Abstract
Main conclusion
HSP60 gene family in pepper was analyzed through bioinformatics along with transcriptional regulation against multiple abiotic and hormonal stresses. Furthermore, the knockdown of CaHSP60-6 increased sensitivity to heat stress.
Abstract
The 60 kDa heat shock protein (HSP60) also known as chaperonin (cpn60) is encoded by multi-gene family that plays an important role in plant growth, development and in stress response as a molecular chaperone. However, little is known about the HSP60 gene family in pepper (Capsicum annuum L.). In this study, 16 putative pepper HSP60 genes were identified through bioinformatic tools. The phylogenetic tree revealed that eight of the pepper HSP60 genes (50%) clustered into group I, three (19%) into group II, and five (31%) into group III. Twelve (75%) CaHSP60 genes have more than 10 introns, while only a single gene contained no introns. Chromosomal mapping revealed that the tandem and segmental duplication events occurred in the process of evolution. Gene ontology enrichment analysis predicted that CaHSP60 genes were responsible for protein folding and refolding in an ATP-dependent manner in response to various stresses in the biological processes category. Multiple stress-related cis-regulatory elements were found in the promoter region of these CaHSP60 genes, which indicated that these genes were regulated in response to multiple stresses. Tissue-specific expression was studied under normal conditions and induced under 2 h of heat stress measured by RNA-Seq data and qRT-PCR in different tissues (roots, stems, leaves, and flowers). The data implied that HSP60 genes play a crucial role in pepper growth, development, and stress responses. Fifteen (93%) CaHSP60 genes were induced in both, thermo-sensitive B6 and thermo-tolerant R9 lines under heat treatment. The relative expression of nine representative CaHSP60 genes in response to other abiotic stresses (cold, NaCl, and mannitol) and hormonal applications [ABA, methyl jasmonate (MeJA), and salicylic acid (SA)] was also evaluated. Knockdown of CaHSP60-6 increased the sensitivity to heat shock treatment as documented by a higher relative electrolyte leakage, lipid peroxidation, and reactive oxygen species accumulation in silenced pepper plants along with a substantial lower chlorophyll content and antioxidant enzyme activity. These results suggested that HSP60 might act as a positive regulator in pepper defense against heat and other abiotic stresses. Our results provide a basis for further functional analysis of HSP60 genes in pepper.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants are sessile organisms and are exposed to various threats, both biotic and abiotic. These stresses individually or in combination result in huge losses in terms of growth, development, yield and sometimes leading to plant’s death (Mittler 2006). Plants continuously confront harsh environment, such as high and low temperatures, drought, salinity, heavy metals, light, flooding, and physical wounding (Al-whaibi 2011; Guo et al. 2016). These stresses negatively affect plant germination (Cheng et al. 2009), growth (Wahid et al. 2007), and loss of photosynthetic pigment (Tan et al. 2011). These stresses also affect the reproductive characteristics by causing male sterility (Young et al. 2004), reduced pollination and fertilization (Guo et al. 2015), and increased premature flower and fruit drop (Tubiello et al. 2007).
