extreme rainfall events affect the soil physical, chemical and biological properties which eventually affect the crop water and nutrient uptake from soil. Due to the closure of stomata (Ahmed et al. 2002), photosynthetic rate and net carbon assimilation decrease under excess soil moisture. Thus, resulting events lead to a reduction in yields (Zhuo and Lin 1995; Ahmed et al. 2002). However, these effects vary from species to species and between genotypes within species (Orchard and Jessop 1984; Umaharan et al. 1997; Pang et al. 2004).
Due to deforestation and fossil fuel utilization, currently atmospheric CO2 is increased to 400 µmol−1. It is projected to increase up to 800 µmol−1 by the end of the century. Elevated CO2 was found to reduce the stomatal conductance, thus increasing the water use efficiency of both C3 and C4 plants. But there are contradictory studies (Long et al. 2006) which reported the effect of elevated CO2 on crop plants. Some studies even reported the reduced nutritional quality of crops due to high CO2 when rising in the nutrient poor soils by reducing the nitrate assimilation (Taub et al. 2008). Elevated CO2 during drought could lead to the induction of reactive oxygen species (ROS) which disturb photosynthesis and respiration. ROS can cause disturbance in the synthesis of carbohydrates, protein, lipids, nucleic acids which are building blocks for plant growth (Ahmad et al. 2018). Leakey (2009) reported, under elevated CO2 conditions, CO2 concentration increases in the bundle sheet cells which lead to reduced photorespiration in the case of C4 plants. However, like C3 plants, C4 plants also exhibit high photosynthetic rates, water use efficiency by reducing stomatal conductance due to elevated CO2, and reduce the effects of drought.
During climate change, phytohormones also play a major role by inducing stress responsive signal transduction mechanisms. For example, ethylene is found to act as signalling pathway among plant growth and environmental variations. During abiotic stress conditions, it controls seed germination, leaf growth, senescence, and ripening. Abscisic acid also induces several physiological mechanisms during drought stress by producing stress responsive genes and controlling transpiration and stomata closure and opening (Kuromori et al. 2018).
Grain quality
The growth environment plays important role in all aspects of seed quality—seed size, seed composition, and germination ability. Several studies showed the effect of environment on grain composition and many of them reported year to year variability and region × year interactions for grain quality traits such as protein and oil concentrations (Hurburgh et al. 1990; Brumm and Hurburgh 2006; Naeve and Huerd 2008;). Drought and heat stress are the two major stresses which affect the size and composition of matured seed both in cereals and legumes due to their negative impact on nutrient uptake, assimilate supply, and remobilization of nutrients (Prasad et al. 2008). In addition to that, these stresses negatively affect the viability of harvested seeds. Seed filling is the most crucial stage and temperature influence the various processes involved in seed filling, ultimately affecting seed quality. The optimum temperature for normal grain filling varies from species to species (Hatfield et al. 2011). Due to high temperature, there will be a decrease in seed size, glucose concentration, and at the same time increase in sucrose and raffinose concentrations in grain. Studies also showed decrease in oil concentration and protein percentage with an increase in temperature (Gibson and Mullen 1996; Pazdernik et al. 1996; Thomas et al. 2003; Naeve and Huerd 2008).
Changes in the environment also has a significant impact on starch biosynthesis and properties (Tester and Karkalas 2001; Thitisaksakul et al. 2012). Changes in planting seasons, higher night temperatures, decreased water availability, and soil quality could significantly affect the starch accumulation and physical properties which in turn affect the downstream uses (Hatfield et al. 2011). The structure and composition of starch are important indicators for quality and nutritive value of cereal products as animal feed and suitability as feedstock for biofuels (Dang and Copeland 2004; Moritz et al. 2005; Svihus et al. 2005; Sun et al. 2011). In addition to the genotypic effect, starch functionality also varies with increasing air and soil temperatures, rainfall pattern, growing locations and environmental stresses (Dang and Copeland 2004). In addition to total starch concentration, minor changes in amylose concentrations could seriously alter the starch gelatinization and pasting properties (Zeng et al. 1997; Hurkman et al. 2003). These changes in amylose concentrations due to high temperatures are more evident in maize, rice, and wheat compare to barley and sorghum (Tester 1997; Tester and Karkalas 2001; Kiseleva et al. 2003; Li et al. 2013a, b). Time and severity of heat stress can also alter the starch granule size, shape, and structure (Liu et al. 2011). For example, when applied before anthesis, the size of wheat A granules were affected disproportionately (Liu et al. 2011). Reduction in granule size was also observed in sorghum, rice, and maize (Lu et al. 1996; Li et al. 2013a, b; Mitsui et al. 2013). On the contrary, low temperatures and cold seasons increase the ratio of amylose to amylopectin in cereals such as rice, maize and wheat (Fergason and Zuber 1962; Asaoka et al. 1984; Dang and Copeland 2004; Labuschagne et al. 2009; Singh et al. 2010). Studies showed starch gelatinization and pasting temperatures also decreased in cold treated cereals (Myllarinen et al. 1998; Aboubacar et al. 2006).
Water stress negatively affects grain physical attributes. Reduced grain weight and grain size and increase in grain hardness was reported under water stress (Pang et al. 2018; Impa et al. 2019). Water stress also affects starch accumulation, leading to changes in starch composition, structure, and functionality (Thitisaksakul et al. 2012). Water stress also decreases the amylose content in wheat, rice, and barley (Cheng et al. 2003; Dai et al. 2009; Singh et al. 2010; Gunaratne et al. 2011a, b). Increased flour swelling power, viscosity, gel hardness, and granular breakdown could also be seen due to water stress (Gunaratne et al. 2011a, b). In addition to flour properties, an increase in grain chalkiness and milling properties can also be seen during water stress. Ali et al. (2010) reported grain oil content was reduced up to 40% in maize due to drought stress, at the same time it increased the oleic acid content by > 25% and reduced the linoleic acid content. Reduced grain starch-lipid content was seen in wheat studies due to water deficit (Singh et al. 2008; Fabian et al. 2011). In addition to heat and water stresses, elevated CO2 and O3 also have a significant impact on grain productivity which in turn affects the starch composition and functionality (Mishra et al. 2013; Piikki et al. 2008).
Sorghum
Production
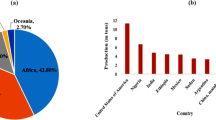
Sorghum is the fifth most important cereal crop in the world. Due to its high photosynthetic efficiency, sorghum can grow both in temperate and tropical regions. It has a short maturity period and can grow both in irrigated and rainfed conditions, thus suitable for subsistence as well as commercial farming. Developing countries, mostly, Africa and Asia account for nearly 90% of sorghum production area. Production-wise, 38.6% was from the Americas, 38.5% from Africa, 18.6% from Asia and remaining 4.3% of sorghum production was from Europe and Oceania (FAOSTAT). Sorghum crop was harvested in nearly 40 million hectares of the world’s area and the total sorghum production in this area was around 57.9 million tonnes in 2019 (Fig. 1).
Spatial distribution of sorghum area (a), production (b) and productivity (c) over Africa and Asia during 1961–2019 based on FAOSTAT 2019. Data
In the past two decades, sorghum production (~ 1%) and production area (2%) was decreased slightly. Over the last decade, sorghum production area and yields increased in Africa (area: 27.5–28.4 M hectares; Yield: 25.6–28.6 M tonnes), whereas it was reduced significantly in Asia (area: 9.3–5.6 M hectares; yield: 10.9–7.8 M tonnes) and Americas (area: 7–5.1 M hectares; yield: 25.1–18.8 M tonnes) (FAOSTAT). Overall productivity is high in commercial systems where sorghum production area is roughly 15%, but produce 40% of global sorghum yields. In contrast, most of the developing world including Africa and Asia grow sorghum extensively, but in low input systems and average yields in these areas remained 0.5–1 tonne per hectare. More than 70% of the sorghum grown in these areas is consumed as food. Especially in Africa, cropping area has increased significantly, but productivity remained low due to the use of marginal, drought-prone lands and poor soils (ICRISAT and FAO 1996). Figure 2 depicts a change in the sorghum production trends over the past few decades in predominantly sorghum growing regions.
Trends of sorghum yields over Africa, Asia, Americas and Australia during 1961–2019 based on FAOSTAT data. Data
Growth conditions
Sorghum is mainly cultivated in drier environments on shallow and deep clay soils. It is more tolerant to alkaline soils and can be grown on soils with a pH between 5.5 and 8.5. The minimum temperature requirement for germination is 7–10 °C. More than 80% of the seeds germinate at 15 °C. The optimum temperature requirement for growth and development is 27–30 °C. Growth and yields can be affected beyond 35 °C. It is a short-day plant with a photoperiod requirement of 10–11 h to induce flower formation. Tropical varieties are more sensitive to photoperiod than short-season varieties. It has a growing season of 115–140 days. Water requirement mainly depends upon growth and environment. Typically, medium to late maturing grain sorghum cultivar requires 450–650 mm of water during growing season (Assefa et al. 2010). Water use is less during the early stages of development and then maximum water use occurs from booting stage to anthesis. Then it gradually decreases during the grain-filling stage. Along with these critical requirements, the production potential of sorghum also depends upon plant population, cultivar choice, fertilizer input, and pest and insect control.
Potential impacts of climate variability and change
Heat stress
Human activities already had a significant impact on global and regional climate, it is evident from Fig. 3 there was approximately 1 °C increase in the surface temperatures across Africa and Asia over the past decade 2009–2019.
Percent change in surface temperature during 1961–2019 over Africa and Asia. Temperature change was calculated based on the baseline climatology, corresponding to the period 1951–1980. Data
Gradual change in surface temperatures also negatively affects the sorghum crop and reduces the yield potential. Generally, the optimum temperature requirement for sorghum crop is 21–35 °C for germination, 26–34 °C for vegetative growth and 21–35 °C for reproductive growth (Maiti 1996). Maximum yields and dry matter can be obtained at 27/22 °C (day/night temperatures). Temperatures above 33/28 °C during panicle development results in floret and embryo abortion (Downes 1972). In general, reproductive stages (panicle initiation, grain filling and grain size) are more sensitive to heat stress compare to the vegetative stage (leaf growth, photosynthesis) (Downes 1972; Craufurd et al. 1998; Hammer and Broad 2003; Prasad et al. 2006). Prasad et al. (2008) found the most sensitive stages for heat stress in grain sorghum were flowering and 10 days before flowering which results in reduced seed set, seed number and yields. In grain sorghum, these most sensitive stages to high temperatures are characterized by a maximum decrease in floret fertility. Meiosis, anthesis, fertilization and embryo formation occur during these periods. As a result, negative impacts like pollen sterility, decreased seed set (Djanaguiraman et al. 2014) and changes in concentration and composition of carbohydrates and starch deficiency (Jain et al. 2007) could be seen during heat stress. Prasad et al. (2008) showed continuous exposure to high temperature (40/30 °C) leads to a delay in panicle emergence by 28 days and flowering by 20 days. Heat stress significantly decreases the plant height at maturity, seed set, seed number and size, but does not have a significant impact on leaf area and leaf dry weight. Maximum seed set decrease was observed when heat stress occurred at the flowering stage (54%) (Prasad et al. 2008). Short periods of heat stress in sorghum during panicle emergence result in a decreased grain-filling rate and duration which in turn leads to smaller seed size. At the same time, an increase in heat stress at the beginning of grain-filling stage leads to a decrease in individual grain weight which was observed both in controlled as well as field grown sorghum. Thus, short periods of heat stress in sorghum significantly affect seed set (Singh et al. 2015) and seed number, whereas season long heat stress has a negative impact on individual seed weight (Prasad et al. 2006) due to reduced grain-filling period (Prasad et al. 2015). However, different genotypes exhibit different responses to heat stress (Nguyen et al. 2013; Djanaguiraman et al. 2014; Singh et al. 2015) and also severity of impact on floret fertility and grain weight varies between tolerant and susceptible sorghum hybrids (Prasad et al. 2015).
