Abstract
Main conclusion
A histological study of Rafflesia patma revealed the simplicity of a flower’s vascular tissue and epidermal features of flower organs, including their structures and pigmentation.
Abstract
Rafflesia is an endophytic holoparasitic plant that infects Tetrastigma. In a previous study, we characterized the shape of the strands of an endophyte (Rafflesia patma Blume) and hypothesized their distribution. In this study, we deepened our analysis by assessing parts of flower tissue sampled during anthesis, performed surface casting of the abaxial and adaxial sides of the perigone lobe to profile their surface features, and histologically characterized the perigone lobe, perigone tube, and central column base, including the anther and cupula region. The objective of these observations was to compare tissues from different organs and the distribution of cells staining positive for tannin, suberin, and lignin. Observable features in this study were vascular and epidermal tissue. We also observed reduced vascular tissue with xylem and vascular parenchyma in multiple organs. The adaxial epidermis found in the perigone lobes and tube had papillate cells, and their function might be to assist with the emission of odor through chemical evaporation. The abaxial epidermis, also found in perigone lobes and tube, had flattened cells. These, combined with the nearby flattened parenchyma cells, especially in the outermost, early perigone lobe, might provide a tougher (stiffer) outer protective barrier for the flower. The accumulation of tannin in perigone lobes might offer protection to the flower from herbivores prior to anthesis. Although a previous observation indicated the possibility of stomata on the surface of Rafflesia flowers, no stomata were found in this study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Members of the Rafflesia genus have the largest individual flowers in the plant kingdom, compared to the titan arum [Amorphophallus titanum (Becc.) Becc. ex Arcang], which has the largest unbranched inflorescence, and the talipot palm (Corypha umbraculifera L.), which has the largest branched inflorescence. Rafflesia has a monoecious and unisexual flower (Nais 2001; Wicaksono et al. 2016; Mursidawati and Irawati 2017). The young flower is covered by a dark bract, which is an extrafloral organ analogous to leaves. Additionally, it is suggested that the diaphragm derived from the petal whorl, while the perigone tube chamber underneath the diaphragm from sepal-petal base (Nikolov et al. 2013). Consequently, the petal-like organ is simply referred to as the perianth, but it is better known as the perigone. Another distinct feature of Rafflesia flowers is their putrid odor and their ability to produce heat to attract pollinators by confusing them, masquerading as decaying flesh. This function is aided by the ramentae, which are protuberances inside the perigonal tube, although the location of the secretory tissue that contributes to the putrid volatile odor is still unclear. Nikolov et al. (2014a) suggested that the ramentae themselves are a secretory tissue. Another possibility is that ramentae might mimic animal fur to attract pollinators (Beaman et al. 1988; Nikolov et al. 2014a). However, Rafflesia species do not have a nectar-secreting organ, making pollination deceptive, as in R. pricei, R. tengku-adlinii, and R. keithii (Nais 2001).
Despite knowledge of their anatomical features, Rafflesia flowers are still enshrouded in mystery. The origin of the flower appears to be an evolutionary adaptation to the current state of possessing a large flower with minimum vegetative endophytic organs, hypotheses that have been supported exclusively by morphological and histological analyses (Nikolov et al. 2014b; Mursidawati et al. 2019), although it is unclear if there are any structural modifications. The parasitic endophyte grows within the vascular cambium layer of its host plant, Tetrastigma (Mursidawati et al. 2019). It is also unclear if modifications to the Rafflesia perigone represent an ancestral leaf-like organ or an as yet unknown homologous organ. Molina et al. (2014) noted the absence of a plastid gene in Rafflesia, which might be a clue about its ancestral origin.
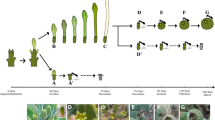
Anthesis in Rafflesia occurs several months after the knob emerges (Fig. 1). Upon blooming, the two uppermost perigone lobes with smooth abaxial surfaces, or early lobes, serve as the lid, which protects the remaining floral organs (Fig. 1c). As the perigone lobes open, the remaining lobes (or the late lobes), display a rough—and certainly never a smooth—abaxial surface (Fig. 1d, e). In R. patma Blume, there may be five or rarely six perigone lobes at anthesis, and all can open almost simultaneously or gradually, with the uppermost lobes opening first followed by the remaining innermost perigone lobes (Mursidawati and Irawati 2017).
Rafflesia patma anthesis (a–e). The knob was initially small (~ 2 cm in diameter) after emerging from the endophyte stage as a protocorm, at which stage the primordial flower was covered by bracts on the cupula (a). As the knob doubled in size (to ~ 4–5 cm in diameter) after about 2 months, the bracts appeared larger than the cupula (b). Within 3–4 weeks, the outer, early perigone lobes emerged from the bracts (diameter was fixed at ~ 20 cm prior to anthesis) (c). 12–48 h later, the outer perigone lobe started to open showing inner, late lobes with a rough abaxial surface (d). Finally, 4–6 h later, the perigone lobes were fully opened, revealing all flower structures; the flower diameter upon anthesis, including the perigone lobe, measured from right to left, was ~ 40 cm (e)
In this study, histological analyses were performed to closely observe the features of each accessory organ of fully blooming flowers throughout anthesis, focusing on the perigone lobes and the lower perigone tube. We used Rafflesia floral organ terminology established by Nais (2001) for general anatomy and by Susatya et al. (2017) for perigone tube anatomy, including the diaphragm window, which is a perigone tube that consists of an upper (distal), middle, and lower (proximal) perigone tube. These analyses provide more information about the morphology and histology of the R. patma flower that might reflect a response to the flower’s functionality (pigmentation, metabolite distribution, differences in epidermal tissues among organs, and organ-specific differences in the morphology of vascular tissue).