On the onset the of stress situation, plants improvised a hormonal balance of ABA, ethylene, salicylic acid (SA), jasmonic acid (JA), and other steroids to enhance stress tolerance and resistance (Wang and Li 2006). Besides these adaptations, at the cellular and molecular levels, plants also tend to enhance the production of special stress-related proteins instead of normal proteins. The specialized stress-related proteins known as heat shock proteins (HSP) are produced through transcription and translation of special HSP genes (Guo et al. 2016; Singh et al. 2016). The up- and down-regulation of these stress-responsive biomolecules, which act as molecular chaperones, are controlled by transcription factors, i.e., heat shock factor (HSF) (Ahuja et al. 2010). Heat shock proteins (HSP) are grouped into different classes based on their molecular weight, such as HSP100, HSP90, HSP70, HSP60, and the small HSP including HSP40, HSP20, and HSP10, respectively (Wang et al. 2004; Kotak et al. 2007). These are responsible for maintaining cell homeostasis, transport of newly synthesized proteins across cell organelles, preventing mis-folded, denatured, and aggregated proteins caused by stress conditions (Balchin et al. 2016). Heat shock proteins (HSP60), as molecular chaperones, assist in folding and refolding of proteins (Hartl et al. 2011; Balchin et al. 2016). Chaperonins share a double ring-like structure that plays an important role in protein functional conformation and transport (Saibil et al. 2013). The group I chaperonin, HSP60, is well studied in prokaryotes, Escherichia coli, both structurally and functionally. ATP binds to the GroEL making the GroEL/GroES (HSP60/HSP10) complex having a barrel like structure, which refolds the substrate into its normal structure. After proper folding the proteins with the use of ATP, GroES detach from GroEL releasing the proper folded protein molecule from the cavity, and the process repeats with the expenditure of ATP (Hartl et al. 2011). Chaperonins have been studied in Arabidopsis (Xu and Huang 2010; Jungkunz et al. 2011), rice (Wang et al. 2014), maize (Prasad et al. 1990), foxtail millet (Singh et al. 2016) and poplar (Yer et al. 2018), however, little in pepper.
Pepper (Capsicum annuum L.) is one of the important solanaceous crop in the world and is used as a major spice in cuisines due to its appealing taste and nutritional value (Kim et al. 2014). Pepper is sensitive to various stresses, particularly to high-temperature stress during the reproductive stage. HSP70 and HSP60 gene families are the most studied chaperones under heat stress (HS) (Hartl et al. 2011), and several reports are available studying these in prokaryotes and some other eukaryotes. However, no systematic study has been reported on the role of HSP60 gene family in pepper. In this study, we identified 16 HSP60 genes in the pepper genome through bioinformatic analysis. Phylogenic relationship and gene-duplication events were studied to know the expansion of HSP60 genes in the pepper along the gene structure, conserved motifs and distribution of these genes on different chromosomes. In a functional analysis, cis-regulatory elements in the promoter region and gene ontology (GO) were also elucidated. Expression analyses of CaHSP60 genes in different vegetative and reproductive plant parts and under multiple environmental stresses were also performed. Based on the expression profile of CaHSP60-6 to heat, other abiotic and hormonal stresses, and presence of multiple stress-related cis-regulatory elements in the promoter region, it was speculated that this gene might be involved in pepper defense to abiotic stresses. Thus, this study aimed to identify the function of CaHSP60-6 and its involvement in different biological processes. We knocked down this gene through virus-induced gene silencing (VIGS) assay and exposed the pepper plants to HS. The results of this study indicated that this gene positively regulates the pepper defense against HS. This study will provide a basis for further insight into this vital gene family in the development of stress tolerance in pepper plants.
Materials and methods
Identification and sequence analysis of CaHSP60 gene family in pepper
To identify the HSP60 gene family members in pepper, profile of “PF00118” from Protein family (Pfam) database (http://pfam.xfam.org/) was blast searched, using the Hidden Markov Model (HMM) via HAMMER 3.0 with default parameters, in the current version of Pepper Genome Platform (PGP) (http://peppergenome.snu.ac.kr/download.php) for CM334 (release 1.55) and Zunla-1 (v 2.0) (Kim et al. 2014; Qin et al. 2014). Initial sequences of CaHSP60 like genes from different pepper genomes were aligned through DNAMAN program. The putative pepper HSP60 genes were examined for the presence of Cpn60_TCP1 domain using SMART (http://smart.embl-heidelberg.de/) and NCBI CDD tool. The confirmed and non-redundant genes (molecular weight (MW) < 69 and > 50 kDa and with Cpn60_TCP1 domain) were named CaHSP60 following the method of Guo et al. (2016).