The impact of heat stress on sorghum grain quality traits was reported only by a few researchers so far (Wu et al. 2016; Impa et al. 2019) and still needs further investigations. It is speculated, like other cereals, accumulation of starch decreases under heat stress in sorghum. Decrease in activities of different enzymes (Ahmadi and Baker 2001; Hurkman et al. 2003; Li et al. 2013a, b) contribute to reduction in starch synthesis and altered amylose to amylopectin ratio. Li et al. (2013a, b) also reported lower starch weight per grain and smaller starch granules under elevated temperatures. Lower starch concentrations under heat stress in grain samples was also found by Johnson et al. (2010) while working on corn and sorghum. Effect of heat stress on starch accumulation also negatively affects the biofuel industry. Heat stressed grain sorghum samples release less sugars due to altered starch accumulation and composition, ultimately causing reduced ethanol production compare to non-stressed sorghum grains (Ananada et al. 2011). However, Impa et al. (2019) reported there was no significant effect of heat stress on starch content, but grain protein decreased under stress with a significant reduction in protein digestibility. The same study reported increase in grain hardness and diameter and a reduction in grain micronutrients under heat stress. Wu et al. (2016) reported a decrease in tannin content under high temperatures, whereas phytates and mineral contents were highly influenced by genotypes compare to growth temperature. Taleon et al. (2012) found a strong effect of abiotic stress factors such as light and temperature on the flavonoid content of black sorghum.
Cold stress
As a tropical crop, sorghum is highly sensitive to chilling stress (Peacock 1982; Rooney 2004). It is sensitive to cold stress than any other cereal. Sorghum production in most of the temperate regions affects by cold temperature stress. Chilling stress can affect the sorghum both in pre-and post-flowering stages thus mitigating the vegetative growth as well as grain-filling period. When planted early in the season with low soil temperatures, sorghum suffers from poor seedling emergence and seedling vigor which results in yield losses (Yu and Tuinstra 2001; Cisse and Ejeta 2003; Burow et al. 2011; Kapanigowda et al. 2013; Maulana and Tesso 2013; Chiluwal 2018). Not only this, emerging seedlings are more prone to soil-borne pathogens such as Pythium and Fusarium spp. (Forbes et al. 1987). As a result, plant population reduce significantly, although this effect varies from genotype to genotype (Tiryaki and Andrews 2001; Franks et al. 2006). Cold stress affects the development and function of chloroplasts (Fracheboud et al. 1999; Allen and Ort 2001), thus reducing photosynthetic capacity and respiration. But in sorghum, Ercoli et al. (2004) found, the photosynthetic rate was severely affected compare to respiration which resulted in reduced leaf area due to loss in leaf turgor. Early season cold stress in sorghum also delays panicle emergence and heading in sorghum (Majora et al. 1982) along with maturity time (Maulana 2011). Moreover, Ercoli et al. (2004) showed N fertilized plants are more sensitive to cold stress than non-fertilized crops. Mid-season cold stress which coincides with the reproductive stage negatively affects the yield components. Cold temperatures at flowering significantly reduce the mean panicle weight, number of seeds per panicle and thousand seed weight (Maulana 2011). These effects are primarily due to the impact of stress on flowering, pollination and fertilization. However, these negative effects depend on genotype and degree of sensitivity to cold stress.
Cold stress in sorghum not only affects the yield but also impacts the grain nutritional quality. Cold stress reduced the grain protein and starch compositions (Ostmeyer et al. 2020). High tannin contents also observed in sorghum genotypes under cold stress. Although grain protein and starch composition differ in genotype to genotype, there was a significant genotype by environment interaction observed in recent studies (Ostmeyer et al. 2020). Ostmeyer et al. (2020) also reported that not only chemical composition, physical traits such as reduction in kernel hardness and diameter also reduced due to chilling stress. Development of early chilling tolerance hybrids found to improve the nutritional quality along with yields. Also, tannin free chilling tolerant hybrids were identified (Chiluwal et al. 2018) which improves the grain quality by enhancing protein digestibility.
Drought stress
With current global climate change trends, there is an increasing frequency of droughts, particularly in arid and semi-arid regions of the world. Although a stress-tolerant crop, sorghum is usually affected by water stress experienced due to drought during pre-and post-flowering stages. Drought stress occurs at these stages results in substantial yield loss in sorghum (Tuinstra et al. 1997; Kebede et al. 2001; Blum 2004). Drought stress at post-flowering stage affects the seed size and number per plant (Rosenow and Clark 1995) by 55 and 36%, respectively, ultimately reducing the grain yield (Assefa et al. 2010).
Generally, a medium to late maturing sorghum cultivar requires 450–650 mm of water during the growing season (Tolk and Howell 2001; FAO 2002), although daily requirements depend on the growth stage. Roughly 1–2.5 mm of water is sufficient for sorghum at the early growth stage to avoid water stress. Later water requirement increases up to 7–10 mm and then it is maximum from booting stage to anthesis (Assefa et al. 2010). Thus, reduced soil moisture below this minimum requirement results in developing water stress. A further study on water use of sorghum reported the addition of every mm of water above 100 mm results in an additional 16.6 kg of grain (Stone, and Schlegel, 2006). Water deficit at certain growth stages results in yield loss in sorghum. So, a well-distributed water supply based on the growth stage is necessary for good grain yield, rather than the amount of total water available throughout the cropping season. Majorly, sorghum is vulnerable to long periods of water stress and susceptible to yield losses. For example, Eck and Musick (1979) showed water stress for 35–42 days from the beginning of boot stage resulted in yield loss of 43 and 54%, respectively. Likewise, Inuyama et al. (1976) reported, 16 and 28 days of water stress during the vegetative stage resulted in 16 and 36% of yield reduction. It shows water stress at the reproductive stage is more sensitive than vegetative stage. Water deficit at this stage prevents the development of pollen and ovules, fertilization and premature abortion of fertilized ovules (Saini 1997; McWilliams 2003). As a result, a number of panicles, seeds per panicle, and individual grain size decrease with drought. Precisely, if the drought stress occurs at the early boot stage, yield loss would be due to reduced seed size and number, but if the stress occurs at later stages, yield loss would be only due to reduced seed size (Eck and Musick 1979). Severe water stress at pre-flowering stage lowers the net photosynthetic rate by reducing PSII and PEPcase activities and by closing stomata (Vinita et al. 1998). Thus, water stress ultimately increases photorespiration and internal oxygen concentration. The resulting formation of reactive oxygen spp. leads to cellular death, thus reducing total dry matter production under drought conditions (Perry et al. 1983; Terbea et al. 1995). Wong et al. (1983) found drought at the vegetative stage accelerates flowering but does not affect the grain-filling period. Manjarrez-Sandoval et al. (1989) reported, microsporogenesis is the most susceptible stage to drought stress in sorghum by causing panicle loss and resulting yield loss. The same study also reported severe drought stress at the microsporogenesis stage does not affect grain yields, because of compensated yields by tillers produced at later stage, especially in long maturity sorghums (Manjarrez-Sandoval et al. 1989).
Impa et al. (2019) showed that terminal water stress decreased the individual grain size and diameter, but increased the grain hardness. This reduced grain size and number might be attributed to decreased grain-filling duration under drought stress which terminates the grain-filling period early (Impa et al. 2019). Pang et al. (2018) also reported, reduced test weight, grain size, and grain hardness in sorghum under low soil moisture. Drought stress, depending on the severity reduces various enzyme activities involved in starch biosynthesis and accumulation (Ahmadi and Baker 2001; Hurkman et al. 2003; Pang et al. 2018), thus reducing the total starch content in the grain. Bing et al. (2014) reported, drought stress at the flowering stage shows a reduction in activities of granular bound starch synthase which is responsible for amylose synthesis, starch branching enzyme activity, that is responsible for amylopectin formation and also starch debranching enzyme activity. However, many studies reported an increase in grain protein content under drought stress (De Souza et al. 2015; Impa et al. 2019; Sarshad et al. 2021), but decrease in protein digestibility, one of the important factors which determine the quality of sorghum feed. On the contrary, a few researchers noticed increased kernel hardness and protein content in irrigated sorghum grain samples (Wu et al. 2008; Njuguna et al. 2018). Zhan et al. (2003) and Wu et al. (2007) showed protein content is inversely proportional to starch content, a property that negatively affects the biofuel industry by reducing ethanol production from sorghum grain samples. Increased protein content may contribute to more starch–protein complexes which in turn results in less starch availability to hydrolytic enzymes to release glucose and less fermentation efficiency for ethanol production (Wu et al. 2007). Ananda et al. (2011) confirmed the same by showing increased ethanol yields from drought stressed grain samples compare to controls, whereas Pang et al. (2018) showed irrigation capacity has a positive impact on final bioethanol yields although less fermentation efficiency was observed for the irrigated grain samples during the first 48 h of fermentation. Thus, these contradictory results support the fact that grain quality not only depends on climate but also on genotype and location and their interactions (Ebadi et al. 2005). Wu et al. (2007) found reduced crude fiber content in drylands grown sorghum compare to irrigated lands. The same study reported there was no significant reduction in mineral content (ash) under drylands, at the same time noticed, location specific increase in mineral content. On the contrary, Impa et al. (2019) showed reduced micronutrient concentration under moisture stress except for grain Fe content. An increase in tannin content was noticed by Njuguna et al. (2018) under less soil moisture compare to higher moisture soils.
Waterlogging (excess moisture)
Waterlogging that occurs mainly due to flash and heavy floods is a major constraint for crop growth and yield due to current management practices and changes in precipitation levels (Polthanee 1997). Many studies (Orchard and Jessop 1984, 1985; Pardales et al. 1991; McDonald et al. 2002) reported the effect of waterlogging on growth and yields in sorghum. Adverse effects of flooding depend on the crop growth stage. The early growth stage was found to be more susceptible compare to the early and late reproductive stages (Orchard and Jessop 1984; Umaharan et al. 1997; Linkemer et al. 1998). Promkhambut et al. (2011) showed flooding applied for 20 days at the early growth stage severely impaired the primary root and shoot growths in sorghum. Moreover, root growth was severely affected than shoot growth. Low radial oxygen loss in sorghum in response to flooding was also observed by McDonald et al. (2002). Due to this oxygen deficit, sorghum experience anaerobic conditions when exposed to prolonged excess moisture stress (Pardales et al. 1991). Promkhambut et al. (2011) observed aerenchyma development on nodal and lateral roots in response to early vegetative and reproductive stage flood conditions, which is an adaptive response to flooding stress (Zaidi et al. 2004). Root aerenchyma development in response to flooding stress, which is high at the vegetative stage than late growth stage in grain sorghum was also reported by Orchard and Jessop (1985). Pardales et al. (1991) observed nodal root development in a few sorghum genotypes under flooding stress, which is an important trait for waterlogging tolerance. This increase in nodal root number was also observed in sweet sorghum genotypes (Promkhambut et al. 2011) with an increase in the duration of flooding. Excess moisture conditions at the vegetative stage led to a reduction in net photosynthetic rate, transpiration, and stomatal conductance in sweet sorghum genotypes as observed by Zhang et al. (2016). Resulting in poor panicle differentiation and seed setting rate showed reduced grain yields in sorghum (Zhang et al. 2019a, b). Thus, excess moisture in soil due to flooding at early vegetative and reproductive stages leads to a reduction in stalk and grain yields due to stunted root and shoot growths (Promkhambut et al. 2011).