Materials and methods
Plant samples
An R. patma plant growing in Pangandaran on a Tetrastigma leucostaphyllum (Dennst.) Alston ex Mabb vine that had been grafted in 2006 into a locally grown vine in Bogor Botanical Garden, and which bloomed on September 5, 2018, was sampled and processed on September 6, 2018. Samples were from two 8-month-old flowers that had emerged on the T. leucostaphyllum vine surface. These samples were also used in previous studies (Wicaksono et al. 2017; Mursidawati et al. 2019). At the time of sampling, local temperature was 24.5 °C at 9 am with 84% relative humidity. Tissue samples were obtained from externally visible organs such as the flower petal-like structure or perigone lobes and internal organs. These included the lower (proximal area) floral perigone tube which houses tiny protuberances or ramentae-like structures, typical of R. patma, the flower anther region, the proximal part of the central column, and the cupula region (Suppl. Figure 1). After sampling, the dissected parts were stored in 70% ethanol in a jar for staining at a later stage. The same method was also performed for a second independent flower (for details of the second flower, see epidermal surface cast below).
Epidermal surface cast
A second R. patma male flower was sampled on September 20, 2018 (local temperature while sampling was 26 °C at 9 am with 79.5% relative humidity). A cast,Footnote 1 which was made with nail polish over an area of approximately 2 × 2 cm2, was left to dry for 10 min then peeled for microscopic observation. Casts for two stages of perigone lobes were prepared: (1) the early perigone lobe, which normally covers the outermost layer prior to blooming, and (2) the late perigone lobe, which opens at a later stage of anthesis (for illustration, see Suppl. Figure 2). Despite these differences, both types of perigone lobes have a rough adaxial surface. Any surface features, including the presence of stomata, trichomes, and cellular morphology on both adaxial and abaxial surfaces, were noted. The same procedure was performed for the flower bract.
Staining and object glass preparation
The floral organs of R. patma were cut into 2 × 2 cm2 segments (details in Suppl. Figure 1) then stained with fastgreen and safranin counterstain as performed previously in Mursidawati and Sunaryo (2012) and Mursidawati et al. (2019), which used a similar method as Ma et al. (1993). The microtome used was Yamato RV-240 (Yamato Kohki Industrial Co., Ltd., Saitama, Japan). Slices were approximately 10 μm thick. Samples were observed under a microscope (Olympus CX31 with built-in yellow lighting) at 40–1000 × magnification using camera software (FlashBus Spectrim FBG 1.2; Integral Technologies, Inc., Indianapolis, USA) to capture screen images.
Results
Microscopy of perigone lobes
The stained R. patma perigone lobes had near-even red coloration of both the early (Fig. 2a) and late (Fig. 2b) perigone lobes. Staining was purple in the central region of samples but bright red in the epidermal region. Safranin-fastgreen staining reveals, through pigmentation, more detail regarding the distribution of metabolites, namely the red color of tannin, suberin, and lignin indicated by safranin, while fastgreen stains cytoplasm green (Ma et al. 1993). Since the cell walls are thin, tannin rather than lignin might explain the red coloration in perigonal tissues. Additionally, lignin does not contribute to coloration, so the rusty color may be the outcome of the accumulation and oxidation of tannin. Both adaxial (Fig. 2e, g) and abaxial (Fig. 2f, h) epidermal tissues are rich in tannins, as shown by bright red coloration. This is in contrast to the purple coloration of the middle region (see Fig. 2a, b), which indicates less accumulation of tannin. This coloration was also visible in the freshly prepared sample of the late perigone lobe (see Suppl. Figure 3; under microscope in Suppl. Figure 4), where the adaxial epidermis was visibly pigmented.
Perigone lobes of R. patma in transverse sections stained with fastgreen-saffranin: early perigone lobe a with extruded-papillate adaxial surface e and smooth abaxial surface with flattened cells f, and late perigone lobe b with flattened-papillate adaxial surface g and rougher abaxial surface h. Vascular bundle was found in the middle of late perigone lobe b, and xylem is located in the middle of the vascular bundle (see longitudinal section c, enlarged in d with black arrow pointing at xylem vessel elements, also in a longitudinal section). Magnifications: 4 × 10 (a, b); 10 × 10 (c, e–h); 40 × 10 (d). Scale bars: 1 mm (a, b), 0.25 mm (c, e–h), and 0.625 mm (d). Lighting source: add-on custom white LED light
Structurally, the adaxial epidermal tissue in the early perigone lobe is extruded and papillate (Fig. 2a, magnified in 2e). In the late perigone lobe, the adaxial epidermal tissue is also papillate but the papillate surface is flatter (Fig. 2b, magnified in 2g). On the other hand, abaxial epidermal tissue in early and late perigone lobes are flattened (Fig. 2a, b, and magnified in 2f and 2h, respectively). However, the abaxial epidermal tissue of the early perigone lobe is flattened more evenly than that of the late perigone lobe (Fig. 2a, magnified in 2f), which has more bulges (Fig. 2b, magnified in 2h). The surface cast peels confirmed these topographic differences (Suppl. Figure 5). The abaxial late perigone lobe is rough with extrusions (Suppl. Figure 5a, magnified further in Suppl. Figure 5b–d) while the abaxial early perigone lobe is smooth and flat with shapes that resemble stomata (Suppl. Figure 5e). However, no actual stomata were observed. Based on texture, the abaxial surface of the late perigone lobe is leathery, compared to the waxy and smooth abaxial surface of the early perigone lobe. Both early and late perigone lobes have the same leathery adaxial surface with a papillate surface (Suppl. Figure 5f). The papillate cells may play roles in mimicry by mimicking animal flesh, or may produce a pungent odor to attract pollinators. During observations, a rotting smell was emitted from the perigone, but no proof of glandular trichomes or cells was observed on either epidermis.