Physico-chemical attributes and bioinformatics analysis of CaHSP60 genes
To compute and analyze the MW, theoretical isoelectric point (pI) and instability index; protein sequences were blasted in ExPASy-ProtParam tool (http://web.expasy.org/ProtParam/) (Gasteiger et al. 2003). For subcellular location prediction, the program WOLF PSORT (https://www.genscript.com/wolf-psort.html) was used (Horton et al. 2007). Exon/intron structure of CaHSP60 genes was determined by blasting fasta format the coding DNA sequences (CDS) to corresponding genomic sequences in the online tool Gene Structure Display Server (GSDS2.0) (http://gsds.cbi.pku.edu.cn/) as described by Kang et al. (2016). For phylogenetic tree construction, protein sequences from different plants and pepper HSP60 were aligned by CUSTALW and the tree was generated by MEGA 6.0, using neighbor-joining (NJ) method with 1000 bootstraps following our previous study of Khan et al. (2018). Gene ontology (GO) enrichment analyses were performed using the online program Blast2Go (http://www.blast2go.com). The results were predicted and analyzed in three categories, i.e., cellular component, molecular function, and biological processes (Ali et al. 2018).
Analysis of conserved motifs and cis-acting elements in the promoter region of CaHSP60 genes
We used MEME online program to determine the conserved motifs in the CaHSP60 genes (http://meme-suite.org/tools/meme), keeping the settings as described by Guo et al. (2016). The conserved domains in the sequences were confirmed by the conserved domain database (CDD) (http://www.ncbi.nlm.nih.gov/cdd/), SMART (http://smart.embl-heidelberg.de/), and EMBL-EBI (https://www.ebi.ac.uk/interpro/).
The cis-regulating elements in the promoter region of CaHSP60 genes were searched in the pepper genome platform (PGP). PlantCARE online tool was used to find out cis-acting elements (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) (Lescot et al. 2002).
Chromosomal location and gene-duplication analysis of CaHSP60 genes
Information about the chromosome and position of CaHSP60 genes on the respective chromosome was retrieved from pepper genome platform (PGP) (http://peppergenome.snu.ac.kr/) as described by Zhang et al. (2016), and the genes mapping diagram on chromosomes was constructed using Map Draw (Liu and Meng 2003). Duplication analysis was performed following the method of Gu et al. (2002), briefly described as follows: (1) the FASTA-alignable region among the two proteins should be more than 70% of the longer protein sequence and (2) the identity between the two protein sequences (I) should be I_30% if the alignable region is longer than 150 amino acid and I ≥ 0.01n + 4.8L−0.32(1 + exp (−L/1000), where n = 6 and L is the alignable length between the two protein sequences of the gene.
Tissue-specific expression of CaHSP60 genes based on RNA-Seq data
Previously published and publically available data (Kim et al. 2014) were used for tissue-specific expression from online server (http://peppergenome.snu.ac.kr/). These data in reads per kilo base per million mapped reads (RPKM) for roots, stems, leaves, and for pericarp and placenta each at mature green (MG), breaker (B), 5 day post-breaker (5B), 10 day post-breaker, 6 day post-anthesis (6DPA), 16 day post-anthesis (16DPA), and 25 day post-anthesis (25DPA) were used for CaHSP60 genes members and the results were normalized to log 2 and heat maps were generated through Heml 1.0 heat map illustrator (Deng et al. 2014).
Plant materials and growth conditions
Pepper thermo-tolerant cultivar R9 (sweet pepper, introduced from the World-Asia Vegetable Research and Development Center, PP0042-51) and thermo-sensitive line B6 from the Laboratory of Vegetable Plant Biotechnology and Germplasm Innovation, Northwest A&F University, China, were used in this study. The previously described methods of Huang et al. (2018) were followed by growing the seedlings in growth chamber (GXZ-380C, Jiangnan Instrument Factory, Ningbo, China) at day (25 °C for 16 h) and night (20 °C for 8 h) with 60% relative humidity and 200 μmol m−2 s−1 illumination intensity.