Sorghum grain quality due to excess moisture in the soil is not reported much due to limited literature. Studies conducted on sweet sorghum (Promkhambut et al. 2011; Zhang et al. 2016) showed crops experience anaerobic conditions due to oxygen depletion in the soil. As a result, nutrient uptake decreases (Setter and Belford 1990). Limited N supply causes stunted growth. Overall, photosynthetic efficiency decreases due to a reduction in chlorophyll content. The resulting senescence conditions impair the relocation of photoassimilates. It impacts carbohydrate accumulation, grain size, and grain nutrient composition along with the yields due to nutrient deficiency experienced by the crop.
Effect of elevated CO2
Studies reported sorghum crop shows a significant reduction in transpiration rate due to elevated CO2 (Pallas 1965; van Bavel 1974) under irrigated conditions like C3 cereals. An increase in stomatal resistance results in reduced water use increased nutrient and water uptake from deeper soils due to increased root mass at every growth phase (Chaudhuri et al. 1986). This allows optimum growth and development in case of sorghum. This characteristic indeed benefits the crop during drought conditions. Elevated CO2 was found to reduce the water use under drought stress, resulting in the availability of soil water for a long time during dehydrated periods. It was found, elevated CO2 increased the growth during the grain-filling period under drought, but decreased the vegetative growth (Ottman et al. 2001). Thus, with continuous carbon gain, an increase in yields was observed for sorghum due to elevated CO2 under drought conditions (Ottman et al. 2001). Torbert et al. (2004) observed around a 30% increase in sorghum biomass production due to elevated CO2. They noticed a substantial rise in C:N ratio due to CO2 enrichment.
Very few studies reported grain quality in sorghum with elevated CO2. De souza et al. (2015) reported there was almost a 60% increase in grain protein content when grown under elevated CO2 and water deficit conditions. Fatty acids in the grain were slightly increased, but no such increase in starch content was found. Thus, elevated CO2, in the case of sorghum was found to be beneficial to mitigate the drought conditions as well as enhanced grain quality.
Potential sorghum adaptation strategies for climate change effects
Sorghum crop improvement programs along with strategic crop adaptation approaches are designed to cope with the negative impacts of climate change and further maintaining the production and income of smallholders. Different adaptation approaches like crop management practices, breeding, and biotechnological approaches could enhance the sorghum grain productivity and quality under extreme climatic scenarios to a great extent. Understanding genetic variation and the development of climate-resilient sorghum genotypes broaden its adaptation and enhance the production in different agroclimatic zones. The following adaptation strategies could benefit the sorghum crop from climate change impacts.
Crop management practices
Better crop management practices are the first step to be taken to improve the sorghum yields under different stress conditions. Generally, yield potential can be enhanced by adapting changes in sowing time, crop cultivars and mixed cropping systems, alteration of planting, and harvesting time, short life cycle cultivars, use of drought and heat-resistant cultivars and implementing different irrigation techniques to tolerate abiotic stresses (Fatima et al. 2020). Adoption of suitable soil, water, and pest management practices maintains crop production under climate change situations. Choosing commercial sorghum hybrids, which possess different stability components (drought, disease and heat tolerance, photosynthetic efficiency, nutrient use efficiency) enhances yield stability across a wide range of environments compared to inbred lines (Boyles et al. 2019). Management of planting geometry such as decreased planting density, increased plant or row to row spacing and skip row configurations helps to better utilize soil moisture in dryland areas (Fatima et al. 2020). It enhances the dry matter accumulation and grain yields with an increase in photo assimilates available. Delay in sowing time helps the crop to escape from heat stress at critical stages of development like flowering and grain filling. The use of genetically pure and quality seed reduces the negative effects of climate change to some extent and provides scope for sustainable nutritional security (Yu and Tian 2018). Making use of agricultural biodiversity is another promising option to reduce future climate change effects. Crop rotation and multi-cropping systems such as combining deep-rooted with shallow-rooted, C3 crops with C4 crops, combining different varieties of crop in the same field may enhance productivity by reducing the adverse effects of climate change (Fatima et al. 2020). Optimum use of fertilizers is also vital for crop growth and productivity as it provides sufficient nutrients to plants and also enhances the fertility of soil (Raza et al. 2019). Sonobe et al. (2010) found the application of silica fertilizer for sorghum crop improved growth under water stress conditions by increasing the root water uptake and reducing the root osmatic potential. Extensive field trials and data collection help to evaluate the impacts of climate change. Future predictions are possible using remote sensing and crop modelling strategies which helps to apply corrective measures to improve the yields for different agroecological zones (Arora 2019).
Breeding and genetic modifications strategies
Plant breeding gives ample opportunities to develop stress-tolerant cultivars that escape extreme weather changes and gain yield benefits. Landraces are a significant source for genetic studies as they contain broad genetic variation. The genetic divergent analysis is an important tool to develop new cultivars with stress resistance (Lopes et al. 2015; Raza et al. 2019). Molecular breeding is a powerful technique which couples breeding with genomic approaches to screening elite germplasms. QTL studies, genomics, and transcriptomic analyses enable to identify the molecular mechanisms responsible for stress tolerance. All these techniques help to develop new cultivars with improved production potential under different climatic change effects (Roy et al. 2011). Genome wide association studies (GWAS) is a powerful tool to identify allelic variants linked with any specific trait (Manolio 2010). GWAS has been extensively used for many crops to exploit the genetic basis for stress resistance under climate change (Mousavi-Derazmahalleh et al. 2018). High throughput phenotyping is extensively used to screen germplasm for various traits of interest. The biotechnological approach uses genetic engineering techniques to develop transgenic plants with different biotic and abiotic stress tolerance. Herewith, important molecular and high throughput approaches used for sorghum crop improvement under different stress scenarios were discussed further.
Heat tolerance
Sorghum, naturally, a heat-tolerant crop, experience heat stress at critical stages which result in reduced yields. At the vegetative stage, it decreases the photosynthetic rate (Djanaguiraman et al. 2014) and when it occurs at the reproductive stage, it reduces pollen viability and impacts fertilization (Djanaguiraman et al. 2014, 2018; Prasad et al. 2015). Genetic variation is available in sorghum to develop heat-tolerant cultivars (Singh et al. 2015, 2016). BTx 623 is one of the heat-resistant cultivars that is being used (Singh et al. 2015). Understanding the genetic control of heat tolerance is a basic requirement for developing the appropriate breeding program. Khizzah et al. (1993) studied sorghum lines and reported heat tolerance is associated with two genes with a simple additive model. GWAS was applied for sorghum by Chen et al. (2017) to identify loci for heat tolerance during the vegetative stage.
14 SNPs that are associated with leaf firing and blotching in sorghum were identified in their study which could serve as candidate gene markers in molecular breeding for heat tolerance under the vegetative stage. Another genome-wide analysis (Nagaraju et al. 2015) of sorghum reported 25 heat shock transcription factors expressed under different abiotic stress conditions. Out of them, Hsf1 was expressed under high-temperature stress and Hsf 5, 6, 10, 13, 19, 23 and 25 expressed under drought stress. These genes provide insights into abiotic stress-tolerance mechanisms under different conditions. Transcriptomic analysis of sorghum under drought and heat stress revealed (Johnson et al. 2014) 4% of genes were differentially expressed for drought and 17% for heat stress. Also, a 7% of unique genes were identified for combined stress response. Identification of these differentially expressed genes could be targeted for improvement of sorghum for heat as well as drought stress tolerance under changing climatic conditions.
Cold tolerance
Improvement of early-stage chilling tolerance hybrids is an important breeding target for improved sorghum productivity (Knoll et al. 2008; Knoll and Ejeta 2008; Fernandez et al. 2015; Chiluwal et al. 2018). Currently, cold tolerant sorghum hybrids are limited compare to other cereals (Yu et al. 2004). Many sorghums originated from semi-arid tropics are sensitive to low temperatures. Detailed physiological studies help to understand the effect of chilling stress on root conductance, shoot growth, and seedling development. Franks et al. (2006) identified Chinese kaoliangs as cold tolerant landraces with improved seedling vigor and emergence under cold stress. Another germplasm from Ethiopian highlands found to retain the growth below base temperature of 10 °C, indicating adaptation to chilling conditions (Tirfessa et al. 2020). Simple sequence repeat markers associated with traits for early season chilling tolerance were identified by Burow et al. (2011). Hybrids developed from inbreds were extensively tested and selected for early-stage chilling tolerance (Chiluwal et al. 2018) with high germination and seedling vigor. Ostmeyer et al. (2020) reported, a promising tannin free hybrid (ARCH11192A/ARCH12012R) with early-stage chilling tolerance significantly enhanced the yields without affecting the grain quality during early-stage chilling stress. These tolerant hybrids found to take a longer duration for flowering and extended grain-filling period which enhances the grain yields without impacting quality.
Bekele et al. (2014) screened a sorghum RIL population by phenotyping to select the traits useful in breeding for chilling tolerance. They also identified potential QTL regions on chromosomes 1,2,3,4 and 6 responsible for cold tolerance, which can be further used for fine mapping and candidate gene identification for early-stage chilling tolerance. Interestingly, QTLs identified in their study corresponds to the QTLs for stay-green which was identified earlier (Harris et al. 2007; Mace et al. 2012). Thus, these QTL hotspots facilitate the development of sorghum varieties with broad abiotic stress tolerance. Marla et al. (2019) studied sorghum NAM population developed from sensitive BTx 623 and three chilling tolerant Chinese lines (Niu Sheng Zui (NSZ; PI 568016), Hong Ke Zi (HKZ; PI 567946), and Kaoliang (Kao; PI 562744)). They found chilling tolerant QTLs were co-mapped with tannin and dwarfing genes. So, it is essential to carefully understand the genetic tradeoff to go for further genomic selection for chilling tolerance as it can negatively affect the grain quality.