A vascular bundle was found in the late perigone lobe (Fig. 2b, magnified longitudinal view in 2c). Oddly, the vascular bundles here only show a xylem vascular element in the middle (Fig. 2d, black arrow), but no signs of tracheids or phloem (which normally appears as an elongated vessel tube separated by a sieve—hence their name, sieve tube cells—with companion cells next to it; Mauseth 1988), and only parenchyma cells (with nuclei) are visible next to xylem. The absence of phloem may signify the holoparasitic nature of Rafflesia, as it does not produce its own nutrients. Thus, xylem works as a distributor of host-derived photosynthate throughout the floral organ. However, the vascular system still requires vascular parenchyma, or xylem parenchyma, due to its function of loading–unloading solutes into/from the transpiration system or leaf, nutrient storage (De Boer and Volkov 2003), additional support structure, preventing water stress, and repair of xylem air embolism (Secchi et al. 2017). In the case of R. patma, lateral water distribution should be equal to lateral nutrient distribution as well, as xylem carries host-borne nutrients.
The xylem trachea elements in higher plants are distinguishable by their thick and modified secondary wall structure, of which there are many types: annular (ring), helical, scalariform, reticulate, and circular bordered pits (Mauseth 1988). The perigone lobe of R. patma appears to have xylem trachea with an annular secondary wall (Fig. 2d, black arrows).
Microscopy of the perigone tube: diaphragm, tube-lobe intersection, and proximal area
Similar to the perigone lobe, the perigone tube has extruded, papillate adaxial epidermal tissue (Fig. 3a, c for perigone tube-lobe intersection; Fig. 3e, h for perigone tube diaphragm or lid; and Fig. 3i for perigone tube proximal region). R. patma has tuberculate-type ramentae located in the inner part of the perigone tube and inner surface of the diaphragm (Susatya et al. 2017; Suppl. Figure 6). Their shape resembles tiny warts (Suppl. Figure 6) rather than protruding filaments as in many Rafflesia species such as R. arnoldii (Susatya 2011; Wicaksono et al. 2016; Susatya et al. 2017). The perigone tube-lobe intersection area is located in the distal part of the perigone tube which also connects directly as well to the perigone tube window or diaphragm. Since the outer epidermis of the perigone tube connects to perigone lobes and has a similar waxy, smooth texture as the early perigone lobe, it also has a flat abaxial epidermis (Fig. 3d, m). The perigone tube diaphragm has a papillate adaxial epidermis similar to the adaxial epidermis of the perigone lobe (Fig. 3e, h). Similarly, the inner epidermis of the proximal perigone tube also has the same papillate epidermal morphology (Fig. 3i). In addition, the tuberculate ramentae have an epidermal thickening (Fig. 3j). It is possible that the papillate epidermis also secretes odor-producing volatiles, but these were not detected in this study. At the window opening (tip of the diaphragm), the epidermal area thickens (Fig. 3g). Unlike in the perigone lobe, tannin accumulation is lower in the perigone tube, shown by a thin red layer and a smaller safranin-stained layer seen in this area.
Transverse sections of various parts of R. patma stained with fastgreen-saffranin: first column a–d reveals the intersection part where the perigone tube and lobe are connected: a papillate adaxial epidermis surface exposed to the axis of the flower (adaxial tube surface), b vascular bundles near the intersection, c adaxial epidermis area exposed to the top, one side belonging to the perigone tube and another to the perigone lobe, and the abaxial epidermis surface (d). Second column e–h reveals the diaphragm part (window part) of the perigone tube: e papillate adaxial epidermis surface and flattened-papillate abaxial epidermis surface with a vascular bundle in the middle, f enlarged view of the vascular bundle with black arrows pointing to the xylem vessel elements, g distal part of the diaphragm, h and another part of diaphragm tissue with ramentae-like protrusions (blue arrow) in the adaxial surface and vascular bundle. Third column i–m reveals the lower perigone tube: i papillate adaxial epidermis surface, j rammentae-like protrusions (blue arrow) on the adaxial epidermis surface, k enlarged view of the vascular bundle with black arrows pointing to the xylem vessel elements, l further enlarged view of the vascular bundle reveals that the bundle is tangentially cut with black arrows pointing to the bent xylem vessel elements’ annular-shaped secondary wall, and m semi-flat abaxial surface (with ripped part caused by microtomy sectioning). Adx adaxial region, In adaxial region in contact with inner or central chamber space, Out abaxial region in contact with the outer space of the flower, Per perigone lobe, Tub perigone tube, VB vascular bundle. Magnifications: a–e, g–k, m 4 × 10, f 10 × 10, and l 40 × 10. Scale bars: a–e, g–k, m 1 mm, f 0.25 mm, and l 0.0625 mm. Lighting source: add-on custom white LED light
The vascular bundles of the perigone tube are similar to those of the perigone lobe, especially in the tube-lobe intersection area (Fig. 3b) where the xylem is at the core and is surrounded by parenchyma tissue. The same type of vascular bundle is also found in the perigone tube diaphragm (Fig. 3e, h). In the lower perigone tube, the structure of the vascular bundles is similar but the xylem is more widely spread (Fig. 3k shows a tangentially sectioned vascular bundle, exposing the structure of the xylem vessel element tubes, enlarged in Fig. 3l; black arrows) instead of bundled in the middle as in the diaphragm and perigone lobe.