Abiotic stress treatments and hormonal applications
Plants were subjected to high-temperature stress as described by Guo et al. (2015, 2016). Briefly, pepper seedlings in the growth chambers at 6–8 true leaves stage were treated with HS of 42 °C for 0.5, 1, 2, 4, and 8 h, respectively, to determine dynamic expression profile of HSP60 genes. Plants grown at 25 °C in the day conditions were considered as controls. For tissue-specific expression, R9 plants treated with HS at 42 °C for 2 h and others at 25 °C were treated as control. Samples from leaves, stems, roots from seedlings, and flowers of adult plants with and without HS were collected.
For other abiotic stresses, pepper seedlings were exposed to low temperature (6 °C) in the growth chamber, and for salt and osmotic stresses, the seedlings were treated with 300 mM NaCl and mannitol for 0, 1, 3, 6, 12, and 24 h. Similarly, for hormonal applications, 50 µM methyl jasmonate (MeJA), 5 mM SA, and 0.57 mM ABA solution were used. Methods of our previous study were followed and samples were collected at 0, 1, 3, 6, , and 24 h post-treatment (hpt) (Ali et al. 2018). The samples after collection were immediately frozen in liquid nitrogen and stored at − 80 °C.
RNA extraction and qRT-PCR analysis
Total-RNA extraction, cDNA synthesis, dilution, and quality checking were done following the protocol of Ali et al. (2018). Primer Premier 6.0 package (http://www.premierbiosoft.com/primerdesign/index.html) was used for designing qRT-PCR gene-specific primers (Table S1). The designed primers were analyzed and confirmed through the NCBI Primer BLAST tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). The pepper CaUbi3 gene was used as a reference gene and 2−ΔΔCT method was used for the determination of relative expression of all the genes.
VIGS assay of CaHSP60-6 in pepper
VIGS technique was used to knockdown the CaHSP60-6 in pepper cultivar R9. The pTRV2:CaHSP60-6 recombinant plasmid was engineered following the method of Liu et al. (2016); briefly, a 337-bp sequence part of CaHSP60-6 was cloned into pTRV2 vector using the forward 5′ GCCGCCCAAGGAATG 3′ and reverse primers 5′ CCGCAAATGTTGAGACCA 3′. Afterwards, the freeze–thaw method was used to transform pTRV1, pTRV2 (negative control), and pTRV2:CaPDS (positive control) with the combined vector pTRV2:CaHSP60-6 into Agrobacterium tumefaciens (GV3101). A. tumefaciens carrying pTRV1 was mixed in a ratio of 1:1 with pTRV2, pTRV2:CaPDS and pTRV2:CaHSP60-6. The agrobacterium-mediated suspensions with pTRV1, pTRV2, pTRV2:CaPDS, and pTRV2:CaHSP60-6 (OD600 = 1.0) were infiltrated and pepper seedlings. Samples from leaves in the control and CaHSp60-6 knockdown plants were collected 6 week post-infiltration to measure the silencing efficiency by qRT-PCR. These experiments were conducted with three biological replicates.
Plant physiological indices
Measurement of excised leaf water loss assay, relative electrolyte leakage, chlorophyll contents, and lipid peroxidation
To determine water loss assay, the top second fully expanded leaves of silenced and control plants at untreated and heat-stressed were detached and placed on blotting papers and weight was recorded every half-hour interval for 8 h, under light conditions and at room temperature. The water loss assay of leaves was calculated as the percentage of fresh weight of the control and silenced plants. Relative electrolyte leakage (REL) was computed by the formula REL % = (EC1/EC2) × 100 according to the method described by Yin et al. (2014).
Total chlorophyll was spectrophotometrically determined in the leaves using an 80% acetone method. Leaves from untreated and stressed plants in control and knockdown plants were collected and incubated overnight in 80% acetone and absorbance was taken at 663 and 646 nm and the chlorophyll content was measured in milligrams per gram FW (Lichtenthaler and Wellburn 1983). Lipid peroxidation was determined by calculating the malondialdehyde (MDA) contents following the method of Campos et al. (2003). Briefly, 0.5 g leaves’ sample was grounded in sodium phosphate buffer (pH 7.8) having 1% polyvinyl pyrrolidone (PVP) on an ice bath. The homogenate was centrifuged at 12,000g, for 20 min at 4 °C, and the supernatant was mixed with 5% TCA having 0.5% 2-thiobarbituric acid (TBA) and boiled for 20 min in a water bath. Absorbance of the cooled and centrifuged solution was measured at 600, 532, and 450 nm.