Drought resistance
As sorghum is majorly grown in arid and semi-arid environments, breeding for drought-resilient cultivars requires an understanding of environmental control over crop growth (Bidinger et al. 1996). The development of drought-tolerant cultivars with the help of genetic improvement not only stabilizes productivity but also provide sustainable production systems. Screening and selection under optimal as well as stress conditions is necessary to select for yield stability, drought tolerance, and expression of drought-tolerant related traits (Richards 1996; Tuinstra et al. 1997). Tuinstra et al. (1997) identified 13 genomic regions related to the post-anthesis drought tolerance in sorghum. Out of them, four QTLs were identified for yield and yield stability, seven for grain weight and development and two for stay-green trait. In the past decade, several post-flowering drought-resistance cultivars were developed in sorghum. Drought-resistant cultivars showed high chlorophyll and photosynthetic efficiency and also stay-green phenotype. These stay-green phenotypes found to improve the grain yields significantly under drought conditions. BTx 642 has been a primary source to stay-green. This stay-green source was widely tested and also used to develop new hybrids with drought resistance (Borrell et al. 2000; Henzell et al. 2010; Jordan et al. 2010). Kassahun et al. (2010) identified similar stay-green loci an important for early-stage drought. Sta-green phenotype found to reduce water uptake and vegetative biomass during pre-flowering growth stages and uses the soil moisture during grain filling for yield benefits (Borrell et al. 2014). Reddy et al. (2007) showed genotypes with stay-green trait also exhibit resistance to lodging and charcoal rot. In addition to that, several QTLs for nodal root angle, root volume, dry weight, fresh weight were identified in past few years (Mace et al. 2012; Rajkumar et al. 2013). Mace et al. (2012) found root angle QTL was co-located with stay-green QTL and linked with grain yield. Jiang et al. (2013) reported reverse genetic approaches such as RNAi / type II CRISPR/CAS systems help to characterize the individual gene functions when expressed under stress conditions. In addition to that, miRNA expression studies (Ram and Sharma 2013) in sorghum reported mi169 is an excellent source to improve drought tolerance in sweet sorghum through genetic engineering.
Waterlogging
Enhancement of hypoxia tolerance is a convincing route to mitigate the waterlogging stress in crop plants. Understanding of molecular and physiological basis for this tolerance plays a key role to breed for waterlogging tolerance by expression of fermentation pathway genes (Dennis et al. 2000). Literature available on this is still inadequate for sorghum. Formation of root aerenchyma (Promkhambut et al. 2011), nodal root development (Pardales et al. 1991), maintaining high leaf air temperature difference (Zhang et al. 2019a), maintaining high antioxidant activity (Zhang et al. 2019b) are some of the waterlogging tolerance mechanisms observed in sorghum. Breeding for the varieties which express these tolerance mechanisms is prerequisite to improve the cultivation of sorghum under waterlogging conditions. Kadam et al. (2017) worked on the transcriptional profiling of aquaporin genes expressed under waterlogging conditions. They noticed tissue-specific, differential expression of AQP genes (PIP2-6, PIP2-7, TIP2-2, TIP4-4, and TIP5-1) under waterlogging conditions. The genetic variation observed in these AQP genes may play important role in breeding for waterlogging stress tolerance.
Improvement of grain quality
Stay-green phenotype was found to maintain higher stem carbohydrates, in addition to higher photosynthetic efficiency, grain yields, and resistance for lodging under drought conditions (Borrell et al. 2000; Burgess et al. 2002; Jordan et al. 2012). Also, leaves of stay-green phenotype found to have high N and nutritional quality (Borrell et al. 2000; Jordan et al. 2012) and predicted to contain high sugar concentrations in leaves and stem. Thus, breeding for stay-green trait also help to improve the nutritional quality of sweet sorghum cultivars along with drought resistance. Moreover, Blümmel et al. (2015) found stay-green sorghum contains higher In vitro organic matter digestibility (IVOMD) and use as animal feed. In addition to that, unpublished results from ICRISAT also showed stay-green trait could also enhance grain major nutritional components (protein, fat, and starch), but this capacity is highly dependent on genotype and genetic background.
Apart from stay-green, many studies have been attempted to improve the grain quality by developing new cultivars (Miller et al. 1996; Rooney et al. 2013) or identifying mutants with unique grain composition (Pedersen et al. 2005; Tesso et al. 2006). One such most popular mutant line is P721Q with high protein digestibility and high lysine content but with agronomically undesirable floury endosperm texture. However, Tesso et al. (2006) reported the possibility of developing sorghum with near-normal endosperm along with high digestibility through traditional breeding approaches. But still, limited breeding efforts were carried out till now to develop agronomically adapted, high protein digestible sorghum cultivars (Duressa et al. 2018). In addition to mutant lines, large genetic variability for protein digestibility (Hicks et al. 2002; Wong et al. 2010; Elkonin et al. 2013) and protein content (Rhodes et al. 2017) were observed by many researchers. This genetic variability could be a potential source to breed cultivars for high protein digestibility and high protein content. Especially, Durra and Durra-bicolor races which were known to contain higher protein levels (Johnson et al. 1968; Rhodes et al. 2017) in their grains are potential breeding targets to develop cultivars with high protein content. Specifically, the durra race was found to have higher water extraction capacity under terminal water stress which gives yield advantage (Vadez et al. 2011). Therefore, this race is possibly useful to maintain yield and quality under a drought stress scenario.
A few researchers (da Silva 2012; Elkonin et al. 2016) reported work on transgenic sorghum where they used RNA silencing technology to silence α and γ kaffirins synthesis to improve protein digestibility along with desirable vitreous endosperm). However, gene silencing was found to be sensitive to environmental conditions (Tuttle et al. 2008; Von Born et al. 2018), making it unsuitable for a wide range of environments Another efficient transformation system was developed for sorghum by Liu et al. (2014) that allowed the development of transgenic sorghum for improved starch content. Particularly, overexpression of two potential target genes, sucrose synthase (Su Sy) and SWEET sugar transporters from maize and Rice (Eom et al. 2015) leads to improved starch content in the grain. Thus, upregulation of these homologous in sorghum may not only improve the starch content but also improves kernel size (Mudge et al. 2016). Nevertheless, these transgenic sorghum lines can serve as donors to transfer these traits into locally adapted cultivars through breeding. Same way, Gilding et al. (2013) identified a mutant allele in the starch metabolic gene, pullulanase with increased digestibility and without any yield tradeoff. Introgressions of this mutant allele into elite sorghum lines through breeding may increase the yields as well as nutritional quality in adapted environments.
Exploitation of genetic variability in sorghum wild relatives is also an important strategy to improve the crop nutritional quality. Abdelhalim et al. (2019) found genetic variability and elevated microelements, protein content, digestibility and lower tannins in their study using Sudanese wild sorghum genotypes. Similar results were also shown by Peleg et al. (2008) in wheat wild genotypes. Recently, Cowan et al. (2019) demonstrated several sorghum wild relatives were less affected by severe drought compare to cultivated sorghums. Thus, introgressions of useful genes from wild sorghum to cultivated sorghum may improve the nutritional quality without compensating agronomical adaptability. Moreover, a wide genetic variability and a few GxE interaction for grain chemical attributes allow the ability to select desirable traits for particular environment (Kaufman et al. 2018). However, grain physical traits such as kernel weight, hardness, and diameter were found to be more prone to GxE interactions (Kaufman et al. 2018) which need to be taken into account while improving the quality under stress conditions.
GWAS is another promising tool to dissect genomic regions for several qualitative traits. In sorghum, GWAS has been used to identify several QTLs for protein, fat (Rhodes et al. 2017), starch (Boyles et al. 2019), minerals (Shakoor et al. 2015) and polyphenols (Rhodes et al. 2014). Recently, Kimani et al. (2020) used GWAS to identify 14 loci for starch content, 492 loci for 17 amino acids and 8 candidate genes for BCAA (branched chain amino acid) biosynthetic pathway. Other than macronutrients, sorghum polyphenols are of major interest recently owing to their importance as healthy antioxidants. A few recent studies on GWAS for polyphenols reported several small and major effect markers as well as many QTLs for variation in tannin levels (Rhodes et al. 2017; Habyarimana et al. 2019). Two novel functional markers for antioxidant activity, identified on chromosome 9 and 10 could be directly used in breeding programs to improve the antioxidant levels in sorghum (Habyarimana et al. 2019). Identified candidate genes could be exploited in molecular breeding programs to improve the grain quality. However, while employing these tools to improve the grain quality under stress scenarios, it is necessary to study the stability of end-use quality traits across different environments.
Conclusion
Climate change is a serious threat to the world agriculture and food production. Climate change affects agroecological systems and crops are more prone to abiotic stresses which results in substantial yield losses. Especially food crops in the arid and semi-arid regions of the world suffer from climate changes tremendously, affecting millions of people in those regions to suffer from malnutrition and hunger. In this scenario, it is essential to focus on climate-resilient crops to minimize the negative effects of climate change. Sorghum is one such climate-resilient crop which is naturally tolerant to abiotic stresses and can grow on marginal lands with minimum input. Also, it is one of the staple foods for people in the arid and semi-arid regions of the world where food security is at greater risk due to climate variability. It is also used as feed, fodder, bioenergy feedstock, and also gained recent popularity as healthy alternative food grain due to its nutritional quality and health benefits. Understanding sorghum sensitivity to different abiotic stresses allows breeding for improved cultivars for climate change vulnerabilities through conventional and molecular approaches. Advanced NGS technologies, high throughput GWAS and genetic engineering approaches identified several candidate genes/QTLs/alleles which would benefit the crop improvement programs under changing climate. So far, tremendous progress has been made to improve sorghum crop yields under stress scenarios. Although several efforts were made to improve the grain quality at the genetic level, maintaining and improving the quality under different stress scenarios is still challenging as grain physical and chemical attributes are prone to environmental changes and to some extent GxE interactions. Thus, further research efforts are still required to enhance the nutritional quality under changing climate. Moreover, in context to climate change, it is also essential for the research community as well as growers to diversify the strategies based on the local environments.