Microscopy of the central column organs: anther chamber, column base, cupula, and bract
Since a male R. patma flower was sampled, it was possible to closely observe the anther region (Fig. 4a–e). R. patma pollen appears to develop in chambers (Fig. 4a–c) inside the dome-shaped anther. This terminology refers to that suggested by Ng (2019). Uninuclear pollen (Fig. 4e), which is the early stage of pollen development immediately after the microsporogenesis stage, was observed. Binuclear pollen, which has undergone nuclear mitosis and contains a generative nucleus and a vegetative nucleus (pollen tube nucleus) during the microgametogenesis stage, was also observed (Fig. 4d). In Fig. 4d, some generative nuclei may have undergone more mitosis (Fig. 4d; red arrow) to produce two generative nuclei. However, this is uncertain given the relatively low resolution of the microscope’s camera. Pollen develops from the microsporocyte into uninuclear tetrad cells via meiosis in microsporogenesis, then the uninuclear cell individually undergoes nuclear mitosis into a generative nucleus (it later undergoes mitosis into two nuclei) and a vegetative nucleus in microgametogenesis (Twell et al. 2006; Biancucci et al. 2015).
Other flower organs of R. patma stained with fastgreen-saffranin: First column a–e shows the anther region with pollen: a anther tip, b enlargement of a showing anther tip with pollen, c another part of enlarged anther tip with young pollen, d pollen of R. patma with uninuclear pollen and binuclear pollen consisting of vegetative nuclei and generative nuclei, and e pollen and microsporocyte cells, the precursors of pollen. Second column f–i shows part of the central column (central disc base) vascular bundle f and vascular bundles of the flower base, the cupula region which is close to the host tissue, g bent vascular bundle, giving it is the appearance of being flattened, h another vascular bundle, with xylem vessel element secondary wall enlarged in (i). Orientations of sectioning: a–e longitudinal, and f–i transverse. Bi binuclear pollen, GN generative nucleus, Mi microsporocyte, Po pollen cell, Un uninuclear pollen, VN vegetative nucleus (pollen tube nucleus). Black arrows: xylem, and red arrows: generative nucleus possibly undergoing mitosis (uncertain due to low resolution). Magnifications: a 4 × 10, b, c, f, h 10 × 10, i 40 × 10, and d, e 100 × 10—enhanced with immersion oil. Scale bars: a 1 mm, b, c, f, h 0.25 mm, and i, d, e 0.0625 mm. Lighting source: Add-on custom white LED light
At the base of the flower’s central column, the vascular bundles are similar to those in the perigone lobe and the xylem tube in the middle of the vascular bundle (Fig. 4f; xylem indicated by black arrows). Underneath, in the cupula region, the observed vascular bundles have a unique xylem formation. Inside each vascular bundle, xylem is concentrically arranged (Fig. 4g, h; enlarged in 4i; xylem indicated by black arrows). The vascular bundle in Fig. 4g appears to be flattened, probably caused by bending of the vasculature (see Suppl. Figure 7).
Some samples were brittle, so it was not possible to obtain stained and sectioned samples of the central disc processes and bract. Fortunately, some surface casts of the flower bract were made in passing. The tissue composition of the bract strongly resembles that of the perigone lobe with a papillate adaxial epidermis and a flat abaxial epidermis, and there is a vascular bundle but its vascular composition is unknown (Suppl. Figure 8). Future studies will need to sample and stain central disc processes and bracts as was performed in this study for other R. patma organs. No stomata were visible on the epidermal casts of both adaxial and abaxial epidermises of bracts (Suppl. Figure 9).
Discussion
Reduced vasculature composition
Rafflesia, which belongs to the Rafflesiaceae, is phylogenetically closely related to the Euphorbiaceae (Wurdack and Davis 2009). Rafflesia has heavily modified organs, namely the flower, and only bracts and perigonal structures can be observed, although these cannot be seen morphologically separated as sepals or petal (but derived from sepal and petal), while a central disc can also be observed in the middle of the flower (Nais 2001; Susatya 2011; Nikolov et al. 2013). In the Rafflesia vegetative state, there is no visible organ differentiation prior to flower emergence, only the association with an endophyte (Mursidawati and Sunaryo 2012), and no collenchyma and sclerenchyma tissue is observed (Nikolov et al. 2014b), although some structures found in this study are similar to those in other flowering plants.