References
Abdelhalim TS, Kamal NM, Hassan AB (2019) Nutritional potential of wild sorghum: grain quality of Sudanese wild sorghum genotypes (Sorghum bicolor L. Moench). Food Sci Nutr 7(4):1529–1539
Aboubacar A, Moldenhauer KAK, McClung AM, Beighley DH, Hamaker BR (2006) Effect of growth location in the United States on amylose content, amylopectin fine structure, and thermal properties of starches of long grain rice cultivars. Cereal Chem 83:93–98
Adhikari U, Nejadhashemi AP, Woznicki SA (2015) Climate change and eastern Africa: a review of impact on major crops. Food Energy Secur 4:110–132
Ahmad Z, Anjum S, Waraich EA, Ayub MA, Ahmad T, Tariq RMS, Ahmad R, Iqbal MA (2018) Growth, physiology, and biochemical activities of plant responses with foliar potassium application under drought stress—a review. J Plant Nutr 41:1734–1743
Ahmadi A, Baker DA (2001) The effect of water stress on the activities of key regulatory enzymes of the sucrose to starch pathway in wheat. Plant Growth Regul 35(1):81–91
Ahmed S, Nawata E, Sakuratani T (2002) Effects of waterlogging at vegetative and reproductive growth stages on photosynthesis, leaf water potential and yield in mungbean. Plant Prod Sci 5:117–123
Ali Q, Ashraf M, Anwar F (2010) Seed composition and seed oil antioxidant activity of maize under water stress. J Am Oil Chem Soc 87(10):1179–1187
Allen DJ, Ort DR (2001) Impacts of chilling temperatures on photosynthesis in warm climate plants. Trends Plant Sci 6:36–42
Ananda N, Vadlani PV, Prasad PV (2011) Evaluation of drought and heat stressed grain sorghum (Sorghum bicolor) for ethanol production. Ind Crops Prod 33(3):779–782
Arora NK (2019) Impact of climate change on agriculture production and its sustainable solutions. Environ Sustain 2:95–96
Asaoka M, Okuno K, Sugimoto Y, Kawakami J, Fuwa H (1984) Effect of environmental temperature during development of rice plants on some properties of endosperm starch. Starch/Stärke 36:189–193
Assefa Y, Staggenborg SA, Prasad VP (2010) Grain sorghum water requirement and responses to drought stress: a review. Crop Manage 9(1):1–11
Baker JT, Boote KJ, Allen LH Jr (1995) Potential climate change effects on rice: carbon dioxide and temperature. In: Rosenzweig C (ed) Climate change and agriculture: analysis of potential international impacts, ASA Spec. Publ. No. 59. ASA, Madison, WI, pp 31–47
Bekele WA, Fiedler K, Shiringani A, Schnaubelt D, Windpassinger S, Uptmoor R, Friedt W, Snowdon RJ (2014) Unravelling the genetic complexity of sorghum seedling development under low-temperature conditions. Plant, Cell Environ 37(3):707–723
Bidinger FR, Hammer GL, Muchow RC (1996) The physiological basis of genotype by environment interaction in crop adaptation. In: Cooper M, Hammer GL (eds) Plant adaptation and crop improvement. CAB International, Wallingford, UK, pp 329–347
Bing YI, Zhou YF, Gao MY, Zhang Z, Yi HAN, Yang GD, Wenjuan XU, Huang RD (2014) Effect of drought stress during flowering stage on starch accumulation and starch synthesis enzymes in sorghum grains. J Integr Agric 13(11):2399–2406
Blum A (2004) Sorghum physiology. In: Nguyen HT, Blum A (eds) Physiology and biotechnology integration for plant breeding. Marcel Dekker Inc., NY, pp 141–223
Blümmel M, Deshpande S, Kholova J, Vadez V (2015) Introgression of staygreen QLT’s for concomitant improvement of food and fodder traits in Sorghum bicolor. Field Crops Res 180:228–237
Boote KJ, Allen LH Jr, Prasad PVV, Baker JT, Gesch RW, Synder AM, Pan D, Thomas JMG (2005) Elevated temperature and CO2 impacts on pollinations, reproductive growth, and yield of several globally important crops. J Agric Meteorol (Tokyo) 60:469–474
Borrell AK, Hammer GL, Henzell RG (2000) Does maintaining green leaf area in sorghum improve yield under drought? II. Dry matter production and yield. Crop Sci 40:1037–1048
Borrell AK, Mullet JE, George-Jaeggli B, van Oosterom EJ, Hammer GL, Klein PE, Jordan DR (2014) Drought adaptation of stay-green sorghum is associated with canopy development, leaf anatomy, root growth, and water uptake. J Exp Bot 65(21):6251–6263
Boyles RE, Brenton Z, Kresovich S (2019) Genetic and genomic resources of sorghum to connect genotype with phenotype in contrasting environments. Plant J 97(1):19–39
Brumm TJ, Hurburgh CR (2006) Changes in long-term soybean compositional patterns. J Am Oil Chem Soc 83:981–983
Burgess MS, Mehuys GR, Madramootoo CA (2002) Nitrogen dynamics of decomposing corn residue components under three tillage systems. Soil Sci Soc Am J 66(4):1350–1358
Burow G, Burke JJ, Xin Z, Franks CD (2011) Genetic dissection of early-season cold tolerance in sorghum (Sorghum bicolor (L.) Moench). Mol Breed 28:391–402
Challinor A, Wheeler T, Craufurd P, Ferro C, Stephenson D (2007) Adaptation of crops to climate change through genotypic responses to mean and extreme temperatures. Agric Ecosyst Environ 119:190–204
Chaudhuri UN, Burnett RB, Kirkham MB, Kanemasu ET (1986) Effect of carbon dioxide on sorghum yield, root growth, and water use. Agric For Meteorol 37:109–122
Chen J, Chopra R, Hayes C, Morris G, Marla S, Burke J, Xin Z, Burow G (2017) Genome-wide association study of developing leaves’ heat tolerance during vegetative growth stages in a Sorghum Association Panel. Plant Genome 10(2):1–15
Cheng W, Zhang G, Zhao G, Yao H, Xu H (2003) Variation in rice quality of different cultivars and grain positions as affected by water management. Field Crops Res 80:245–252
Chiluwal A (2018) Physiological and genetic characterization of sorghum exposed to early season chilling and terminal heat and drought stress. Doctoral dissertation. Kansas State University
Chiluwal A, Bheemanahalli R, Perumal R, Asebedo AR, Bashi E, Lamsal A, Sebela D, Shetty NJ, Jagadish SVK (2018) Integrated aerial and destructive phenotyping differentiates chilling stress tolerance during early seedling growth in sorghum. Field Crops Res 227:1–10
Cisse N, Ejeta G (2003) Genetic variation and relationships among seedling vigor traits in sorghum. Crop Sci 43:824–828
Cowan MF, Blomstedt CK, Norton SL, Henry RJ, Møller BL, Gleadow R (2020) Crop wild relatives as a genetic resource for generating low-cyanide, drought-tolerant Sorghum. Environ Exp Bot 169:103884
Crafts-Brandner SJ, Salvucci ME (2002) Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiol 129(4):1773–1780
Craufurd PQ, Qi A, Ellis RH, Summerfield RJ, Roberts EH, Mahalakshmi V (1998) Effect of temperature on time to panicle initiation and leaf appearance in sorghum. Crop Sci 38:942–947
Da Silva LS (2012) Transgenic sorghum: effects of altered kafirin synthesis on kafirin polymerisation, protein quality, protein body structure and endosperm texture, Doctoral dissertation, University of Pretoria
Dai Z, Yin Y, Wang Z (2009) Starch granule size distribution from seven wheat cultivars under different water regimes. Cereal Chem 86:82–87
Dang JMC, Copeland L (2004) Genotype and environmental influences on pasting properties of rice flour. Cereal Chem 81:486–489
De Souza AP, Cocuron JC, Garcia AC, Alonso AP, Buckeridge MS (2015) Changes in whole-plant metabolism during the grain-filling stage in sorghum grown under elevated CO2 and drought. Plant Physiol 169(3):1755–1765
Dennis ES, Dolferus R, Ellis M, Rahman M, Wu Y, Hoeren FU, Grover A, Ismond KP, Good AG, Peacock WJ (2000) Molecular strategies for improving waterlogging tolerance in plants. J Exp Bot 51(342):89–97
Dilley M, Chen RS, Deichmann U, Lerner-Lam AL, Arnold M (2005) Natural disaster hotspots: a global risk analysis. World Bank, Washington
Djanaguiraman M, Prasad PVV, Murugan M, Perumal M, Reddy UK (2014) Physiological differences among sorghum (Sorghum bicolor L. Moench) genotypes under high temperature stress. Environ Exp Bot 100:43–54
Djanaguiraman M, Perumal R, Jagadish SVK, Ciampitti IA, Welti R, Prasad PVV (2018) Sensitivity of sorghum pollen and pistil to high-temperature stress. Plant Cell Environ 41:1065–1082
Downes RW (1972) Effect of temperature on the phenology and grain yield of Sorghum bicolor. Aust J Agric Res 23:585–594
Duressa D, Weerasoriya D, Bean SR, Tilley M, Tesso T (2018) Genetic basis of protein digestibility in grain sorghum. Crop Sci 58(6):2183–2199
Ebadi MR, Pourreza J, Jamalian J, Edriss MA, Samie AH, Mirhadi SA (2005) Amino acid content and availability in low, medium and high tannin sorghum grain for poultry. Int J Poult Sci 4(1):27–31
Eck HV, Musick JC (1979) Plant water stress effect on irrigated sorghum. I. Effect on yield. Crop Sci 19:586–592
Elkonin LA, Italianskaya JV, Fadeeva IY, Bychkova VV, Kozhemyakin VV (2013) In vitro protein digestibility in grain sorghum: effect of genotype and interaction with starch digestibility. Euphytica 193(3):327–337
Elkonin LA, Italianskaya JV, Domanina IV, Selivanov NY, Rakitin AL, Ravin NV (2016) Transgenic sorghum with improved digestibility of storage proteins obtained by agrobacterium-mediated transformation. Russ J Plant Physiol 63(5):678–689
Eom JS, Chen LQ, Sosso D, Julius BT, Lin IW, Qu XQ, Braun DM, Frommer WB (2015) SWEETs, transporters for intracellular and intercellular sugar translocation. Curr Opin Plant Biol 25:53–62
Ercoli L, Mariotti M, Masoni A, Arduini I (2004) Growth responses of sorghum plants to chilling temperature and duration of exposure. Eur J Agron 2:93–103
Fabian A, Jager K, Rakszegi M, Barnabas B (2011) Embryo and endosperm development in wheat (Triticum aestivum L.) kernels subjected to drought stress. Plant Cell Rep 30:551–563
FAO (2002) Crop water management. Online. AGLW Water Management Group, United Nations FAO, Rome, Italy
FAO (2005) Special event on impact of climate change, pests and diseases on food security and poverty reduction. Background document. In: 31st session of the Committee on World Food Security FAO, Rome, Italy. ftp://ftp.fao.org/docrep/fao/meeting/009/j5411e.pdf
FAO (2008) Press release: Agriculture in the near east likely to suffer from climate change. Rome/Cairo, 3 March 2008. http://www.fao.org/newsroom/en/news/2008/1000800/index.html
Fatima Z, Ahmed M, Hussain M, Abbas G, Ul-Allah S, Ahmad S, Ahmed N, Ali MA, Sarwar G, ul Haque E, Iqbal P (2020) The fingerprints of climate warming on cereal crops phenology and adaptation options. Sci Rep 10(1):1–21
Fergason VL, Zuber MS (1962) Influence of environment on amylose content of maize endosperm. Crop Sci 2(3):209–211
Fernandez MGS, Schoenbaum GR, Goggi AS (2015) Novel germplasm and screening methods for early cold tolerance in sorghum. Crop Sci 54:2631–2638
Fischer G, Shah M, Tubiello FN, van Velthuizen H (2005) Socio-economic and climate change impacts on agriculture: an integrated assessment of agriculture, 1990–2080. Phil Trans R Soc B 360:2067–2083
Forbes GA, Ziv O, Frederiksen RA (1987) Resistance in sorghum to seedling disease caused by Pythium arrhenomanes. Plant Dis 71:145–148
Fracheboud Y, Haldimann P, Leipner J, Stamp P (1999) Chlorophyll fluorescence as a selection tool for cold tolerance of photosynthesis in maize (Zea mays L.). J Exp Bot 50:1533–1540
Franks CD, Burow GB, Burke JJ (2006) A comparison of US and Chinese sorghum germplasm for early season cold tolerance. Crop Sci 46:1371–1376
Garrity D, O’Toole J (1994) Screening rice for drought resistance at the reproductive phase. Field Crops Res 39:99–110
Gibson LR, Mullen RE (1996) Soybean seed quality reductions by high day and night temperature. Crop Sci 36:1615–1619
Gilding E, Frere CH, Cruickshank A, Rada AK, Prentis PJ, Mudge AM, Mace ES, Jordan DR, Godwin ID (2013) Allelic variation at a single gene increases food value in a drought-tolerant staple cereal. Nat Commun 4(1):1–6
Godfray HCJ, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, Pretty J, Robinson S, Thomas SM, Toulmin C (2010) Food security: the challenge of feeding 9 billion people. Science 327(5967):812–818
Gunaratne A, Bentota A, Cai YZ, Collado L, Corke H (2011a) Functional, digestibility, and antioxidant properties of brown and polished rice flour from traditional and new-improved varieties grown in Sri Lanka. Starch/Stärke 63(8):485–492
Gunaratne A, Ratnayaka UK, Sirisena N, Ratnayaka J, Kong X, Arachchi LV, Corke H (2011b) Effect of soil moisture stress from flowering to grain maturity on functional properties of Sri Lankan rice flour. Starch/Stärke 63:283–290
Habyarimana E, Dall’Agata M, De Franceschi P, Baloch FS (2019) Genome-wide association mapping of total antioxidant capacity, phenols, tannins, and flavonoids in a panel of Sorghum bicolor and S. bicolor × S. halepense populations using multi-locus models. PLoS ONE 14(12):e0225979
Hammer GL, Broad IJ (2003) Genotype and environment effects on dynamics of harvest index during grain filling in sorghum. Agron J 95:199–206
Harris K, Subudhi PK, Borrell A, Jordan D, Rosenow D, Nguyen H, Klein P, Klein R, Mullet J (2007) Sorghum stay-green QTL individually reduce post-flowering drought-induced leaf senescence. J Exp Bot 58(2):327–338
Hatfield JL, Boote KJ, Kimball BA, Ziska LH, Izaurralde RC, Ort D, Thomson AM, Wolfe D (2011) Climate impacts on agriculture: implications for crop production. Agron J 103:351–370
Henzell RG, Hare BR, Jordan DR, Fletcher DS, McCosker AN, Bunker G, Persley DS (2010) Sorghum breeding in Australia: public and private endeavours. In: Borrell AK, Henzell RG (eds) Proceedings of the 4th Australian Sorghum Conference. Kooralbyn, Australia
Hicks C, Tuinstra MR, Pedersen JF, Dowell FE, Kofoid KD (2002) Genetic analysis of feed quality and seed weight of sorghum inbred lines and hybrids using analytical methods and NIRS. Euphytica 127(1):31–40
Hurburgh CR, Brumm TJ, Guinn JM, Hartwig RA (1990) Protein and oil patterns in U.S. and world soybean markets. J Am Oil Chem Soc 67:966–973
Hurkman WJ, McCue KF, Altenbach SB, Korn A, Tanaka CK, Kothari KM, Johnson EL, Bechtel DB, Wilson JD, Anderson OD, DuPont FM (2003) Effect of temperature on expression of genes encoding enzymes for starch biosynthesis in developing wheat endosperm. Plant Sci 164:873–881
Impa SM, Perumal R, Bean SR, Sunoj VJ, Jagadish SK (2019) Water deficit and heat stress induced alterations in grain physico-chemical characteristics and micronutrient composition in field grown grain sorghum. J Cereal Sci 86:124–131
International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), Food and Agriculture Organization (FAO) of the United Nations (1996) The World Sorghum and Millet Economies: Facts, Trends and Outlook; Food and Agriculture Organization: Rome, Italy. Available online: http://oar.icrisat.org/1024/
Inuyama S, Musick JT, Dusek DA (1976) Effect of plant water deficit at various growth stages on growth, grain yield and leaf water potential of irrigated grain sorghum. Proc Crop Sci Soc Jpn 45:298–307
IPCC-Intergovernmental Panel on Climate Change (2007) Climate change 2007: synthesis report. https://www.ipcc.ch/report/ar4/syr/ Accessed on January 19, 2021
Jain M, Prasad PVV, Boote KJ, Allen LH Jr, Chourey PS (2007) Effect of season-long high temperature growth conditions on sugar-to-starch metabolism in developing microspores of grain sorghum (Sorghum bicolor L. Moench). Planta 227:67–79
Jiang W, Zhou H, Bi H, Fromm M, Yang B, Weeks DP (2013) Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in arabidopsis, tobacco, sorghum and rice. Nucleic Acids Res 41(20):e188. https://doi.org/10.1093/nar/gkt780
Johnson VA, Schmidt JW, Mattern PJ (1968) Cereal breeding for better protein impact. Econ Bot 22:16–25
Johnson WB, Ratnayake WS, Jackson DS, Lee KM, Herrman TJ, Bean SR, Mason SC (2010) Factors affecting the alkaline cooking performance of selected corn and sorghum hybrids. Cereal Chem 87(6):524–531
Johnson SM, Lim FL, Finkler A, Fromm H, Slabas AR, Knight MR (2014) Transcriptomic analysis of Sorghum bicolor responding to combined heat and drought stress. BMC Genomics 15(1):456
Jordan DR, Mace ES, Henzell RG, Klein PE, Klein RR (2010) Molecular mapping and candidate gene identification of the Rf2 gene for pollen fertility restoration in sorghum [Sorghum bicolor (L.) Moench]. Theor Appl Genet 120:1279–1287
Jordan DR, Hunt CH, Cruickshank AW, Borrell AK, Henzell RG (2012) The relationship between the stay-green trait and grain yield in elite sorghum hybrids grown in a range of environments. Crop Sci 52(3):1153–1161
Kadam S, Abril A, Dhanapal AP, Koester RP, Vermerris W, Jose S, Fritschi FB (2017) Characterization and regulation of aquaporin genes of sorghum [Sorghum bicolor (L.) Moench] in response to waterlogging stress. Front Plant Sci 8:862
Kapanigowda MH, Perumal R, Aiken RM, Herald TJ, Bean SR, Little CR (2013) Analyses of sorghum (Sorghum bicolor (L.) Moench) lines and hybrids in response to early-season planting and cool conditions. Can J Plant Sci 93:773–784
Kassahun B, Bidinger FR, Hash CT, Kuruvinashetti MS (2010) Stay-green expression in early generation sorghum [Sorghum bicolor (L.) Moench] QTL introgression lines. Euphytica 172(3):351–362
Kaufman RC, Wilson JD, Bean SR, Galant AL, Perumal RR, Tesso T, Herald T, Shi YC (2018) Influence of genotype× location interaction on grain sorghum grain chemistry and digestibility. Agron J 110(5):1681–1688
Kebede H, Subudhi PK, Rosenow DT, Nguyen HT (2001) Quantitative trait loci influencing drought tolerance in grain sorghum (Sorghum bicolor L. Moench). Theor Appl Genet 103:266–276
Khizzah BW, Miller FR, Newton RJ (1993) Inheritance and heritability of heat tolerance in several sorghum cultivars during the reproductive phase. Afr Crop Sci J 1(2)
Kimani W, Zhang LM, Wu XY, Hao HQ, Jing HC (2020) Genome-wide association study reveals that different pathways contribute to grain quality variation in sorghum (Sorghum bicolor). BMC Genomics 21(1):112
Kiseleva VI, Tester RF, Wasserman LA, Krivandin AV, Popov AA, Yuryev VP (2003) Influence of growth temperature on the structure and thermodynamic parameters of barley starches. Carbohydr Polym 51:407–415
Knoll J, Ejeta G (2008) Marker-assisted selection for early-season cold tolerance in sorghum: QTL validation across populations and environments. Theor Appl Genet 116:541–553
Knoll J, Gunaratna N, Ejeta G (2008) QTL analysis of early-season cold tolerance in sorghum. Theor Appl Genet 116:577–587
Krupa KN, Dalawai N, Shashidhar HE, Harinikumar KM (2017) Mechanisms of drought tolerance in Sorghum: a review. Int J Pure Appl Biosci 5(4):221–237
Kuromori T, Seo M, Shinozaki K (2018) ABA transport and plant water stress responses. Trends Plant Sci 23(6):513–522
Labuschagne MT, Elago O, Koen E (2009) The influence of temperature extremes on some quality and starch characteristics in bread, biscuit and durum wheat. J Cereal Sci 49:184–189
Leakey AD (2009) Rising atmospheric carbon dioxide concentration and the future of C4 crops for food and fuel. Proc R Soc B Biol Sci 276(1666):2333–2343
Li EP, Hasjim J, Singh V, Tizzotti M, Godwin ID, Gilbert RG (2013a) Insights into sorghum starch biosynthesis from structure changes induced by different growth temperatures. Cereal Chem 90:223–230
Li YF, Wu Y, Hernandez-Espinosa N, Peña RJ (2013b) Heat and drought stress on durum wheat: Responses of genotypes, yield, and quality parameters. J Cereal Sci 57(3):398–404
Linkemer G, Board JE, Musgrave ME (1998) Waterlogging effects on growth and yield components in late- planted soybean. Crop Sci 38:1576–1584
Liu P, Guo W, Jiang Z, Pu H et al (2011) Effects of high temperature after anthesis on starch granules in grains of wheat (Triticum aestivum L.). J Agric Sci 149:159–169
Liu G, Campbell BC, Godwin ID (2014) Sorghum genetic transformation by particle bombardment. Cereal genomics. Humana Press, Totowa, NJ, pp 219–234
Lobell DB, Burke MB, Tebaldi C, Mastrandrea MD, Falcon WP, Naylor RL (2008) Prioritizing climate change adaptation needs for food security in 2030. Science 319(5863):607–610
Long SP, Ainsworth EA, Leakey ADB, Nosberger J, Ort DR (2006) Food for thought: lower-than-expected crop yield stimulation with rising CO2 concentrations. Science 312:1918–1921
Lopes MS, El-Basyoni I, Baenziger PS, Singh S, Royo C, Ozbek K, Aktas H, Ozer E, Ozdemir F, Manickavelu A (2015) Exploiting genetic diversity from landraces in wheat breeding for adaptation to climate change. J Exp Bot 66:3477–3486
Lu TJ, Jane JL, Keeling PL, Singletary GW (1996) Maize starch fine structures affected by ear developmental temperature. Carbohydr Res 282:157–170
Mace E, Singh V, Van Oosterom E, Hammer G, Hunt C, Jordan D (2012) QTL for nodal root angle in sorghum (Sorghum bicolor L. Moench). co-locate with QTL for traits associated with drought adaptation. Theor Appl Genet 124:97–109
Maiti RK (1996) Sorghum science. Science Publishers, Lebanon, NH
Majora DJ, Hammana WM, Rooda SB (1982) Effects of short-duration chilling temperature exposure on growth and development of sorghum. Field Crops Res 5:129–136