The perigonal structures of R. patma appear to follow observations made by Endriss (1902 cit. Rutherford 1970) and Harms (1935 cit. Rutherford 1970), who stated that the R. patma perigone consists of an adaxial epidermis, undifferentiated mesophyll, and an abaxial epidermis. The vascular tissues found in many organs are mostly xylem surrounded by parenchyma tissue. This vascular system could be analogous to amphicribral vascular bundles found in angiosperm flowers such as Magnolia ovules (Liu et al. 2014) and Papaver somniferum Linn. capsules and receptacles (Kapoor 1973). As Rafflesia is holoparasitic, which implies that it has no photosynthetic organs, it is understandable that it does not require phloem for nutrient distribution. To substitute phloem, vascular parenchyma tissue is available to support xylem functions as well as to aid nutrient distribution to the cortex parenchyma tissues. There are known holoparasitic plants that still possess phloem in their vascular bundles, such as Cytinus (Cytinaceae), which has fully developed phloem on its endophytic vascular bundles which reside among the host xylem region of the stem (de Vega et al. 2007). Some plants in the Orobanchaeae like Orobanche, Epifagus, and Aeginetia have a parasitic-to-host phloem interaction (Walsh and Popovich 1977; Dörr et al. 1995; Rajanna et al. 2005). According to de Vega et al. (2007), in Cytinus, no parasitic xylem-host xylem and parasitic phloem-host phloem are detected, so it is possible that the haustorial/endophytic parenchyma tissue serves as a transfer vessel in which specific parenchyma cells mediate water transport between host-parasite xylem, and other specific parenchyma cells capture photoassimilates from host phloem, transferring them to parasitic phloem. In other words, if the parasitic phloem is connected to the host phloem, as in the Orobanchaceae, it is possible that the parasitic plant draws nutrients directly from the host phloem while water is transported via parasitic xylem to host xylem, i.e., there is a complete functional separation, as in normal non-parasitic higher plants.
The xylem of R. patma vascular bundles, which is surrounded by parenchyma, suggests a further reductionist, minimalistic organ of Rafflesia which was previously thought to occur exclusively in endophytes, i.e., the vegetative state of the plant (Nikolov et al. 2014b). Our study reveals the minimalistic features also seen in many parts of the generative state of the plant. Normally, in higher plants, the vascular tissue consists of xylem (vessel element, tracheid, and fibers), phloem (sieve tube and companion cell), and vascular parenchyma (for both xylem and phloem) (Mauseth 1988; Beck 2012). In R. patma, there is only a xylem vessel element, no phloem, and one type of vascular parenchyma. This vascular parenchyma might be actively involved in the distribution of water and nutrients throughout the flower.
According to Nikolov et al. (2014a), Rafflesia has a concentric vasculature in the middle part of the flower, from below the gonad to the intersection of both lobes and bracts, and forms a single line in bracts and perigone lobes, all of which were assumed from transverse sectioning (i.e., the flower was cut on a horizontal plane). However, based on longitudinal sectioning performed in this study, the vasculature appears to be bent into random directions, similar to Sapria himalayana Griff. although its relative, Rhizanthes lowii Beccari (Harms), appears to have a more orderly vasculature (Nikolov et al. 2014a). Nevertheless, the vasculature appears to have ramified from the cupula region to other flower organs. The vascular bundles of the cupula region have concentrically arranged xylem inside the vascular bundle with vascular parenchyma filling the rest of the bundle (Fig. 4g–i). In contrast, on other organs such as the perigone lobes and tube, xylem is packed in the middle and is surrounded by vascular parenchyma tissue (Figs. 2b–d, 3b, e–f, h, k, l, 4f). If the hypothesized ramified vasculature is true, it explains the cupula’s vascular bundles in which each xylem vessel element on the concentric ring in the cupula’s vascular bundles will branch to individually arranged vascular bundles in the other R. patma organs.
Pigmentation
Using saffranin and fastgreen staining, tissue containing tannin, suberin, and lignin will be colored red, while the remaining cytoplasm will stain green. In perigone lobes, nearly all parts are colored red but some small zones of tissue are less red but a bit purplish (Fig. 2a, b) where fewer metabolites (in this case, tannin) accumulated. In other organs, such as the perigone tube, central column, and cupula, only epidermis tissue stained red while the remaining tissue stained blue-green. Fastgreen only stains the cytoplasm. The accumulation of tannin in the perigone lobes could be used as protection against herbivory because they protect the other inner flower organs before anthesis.
Pigmentation seems to characterize only the epidermal region of any flower organ. In Dendrobium, the cells that accumulate anthocyanin pigments are distributed in both adaxial and abaxial epidermises (Mudalige et al. 2003). It is unclear how Rafflesia flowers develop pigmentation and how pigments are distributed because pigment biosynthetic pathways have not yet been studied. In R. hasseltii, phenolic compounds (leucoanthocyanin/tannin, catechin, and phenolic acid) were detected in the flower bract at an almost threefold higher concentration than in the root and stem bark of its host, T. leucostaphylum (Sofiyanti 2008). However, it is unclear if metabolic content is derived from self-biosynthesis by Rafflesia. The phenolic or flavonoid metabolism pathway, which results in the formation of tannin and catechin, shares the same metabolic pathway as anthocyanin, and produces brown coloration (Shoji 2007). In July of 2018, white flowers of an unknown Rafflesia species bloomed in Pagar village, Bengkulu, Indonesia. Although the color of its perigone and outer window was pale white, the inner wall of the central tube was orange (Suppl. Figure 10). It is possible that this lack of coloration reveals a variegated version of Rafflesia in which there is less production and accumulation of anthocyanins, tannins, catechins, or other flavonoid pigments in the epidermal layer of perigone lobes, but with an apparently normal epidermis on the inner side of the perigone tube.