Manjarrez-Sandoval P, Gonzalez-Hernandez AV, Mendoza-Onofret EL, Engleman EM (1989) Drought stress effects on the grain-yield and panicle development of sorghum. Can J Plant Sci 69:631–641
Manolio TA (2010) Genomewide association studies and assessment of the risk of disease. N Engl J Med 363:166–176
Marla SR, Burow G, Chopra R, Hayes C, Olatoye MO, Felderhoff T, Hu Z, Raymundo R, Perumal R, Morris GP (2019) Genetic architecture of chilling tolerance in sorghum dissected with a nested association mapping population. G3 Genes Genomes Genet 9(12):4045–4057
Maulana F (2011) Analysis of cold tolerance in sorghum [Sorghum bicolor (L.) Moench]. Doctoral dissertation. Kansas State University
Maulana F, Tesso TT (2013) Cold temperature episode at seedling and flowering stages reduces growth and yield components in sorghum. Crop Sci 53:564–574
McDonald MP, Galwey NW, Colmer TD (2002) Similarity and diversity in adventitious root anatomy as related to root aeration among a range of wetland and dryland grass species. Plant Cell Environ 25:441–451
McWilliams D (2003) Drought strategies for corn and grain sorghum. Coop Ext. Circ. 580. New Mexico State Univ., Las Cruces, NM
Miller FR, Prihoda KL, Rooney LW, Rosenow DT, Waniska RD (1996) Registration of a food quality sorghum restorer parent, Tx2907. Crop Sci 36:479
Mishra AK, Rai R, Agrawal SB (2013) Differential response of dwarf and tall tropical wheat cultivars to elevated ozone with and without carbon dioxide enrichment: growth, yield and grain quality. Field Crops Res 145:21–32
Mitsui T, Shiraya T, Kaneko K, Wada K (2013) Proteomics of rice grain under high temperature stress. Front Plant Sci 4:36
Moritz JS, Parsons AS, Buchanan NP, Calvalcanti WB, Cramer KR, Beyer RS (2005) Effect of gelatinizing dietary starch through feed processing on zero- to three-week broiler performance and metabolism. J Appl Poult Res 14:47–54
Mousavi-Derazmahalleh M, Bayer PE, Hane JK, Babu V, Nguyen HT, Nelson MN, Erskine W, Varshney RK, Papa R, Edwards D (2018) Adapting legume crops to climate change using genomic approaches. Plant Cell Environ 42:6–19
Mudge SR, Campbell BC, Mustapha NB, Godwin ID (2016) Genomic approaches for improving grain quality of sorghum. In: Rakshit S, Wang Y-H (eds) The sorghum genome. Springer, Cham, pp 189–205
Myllarinen P, Schulman AH, Salovaara H, Poutanen K (1998) The effect of growth temperature on gelatinization properties of barley starch. Acta Agric Scand B Soil Plant Sci 48:85–90
Naeve SL, Huerd SC (2008) Year, region, and temperature effects on the quality of Minnesota’s soybean crop. Agron J 100:690–695
Nagaraju M, Sudhakar Reddy P, Anil Kumar S, Srivastava RK, Kavi Kishor PB, Rao DM (2015) Genome-wide scanning and characterization of Sorghum bicolor L. heat shock transcription factors. Curr Genomics 16(4):279–291
Nguyen CT, Singh V, van Oosterom EJ, Chapman SC, Jordan DR, Hammer GL (2013) Genetic variability in high temperature effects on seed-set in sorghum. Funct Plant Biol 40:439–448
Njuguna VW, Cheruiyot EK, Mwonga S, Rono JK (2018) Effect of genotype and environment on grain quality of sorghum (Sorghum bicolor L. Moench) lines evaluated in Kenya. Afr J Plant Sci 12(12):324–330
Orchard PW, Jessop RS (1984) The response of sorghum and sunflower to short-term waterlogging. I. Effects of stage of development and duration of waterlogging on growth and yield. Plant Soil 81:119–132
Orchard PW, Jessop RS (1985) The response of sorghum and sunflower to short-term waterlogging. II. Root growth effects. Plant Soil 88:421–430
Ostmeyer T, Bheemanahalli R, Srikanthan D, Bean S, Peiris KH, Madasamy P, Perumal R, Jagadish SK (2020) Quantifying the agronomic performance of new grain sorghum hybrids for enhanced early-stage chilling tolerance. Field Crops Res 258:107955
Ottman MJ, Kimball BA, Pinter PJ Jr, Wall GW, Vanderlip RL, Leavitt SW, LaMorte RL, Matthias AD, Brooks TJ (2001) Elevated CO2 effects on sorghum growth and yield at high and low soil water content. New Phytol 150:261–273
Pallas JE Jr (1965) Transpiration and stomatal opening with changes in carbon content of the air. Science 147:171–173
Pang J, Zhou M, Mendham N, Shabala S (2004) Growth and physiological responses of six barley genotypes to waterlogging and subsequent recovery. Aust J Agr Res 55:895–906
Pang B, Zhang K, Kisekka I, Bean S, Zhang M, Wang D (2018) Evaluating effects of deficit irrigation strategies on grain sorghum attributes and biofuel production. J Cereal Sci 79:13–20
Pardales JR Jr, Kono Y, Yamauchi A (1991) Response of the different root system components of sorghum to incidence of waterlogging. Environ Exp Bot 31:107–115
Pazdernik DL, Halgerson PSJ, JL, Orf JH, (1996) Effect of temperature and genotype on the crude glycinin fraction (11S) of soybean and its analysis by near-infrared reflectance spectroscopy (near-IRS). J Agric Food Chem 44:2278–2281
Peacock JM (1982) Response and tolerance of sorghum to temperature stress. Sorghum in the Eighties: Proceedings of the International Symposium on Sorghum. 2-7 Nov 81. International Crops Research lnstltute for the Semi-Arid Tropics, Patancheru, A.P. India
Pedersen JF, Bean SR, Graybosch RA, Park SH, Tilley M (2005) Characterization of waxy grain sorghum lines in relation to granule bound starch synthase. Euphytica 144:51–156
Peleg Z, Saranga Y, Yazici A, Fahima T, Ozturk L, Cakmak I (2008) Grain zinc, iron and protein concentrations and zinc-efficiency in wild emmer wheat under contrasting irrigation regimes. Plant Soil 306(1):57–67
Peng W, Berry EM, Ferranti P, Berry E, Anderson J (2019) The concept of food security. Encycl Food Secur Sustainability 2:1–7
Perry SW, Krieg DR, Hutmacher RB (1983) Photosynthetic rate control in cotton. Plant Physiol 73:662–665
Piikki K, De Temmerman L, Ojanpera K, Danielsson H, Pleijel H (2008) The grain quality of spring wheat (Triticum aestivum L.) in relation to elevated ozone uptake and carbon dioxide exposure. Eur J Agron 28:245–254
Polthanee A (1997) Indigenous farming practices and knowledge in Northeast Thailand. Khon Kaen Publishing, Khon Kaen
Prasad PVV, Craufurd PQ, Summerfield RJ, Wheeler TR (2000) Effects of short episodes of heat stress on flower production and fruit-set of groundnut (Arachis hypogaea L.). J Exp Bot 51:777–784
Prasad PVV, Boote KJ, Allen LH Jr, Thomas JMG (2003) Supra-optimal temperatures are detrimental to peanut (Arachis hypogaea L) reproductive processes and yield at ambient and elevated carbon dioxide. Glob Change Biol 9:1775–1787
Prasad PVV, Boote KJ, Allen LH Jr (2006) Adverse high temperature effects on pollen viability, seed-set, seed yield and harvest index of grain sorghum [Sorghum bicolor (L.) Moench] are more severe at elevated carbon dioxide due to higher tissue temperatures. Agric For Meteorol 139:237–251
Prasad PVV, Pisipati SR, Mutava RN, Tuinstra MR (2008) Sensitivity of grain sorghum to high temperature stress during reproductive development. Crop Sci 48:1911–1917
Prasad PVV, Djanaguiraman M, Perumal R, Ciampitti IA (2015) Impact of high temperature stress on floret fertility and individual grain weight of grain sorghum: sensitive stages and thresholds for temperature and duration. Front Plant Sci 6:820
Promkhambut A, Polthanee A, Akkasaeng C, Younger A (2011) Growth, yield and aerenchyma formation of sweet and multipurpose sorghum (Sorghum bicolor L. Moench) as affected by flooding at different growth stages. Aust J Crop Sci 5(8):954
Rajkumar BF, Kavil SP, Girma Y, Arun SS, Dadakhalandar D, Gurusiddesh BH, Patil AM, Thudi M, Bhairappanavar SB, Narayana YD, Krishnaraj PU, Khadi BM, Kamatar MY (2013) Molecular mapping of genomic regions harbouring QTLs for root and yield traits in sorghum (Sorghum bicolor L. Moench). Physiol Mol Biol Plants 19:409–419
Ram G, Sharma AD (2013) In silico analysis of putative miRNAs and their target genes in sorghum (Sorghum bicolor). Int J Bioinf Res Appl 9(4):349–364
Ray SS, Dadhwal VK, Navalgund RR (2002) Performance evaluation of an irrigation command area using remote sensing: a case study of Mahi command, Gujarat, India. Agric Water Manage 56(2):81–91
Raymundo R, Asseng S, Robertson R, Petsakos A, Hoogenboom G, Quiroz R, Hareau G, Wolf J (2018) Climate change impact on global potato production. Eur J Agron 100:87–98
Raza A, Razzaq A, Mehmood SS, Zou X, Zhang X, Lv Y, Xu J (2019) Impact of climate change on crops adaptation and strategies to tackle its outcome: a review. Plants 8(2):34
Reddy BVS, Sharma HC, Thakur RP, Ramesh S, Ashok Kumar A (2007) Characterization of ICRISAT bred sorghum hybrid parents (Set II). Int Sorghum Millets Newslett 48:1–123
Reynolds TW, Waddington SR, Anderson CL, Chew A, True Z, Cullen A (2015) Environmental impacts and constraints associated with the production of major food crops in Sub-Saharan Africa and South Asia. Food Secur 7:795–822
Rhodes DH, Hoffmann L Jr, Rooney WL, Ramu P, Morris GP, Kresovich S (2014) Genome-wide association study of grain polyphenol concentrations in global sorghum [Sorghum bicolor (L) Moench] germplasm. J Agric Food Chem 62(45):10916–10927
Rhodes DH, Hoffmann L, Rooney WL, Herald TJ, Bean S, Boyles R, Brenton ZW, Kresovich S (2017) Genetic architecture of kernel composition in global sorghum germplasm. BMC Genomics 18(1):1–8
Richards RA (1996) Defining selection criteria to improve yield under drought. Plant Growth Regul 20:157–166
Rooney WL (2004) Sorghum improvement-integrating traditional and new technology to produce improved genotypes. Adv Agron 83:37–109
Rooney WL, Rooney LW, Awika J, Dykes L (2013) Registration of Tx3362 Sorghum germplasm. J Plant Regist 7:104–107
Rosenow DT, Clark LE (1995) Drought and lodging resistance for a quality sorghum crop. In: Proceedings of the 5th annual corn and sorghum industry research conference (Chicago, IL, 6–7 December 1995). American Seed Trade Association, Chicago, IL, pp 82–97
Rosenzweig C, Iglesius A, Yang XB, Epstein PR, Chivian E (2001) Climate change and extreme weather events—implications for food production, plant diseases, and pests. Global Change Hum Health 2:90–104
Roy SJ, Tucker EJ, Tester M (2011) Genetic analysis of abiotic stress tolerance in crops. Curr Opin Plant Biol 14:232–239
Roy JP, Tschakert H, Waisman S, Abdul Halim P, Antwi-Agyei P, Dasgupta B, Hayward M, Kanninen D, Liverman C, Okereke PF, Pinho K, Riahi AG, Suarez Rodriguez (2018) Sustainable development, poverty eradication and reducing inequalities. In: Masson-Delmotte V, Zhai P, Pörtner H-O, Roberts D, Skea J, Shukla PR, Pirani A, Moufouma-Okia W, Péan C, Pidcock R, Connors S, Matthews JBR, Chen Y, Zhou X, Gomis MI, Lonnoy E, Maycock T, Tignor M, Waterfield T (eds) Global warming of 1.5°C. An IPCC special report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. IPCC, Geneva
Saini HS (1997) Effects of water stress on male gametophyte development in plants. Sex Plant Reprod 10:67–73
Sarshad A, Talei D, Torabi M, Rafiei F, Nejatkhah P (2021) Morphological and biochemical responses of Sorghum bicolor (L.) Moench under drought stress. SN Appl Sci 3(1):1–12
Setter T, Belford B (1990) Waterlogging: how it reduces plant growth and how plants can overcome its effects. J Dept Agric Western Austr 31(2):51–55
Shakoor N, Ziegler G, Dilkes BP, Brenton Z, Boyles R, Connolly EL, Kresovich S, Baxter I (2015) Integration of experiments across diverse environments identifies the genetic determinants of variation in Sorghum bicolor seed element composition. bioRxiv, p 019083
Siddique KHM, Loss SP, Regan KL, Jettner RL (1999) Adaptation and seed yield of cool season grain legumes in Mediterranean environments of south-western Australia. Aust J Agric Res 50:375–438
Singh S, Singh G, Singh P, Singh N (2008) Effect of water stress at different stages of grain development on the characteristics of starch and protein of different wheat varieties. Food Chem 108:130–139
Singh S, Gupta AK, Gupta SK, Kaur N (2010) Effect of sowing time on protein quality and starch pasting characteristics in wheat (Triticum aestivum L.) genotypes grown under irrigated and rain-fed conditions. Food Chem 122:559–565
Singh V, Nguyen CT, van Oosterom EJ, Chapman SC, Jordan DR, Hammer GL (2015) Sorghum genotypes differ in high temperature responses for seed set. Field Crops Res 171:32–40
Singh V, Nguyen CT, Yang Z, Chapman SC, van Oosterom EJ, Hammer GL (2016) Genotypic differences in effects of short episodes of high-temperature stress during reproductive development in sorghum. Crop Sci 56:1561–1572
Sonobe K, Hattori T, An P, Tsuji W, Eneji AE, Kobayashi S, Kawamura Y, Tanaka K, Inanaga S (2010) Effect of silicon application on sorghum root responses to water stress. J Plant Nutr 34(1):71–82
Srivastava A, Kumar SN, Aggarwal PK (2010) Assessment on vulnerability of sorghum to climate change in India. Agric Ecosyst Environ 138(3–4):160–169
Stone LR, Schlegel AJ (2006) Yield-water supply relationship of grain sorghum and winter wheat. Agron J 98:1359–1366
Stone PJ, Wilson DR, Reid JB, Gillespie RN (2001) Water deficit effects on sweet corn. I. Water use, radiation use efficiency, growth, and yield. Austr J Agric Res 52(1):103–113
Sun MM, Abdula SE, Lee HJ, Cho YC, Han LZ, Koh HJ, Cho YG (2011) Molecular aspect of good eating quality formation in Japonica rice. PLoS ONE 6:e18385
Svihus B, Uhlen AK, Harstad OM (2005) Effect of starch granule structure, associated components and processing on nutritive value of cereal starch: a review. Anim Feed Sci Technol 122:303–320
Taleon V, Dykes L, Rooney WL, Rooney LW (2012) Effect of genotype and environment on flavonoid concentration and profile of black sorghum grains. J Cereal Sci 56(2):470–475
Taub DR, Miller B, Allen H (2008) Effects of elevated CO2 on the protein concentration of food crops: a meta-analysis. Global Change Biol 14(3):565–575
Terbea M, Vranceanu AV, Petcu E, Craiciu DS, Micut G (1995) Physiological response of sunflower plants to drought. Rom Agric Res 3:61–67
Tesso T, Ejeta G, Chandrashekar A, Huang CP, Tandjung A, Lewamy M, Axtell JD, Hamaker BR (2006) A novel modified endosperm texture in a mutant high-protein digestibility/high-lysine grain sorghum (Sorghum bicolor (L.) Moench). Cereal Chem J 83:194–201
Tester RF (1997) Influence of growth conditions on barley starch properties. Int J Biol Macromol 21(1–2):37–45
Tester RF, Karkalas J (2001) The effects of environmental conditions on the structural features and physico-chemical properties of starches. Starch/Stärke 53:513–519
Thitisaksakul M, Jimenez RC, Arias MC, Beckles DM (2012) Effects of environmental factors on cereal starch biosynthesis and composition. J Cereal Sci 56:67–80
Thomas H, Howarth CJ (2000) Five ways to stay green. J Exp Bot 51:329–337
Thomas JMG, Boote KJ, Allen LH Jr, Gallo-Meagher M, Davis JM (2003) Elevated temperature and carbon dioxide effects on soybean seed germination and transcript abundance. Crop Sci 43:1548–1557
Tirfessa A, McLean G, Mace E, van Oosterom E, Jordan D, Hammer G (2020) Differences in temperature response of phenological development among diverse Ethiopian sorghum genotypes are linked to racial grouping and agroecological adaptation. Crop Sci 60(2):977–990
Tiryaki I, Andrews DJ (2001) Germination and seedling cold tolerance in sorghum: II. Parental lines and hybrids. Agron J 93:1391–1397
Tolk JA, Howell TA (2001) Measured and simulated evapotranspiration of grain sorghum with full and limited irrigation in three high plains soils. Trans ASAE 44:1553–1558
Torbert HA, Prior SA, Rogers HH, Runion GB (2004) Elevated atmospheric CO2 effects on N fertilization in grain sorghum and soybean. Field Crops Res 88(1):7–67
Tuinstra MR, Grote EM, Goldsbrough PB, Ejeta G (1996) Identification of quantitative trait loci associated with pre-flowering drought tolerance in sorghum. Crop Sci 36:1337–1344
Tuinstra MR, Grote EM, Goldbrough PM, Ejeta G (1997) Genetic analysis of post-flowering drought tolerance and components of grain development in Sorghum bicolor L. Moench. Mol Breed 3:439–448
Tuttle JR, Idris AM, Brown JK, Haigler CH, Robertson D (2008) Geminivirus-mediated gene silencing from cotton leaf crumple virus is enhanced by low temperature in cotton. Plant Physiol 148(1):41–50
Umaharan P, Ariyanayagam RP, Haque SQ (1997) Effect of short-term waterlogging applied at various growth phases on growth, development and yield in Vigna unguiculata. J Agr Sci 128:189–198
UN Report (2019) World population prospects—2019 highlights. https://www.un.org/development/desa/publications/world-population-prospects-2019-highlights.html. Accessed on 23 January 2021
Vadez V, Krishnamurthy L, Hash CT, Upadhyaya HD, Borrell AK (2011) Yield, transpiration efficiency, and water-use variations and their interrelationships in the sorghum reference collection. Crop Pasture Sci 62(8):645–655
Van Bavel CHM (1974) Anti-transpirant action of carbon dioxide on intact sorghum plants. Crop Sci 14:208–212
Vinita J, Bhargava S, Streb P, Feierabend J (1998) Comparative effect of water, heat and light stresses on photosynthetic reactions in Sorghum bicolor (L.) Moench. J Exp Bot 49(327):1715–1721
Von Born P, Bernardo-Faura M, Rubio-Somoza I (2018) An artificial miRNA system reveals that relative contribution of translational inhibition to miRNA-mediated regulation depends on environmental and developmental factors in Arabidopsis thaliana. PLoS ONE 13(2):e0192984
Wong R, Mufloz OA, Mendoza LE (1983) Water stress effects on vegetative, reproductive and efficiency traits of sorghum varieties I in Spanish. Agrociencia 51:101–114
Wong JH, Marx DB, Wilson JD, Buchanan BB, Lemaux PG, Pedersen JF (2010) Principal component analysis and biochemical characterization of protein and starch reveal primary targets for improving sorghum grain. Plant Sci 179(6):598–611
Wu X, Zhao R, Bean SR, Seib PA, McLaren JS, Madl RL, Tuinstra M, Lenz MC, Wang D (2007) Factors impacting ethanol production from grain sorghum in the dry-grind process. Cereal Chem 84(2):130–136
Wu X, Zhao R, Liu L, Bean S, Seib PA, McLaren J, Madl R, Tuinstra M, Lenz M, Wang D (2008) Effects of growing location and irrigation on attributes and ethanol yields of selected grain sorghums. Cereal Chem 85(4):495–501
Wu G, Johnson SK, Bornman JF, Bennett SJ, Singh V, Simic A, Fang J (2016) Effects of genotype and growth temperature on the contents of tannin, phytate and in vitro iron availability of sorghum grains. PLoS ONE 11:1–12
Xin Z, Aiken R, Burke J (2008) Genetic diversity of transpiration efficiency in sorghum. Field Crops Res 111:74–80
Yu S, Tian L (2018) Breeding major cereal grains through the lens of nutrition sensitivity. Mol Plant 11:23–30
Yu J, Tuinstra MR (2001) Genetic analysis of seedling growth under cold temperature stress in grain sorghum. Crop Sci 41:1438–1443
Yu J, Tuinstra MR, Claassen MM, Gordon WB, Witt MD (2004) Analysis of cold tolerance in sorghum under controlled environment conditions. Field Crops Res 85:21–30
Zaidi PH, Rafique S, Rai PK, Singh NN, Srinivasan G (2004) Tolerance to excess moisture in maize (Zea mays L.): susceptible crop growth stage and identification of tolerant genotypes. Field Crops Res 90:189–202
Zeng M, Morris CF, Batey IL, Wrigley CW (1997) Sources of variation for starch gelatinization, pasting, and gelation properties in wheat. Cereal Chem 74:63–71
Zhan X, Wang D, Tuinstra MR, Bean S, Seib PA, Sun XS (2003) Ethanol and lactic acid production as affected by sorghum genotype and location. Ind Crops Prod 18(3):245–255
Zhang F, Wang Y, Yu H, Zhu K, Zhang Z, Zou FLJ (2016) Effect of excessive soil moisture stress on sweet sorghum: physiological changes and productivity. Pak J Bot 48(1):1–9
Zhang R, Zhou Y, Yue Z, Chen X, Cao X, Ai X, Jiang B, Xing Y (2019a) The leaf-air temperature difference reflects the variation in water status and photosynthesis of sorghum under waterlogged conditions. PLoS ONE 14(7):e0219209
Zhang RD, Zhou YF, Yue ZX, Chen XF, Cao X, Xu XX, Xing YF, Jiang B, Ai XY, Huang RD (2019b) Changes in photosynthesis, chloroplast ultrastructure, and antioxidant metabolism in leaves of sorghum under waterlogging stress. Photosynthetica 57(4):1076–1083
Zhuo W, Lin X (1995) Effects of waterlogging at different growth stages on physiological characteristic and seed yield of winter rape (Brassica napus L.). Field Crops Res 44:103–110
Author information
Authors and Affiliations
Contributions
Keerthi Chadalavada: conducted literature search and drafted the manuscript; Ranjitha Kumari B.D and Senthil Kumar T: conceptualization and draft correction.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chadalavada, K., Kumari, B.D.R. & Kumar, T.S. Sorghum mitigates climate variability and change on crop yield and quality. Planta 253, 113 (2021). https://doi.org/10.1007/s00425-021-03631-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-021-03631-2