Epidermal tissue functionality of floral organs and the elusive stomata: do Cammerloher’s stomata exist?
The adaxial epidermis of R. patma and the epidermis layer of the inner linings of R. patma flower organs in this study nearly all consisted of papillate cells. This cell shape is similar to Dendrobium which has a velvety surface with papillate and dome-shaped cells (Mudalige et al. 2003). This velvety, leathery structure might be to mimic animal skin and, combined with its odor, might be used to attract potential pollinators. Therefore, it is possible that there is odor-emitting chemical secretion from epidermal cells, similar to Amorphophallus titanum, such as methyl thiolacetate, dimethyl trisulfide, and isovaleric acid (Shirasu et al. 2010). However, we were unable to locate secretory cells in this study. In comparison to the papillate adaxial epidermis, the abaxial epidermis of R. patma flower organs are flatter (except in the late perigone lobe), especially in the early perigone lobe and epidermis layer of outer linings of the flower which are composed of a flat-celled epidermis and next to it, the parenchyma cells are also flatter (see Figs. 2a, f, 3d, m) forming a thick, dense barrier, compared to the adaxial epidermis of the perigone lobe and epidermis layer of inner linings of the flower (see Figs. 2a, e, 3a, i) which are normal, and not flattened. This strategy for the abaxial epidermis and outer lining epidermis could serve as a stronger and more effective protective barrier against any threat from the outside as the stacked flat structures might increase the stiffness of the tissue part since the distance between cell walls is reduced, increasing immediate resistance by the combined rigidity of the cell walls upon bending and or piercing by a foreign object.
A microscopic observation of the R. patma surface indicated the absence of stomata or stomata-like structures. A study by Cammerloher (1920) revealed that R. rochussenii has abnormal stomata with three or more guard cells located in the adaxial epidermis of the perigone lobe, suggesting that stomata also play a role in odor distribution (release of volatile substances) due to extensive opening of the stomata by guard cells. However, Schmucker (1966) stated that stomata were observed only in small numbers, or were absent in Rafflesia, but available in Rhizanthes, another genus in the Rafflesiaceae, and also in Cytinus, Pilostyles, and Mitrastemon, all holoparasites and former members of the Rafflesiaceae. Nevertheless, the reports of stomatal sightings by both Cammerloher and Schmucker are unclear and no actual microscopic images were provided in their reports, only hand drawings or illustrations.
Due to the nature of Rafflesia, which lacks photosynthetic ability shown by the absence of a chloroplast genome in plastids (Molina et al. 2014), stomata in Rafflesia might only be useful for gas exchange for cellular respiration, water transpiration, and/or evaporation of odor volatiles, rather than supporting photosynthesis in the form of CO2 intake. We were unable to confirm the presence of stomata in R. patma in this study. The only structure similar to illustrations by Cammerloher (1920) was the abaxial cast of the early perigone lobe. It is unsure if Cammerloher’s stomata exist in all Rafflesia species, or only in some, or if they are simply epidermis cells (Suppl. Figure 5e; compare to picture inspired by Cammerloher (1920) in Suppl. Figure 11).
If the stomata with multicellularized guard cells, as observed by Cammerloher (1920), exist, it is possible that the guard cells surrounding the stomata allow physically larger stomata to open, equivalent to several stomata combined in the same area (Suppl. Figure 11). More studies on additional Rafflesia species and employing scanning electron microscopy (SEM) might find the elusive stomata in Rafflesia.
Limitations and suggestions for future research
Even though we had two flower samples, several flower organs were very fragile. The central disc processes (i.e., the spiky protuberances) were destroyed during staining. In addition, the bract was too brittle to sample. Better approaches need to be developed in the future to handle Rafflesia flower samples, which are rare, with greater care, to obtain better results.
During vascular observation, we often discovered vascular bundles with different shapes and arrangements. Care is needed to determine if these are different types of vascular bundles, or simply bent vascular tubes or points where the vascular bundle ends and the vascular bundle appears smaller (see Suppl. Figure 7). In Nikolov et al. (2014a), Rafflesia vasculature was spread across the flower but it likely ends at some points in the flower organ.
In this study, saffranin-fastgreen staining indicated areas of tissue containing metabolites such as tannin, suberin, and lignin since saffranin stains cells with these metabolites red (Ma et al. 1993). However, this only provides qualitative data for metabolite assessment and is likely to be inaccurate. In the future, utilization of gas chromatography–mass spectrometry (GC–MS), high performance liquid chromatography (HPLC), and molecular identification are to be encouraged to provide detailed information regarding the Rafflesia metabolites inside tissues or even produced as volatiles.
In order to search for stomata, we initially wanted to assess two separated options: a paradermal microtomy procedure and the creation of a surface cast with nail polish. These two procedures have their own advantages and disadvantages. Paradermal microtomy may provide better imagery although it requires more time, which can damage specimen tissues, and special care is required for samples with extremely thin epidermis layers, as in R. patma. A surface cast provides a quicker solution, but the results might vary and the casts can be damaged upon peeling. Unfortunately, we were unable to perform paradermal microtomy because the R. patma epidermis is very thin and consists only of a single layer of cells. In the future, SEM imagery can be used for paradermal observations. Other than stomata, this thorough observation can be performed to attempt to locate odor-secreting cells in the epidermis.
There are many aspects still left unanswered related to Rafflesia, including how the plant evolved and adapted into a flower with such compact organ systems. A previous study (Mursidawati et al. 2019) revealed that R. patma forms clumps of cells hidden within the host vascular cambium layer instead of forming a haustorial-like or endophytic network. This condition might allow R. patma to use minimal resources during its vegetative stage, and then grow into a massive flower when the time for anthesis arrives. In this study, the flower itself displayed a minimalistic vascular system with only xylem and parenchyma tissue. A possible way to observe Rafflesia tissue development in the future might be via seed germination and tissue culture.
Conclusion
This R. patma study revealed details of its vascular system and pigmented area of the tissue. R. patma has simple, reduced vascular tissue that consists of xylem vessel elements and vascular parenchyma tissue. Most of the stained R. patma metabolites, in this case tannin, accumulated in perigone lobes as shown by red-stained tissues following saffranin-fastgreen staining. The accumulated tannin might serve as protection against herbivores prior to anthesis. The papillate surface of the adaxial epidermis might contain odor-secreting cells, but this is still unconfirmed, while the flat abaxial epidermis is mostly used as a barrier for protection. No stomata were found in this study. Better sampling methods are suggested to obtain samples from all organs with greater care, and also to better histologically profile different cells in a tissue.
Notes
Nail polish was used to create a cast over epidermal tissue as a semi non-destructive sampling method. As the nail polish solidified, the “peel” carried the printed (casted) features of the tissue surface providing details of the observable tissue and/or cell surface without the need to cut those cells and/or tissue.
References
Beaman RS, Decker PJ, Beaman JH (1988) Pollination of Rafflesia (Rafflesiaceae). Am J Bot 75:1148–1162. https://doi.org/10.1002/j.1537-2197.1988.tb08828.x
Beck CB (2012) An introduction to plant structure and development: plant anatomy for the twenty-first century, 2nd edn. Cambridge University Press, Cambridge
Biancucci M, Mattioli R, Forlani G, Funck D, Costantino P, Trovato M (2015) Role of proline and GABA in sexual reproduction of angiosperms. Front Plant Sci 6:680. https://doi.org/10.3389/fpls.2015.00680
Cammerloher H (1920) Der Spaltöffnungsapparat von Brugmansia und Rafflesia. Österr Bot Z 69(7–8):153–164 (in German)
de Boer AH, Volkov V (2003) Logistics of water and salt transport through the plant: structure and functioning of the xylem. Plant Cell Environ 26:87–101. https://doi.org/10.1046/j.1365-3040.2003.00930.X
de Vega C, Ortiz PL, Arista M, Talavera S (2007) The endophytic system of Mediterranean Cytinus (Cytinaceae) developing on five host Cistaceae species. Ann Bot 100:1209–1217. https://doi.org/10.1093/aob/mcm217
Dörr I, Kollmann R (1995) Symplasmic sieve element continuity between Orobanche and its host. Bot Acta 108:47–55. https://doi.org/10.1111/j.1438-8677.1995.tb00830.x
Kapoor LD (1973) Constitution of amphicribral vascular bundles in capsule of Papaver somniferum linn. Bot Gaz 134:161–165. https://doi.org/10.1086/336698
Liu WZ, Hilu K, Wang YL (2014) From leaf and branch into a flower: Magnolia tells the story. Bot Stud 55:28. https://doi.org/10.1186/1999-3110-55-28
Ma Y, Sawhney VK, Steeves TA (1993) Staining of paraffin-embedded plant material in safranin and fast green without prior removal of the paraffin. Can J Bot 71:996–999. https://doi.org/10.1139/b93-114
Mauseth JD (1988) Plant anatomy. The Benjamin/Cummings Publishing Company Inc, San Francisco
Molina J, Hazzouri KM, Nickrent D, Geisler M, Meyer RS, Pentony MM, Flowers JM, Pelser P, Barcelona J, Inovejas SA, Uy I (2014) Possible loss of the chloroplast genome in the parasitic flowering plant Rafflesia lagascae (Rafflesiaceae). Mol Biol Evol 31:793–803. https://doi.org/10.1093/molbev/msu051
Mudalige RG, Kuehnle AR, Amore TD (2003) Pigment distribution and epidermal cell shape in Dendrobium species and hybrids. HortScience 38:573–577
Mursidawati S, Irawati (2017) Biologi konservasi Rafflesia. LIPI Press, Jakarta (in Bahasa Indonesia)
Mursidawati S, Sunaryo S (2012) Studi anatomi endofitik Rafflesia patma di dalam inang Tetrastigma sp. Buletin Kebun Raya 15:71–80 (in Bahasa Indonesia)
Mursidawati S, Wicaksono A, Teixeira da Silva JA (2019) Development of the endophytic parasite, Rafflesia patma Blume, amongst host plant (Tetrastigma leucostaphylum (Dennst.) Alston) vascular cambium tissue. S Afr J Bot 123:382–386. https://doi.org/10.1016/j.sajb.2019.03.028
Nais J (2001) Rafflesia of the world. Sabah: Sabah Parks in association with Natural History Publications (Borneo), Kota kinabalu
Ng FSP (2019) Is Rafflesia an angiosperm? J Trop For Sci 31:286–297. https://doi.org/10.26525/jtfs2019.31.3.286
Nikolov LA, Endress PK, Sugumaran M, Sasirat S, Vessabutr S, Kramer EM, Davis CC (2013) Developmental origins of the world's largest flowers, Rafflesiaceae. Proc Natl Acad Sci 110:18578–18583. https://doi.org/10.1073/pnas.1310356110
Nikolov LA, Staedler YM, Manickam S, Schönenberger J, Endress PK, Kramer EM, Davis CC (2014a) Floral structure and development in Rafflesiaceae with emphasis on their exceptional gynoecia. Am J Bot 101:225–243. https://doi.org/10.3732/ajb.1400009
Nikolov LA, Tomlinson PB, Manickam S, Endress PK, Kramer EM, Davis CC (2014b) Holoparasitic Rafflesiaceae possess the most reduced endophytes and yet give rise to the world’s largest flowers. Ann Bot 114:233–242. https://doi.org/10.1093/aob/mcu114
Rajanna L, Shivamurthy GR, Niranjana R, Vijay CR (2005) Occurrence of phloem in the haustorium of Aeginetia pedunculata wall—a root holoparasite of Orobanchaceae. Taiwania 50:109–116
Rutherford RJ (1970) The anatomy and cytology of Pilostyles thurberi gray (Rafflesiaceae). Aliso 7:263–288
Schmucker T (1966) High parasites, Orobanchaceae (no. Trans-1789). Army biological labs frederick Md. https://apps.dtic.mil/dtic/tr/fulltext/u2/833284.pdf. Accessed 12 May 2020
Secchi F, Pagliarani C, Zwieniecki MA (2017) The functional role of xylem parenchyma cells and aquaporins during recovery from severe water stress. Plant Cell Environ 40:858–871. https://doi.org/10.1111/pce.12831
Shirasu M, Fujioka K, Kakishima S, Nagai S, Tomizawa Y, Tsukaya H, Murata J, Manome Y, Touhara K (2010) Chemical identity of a rotting animal-like odor emitted from the inflorescence of the titan arum (Amorphophallus titanum). Biosci Biotechnol Biochem 74:2550–2554. https://doi.org/10.1271/bbb.100692
Shoji T (2007) Polyphenols as natural food pigments changes during food processing. Am J Food Technol 2:570–581
Sofiyanti N, Wahibah NN, Purwanto D, Syahputra E, Mat-Salleh K (2008) Alkaloid and phenolic compounds of Rafflesia hasseltii Suringar and its host Tetrastigma leucostaphylum (Dennst.) Alston ex Mabb. in Bukit Tigapuluh National Park, Riau: a preliminary study. Biodiversitas 9:17–20. https://doi.org/10.13057/biodiv/d090105
Susatya A (2011) Rafflesia pesona bunga terbesar di dunia. Direktorat Kawasan dan Bina Hutan Lindung, Bengkulu (in Bahasa Indonesia)
Susatya A, Hidayati SN, Mat-Salleh K, Mahyuni R (2017) Ramenta morphology and its variations in Rafflesia (Rafflesiaceae). Flora 230:39–46. https://doi.org/10.1016/j.flora.2017.03.001
Twell D, Oh SA, Honys D (2006) Pollen development, a genetic and transcriptomic view. In: Malhó R (ed) The pollen tube. Plant cell monographs, vol 3. Springer, Berlin, pp 15–45
Walsh MA, Popovich TM (1977) Some ultrastructural aspects of metaphloem sieve elements in the aerial stem of the holoparasitic angiosperm Epifagus virginiana (Orobanchaceae). Am J Bot 64:326–336. https://doi.org/10.1002/j.1537-2197.1977.tb15734.x
Wicaksono A, Mursidawati S, Sukamto LA, Teixeira da Silva JA (2016) Rafflesia spp.: propagation and conservation. Planta 244:289–296. https://doi.org/10.1007/s00425-016-2512-8
Wicaksono A, Teixeira da Silva JA, Mursidawati S (2017) Dispersal of Rafflesia patma Blume endophyte in grafted host plant (Tetrastigma leucostaphylum (Dennst.) Alston). J Plant Develop 24:145–150
Wurdack KJ, Davis CC (2009) Malpighiales phylogenetics: gaining ground on one of the most recalcitrant clades in the angiosperm tree of life. Am J Bot 96:1551–1570. https://doi.org/10.3732/ajb.0800207
Acknowledgements
We are grateful to Dr. Irawati, Dr. Sunaryo and Mr. Ujang Hafid from the Center for Biological Research and Development Cibinong Science Center of LIPI for support and technical assistance, and to Ms. Sri Hartini and Mr. Rubono who maintain the Rafflesia plant collection in Bogor Botanical Garden. We also thank Mr. Heri Santoso, CEO of Genbinesia Foundation for providing some deep discussion about Rafflesia structures and evolution. We also thank Mr. Raffles Yolanda (Pagar Village, Bengkulu, Indonesia) for providing us with a photo of the “albino” Rafflesia. The work in this paper was presented at the Symposium of Flora Malesiana 11, Bandar Seri Begawan, Brunei Darussalam from June 30 to July 5, 2019.
Author information
Authors and Affiliations
Contributions
AW and SM conceived the experimental idea. SM provided access to samples and was involved in sample preparation. AW performed the laboratory work and analyses under the supervision of SM and JATS. All authors contributed to the analysis of results and writing all drafts and revisions of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare no conflicts of interest.
Additional information
Communicated by Anastasios Melis.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mursidawati, S., Wicaksono, A. & Teixeira da Silva, J.A. Rafflesia patma Blume flower organs: histology of the epidermis and vascular structures, and a search for stomata. Planta 251, 112 (2020). https://doi.org/10.1007/s00425-020-03402-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-020-03402-5