Measurement of H2O2 content, histochemical detection of ROS, and antioxidant enzymes activity
H2O2 was determined according to Sergiev et al. (1997). First, the standard solution was prepared to obtain a curve by dilution of 100 µM H2O2 and 0.1% trichloroacetic acid (TCA) in the ratio of 0:1; 0.1:0.9; 0.2:0.8; 0.4:0.6; 0.6:0.4; 0.8:0.2; and 1:0 mL in 3 mL of potassium phosphate buffer and potassium iodide (KI) solution. Samples were homogenized with 0.1% TCA (w/v). The solution was centrifuged for 15 min at 12,000g and the supernatant was added to 1 M KI and 10 mM potassium phosphate buffer pH 7. The mixture was gently mixed and absorbance was measured at 390 nm. Superoxide radical (O2) was estimated in leaves by staining with 0.1% nitro blue tetrazolium (NBT) in 50 mM potassium phosphate buffer (pH 7.8) as described by Liu et al. (2016). Hydrogen peroxide (H2O2) was detected by the 3,3 diaminobenzidine (DAB) method (Feng et al. 2019) according to Su et al. (2014).
Antioxidant enzymes, superoxide dismutase (SOD), and peroxidase (POD) activities were estimated following the protocol of Guo et al. (2012). For SOD activity, the enzyme mixture contained 130 mM methionine (MET), 0.1 mM EDTA-Na2, 0.75 mM NBT, 0.02 mM riboflavin, and 0.05 mM phosphate buffer was used and the activity was recorded at 560 nm. POD activity was calculated by mixing the crude enzyme extract with 0.2% guaiacol and 0.3% H2O2 diluted with 0.2 mM phosphate buffer (pH 7.0) and absorbance was noted at 470 nm at 30 s intervals for 3 min reaction time.
Statistical analysis
The data were analyzed by SPSS package (SPSS version 23.0, SPSS Inc., Chicago, IL, USA). Analysis of variance (ANOVA) and least significant difference (LSD; P < 0.05) test were employed to compare the significant differences among the given treatments using standard deviation (SD) of three replications in all measured parameters. Data were presented as graphs that were constructed by GraphPad Prism 7.0 (GraphPad Software, Inc., LA Jolla, CA, USA).
Results
Identification, sequence analysis, and annotation of CaHSP60 genes family in pepper
Initially, 32 and 43 HSP60-like genes were extracted from the pepper genome platform for CM334 and Zunla-1, respectively, employing the Hidden Markov Model (HMM) profile of HSP60 (accession no: PF 00118). After alignment by DNAMAN, similar sequences were considered as a single gene; as a result, 36 sequences were obtained. Furthermore, based on the SMART scan and NCBI CDD tool, 20 candidate sequences (molecular weight > 69 and < 50 kDa and without Cpn60_TCP1 domain) were removed. Finally, we obtained a total of 16 HSP60 genes which were named CaHSP60-1 to 16 based on the molecular weight. Subsequently, primers pairs (Table S2) were designed to amplify and confirm the sequence through cloning.
Physico-chemical attributes and bioinformatics analysis of CaHSP60 genes
Considerable variations were recorded for the physico-chemical properties of HSP60 genes in pepper (Table 1), such as CDS. The length of HSP60 genes ranged from 1509 bp (CaHSP60-1) to 1923 bp (CaHSP60-16). Similarly, the molecular weights ranged from 502 amino acids (aa) in CaHSP60-1 to 640 (aa) in CaHSP60-16. The predicted PI values were between 5.28 (CaHSP60-11) and 6.99 (CaHSP60-9), whereas the predicated instability index showed that 14 out of 16 CaHSP60s (87%) were stable protein (instability index < 40) (Table 1).
Intron/exon analysis revealed that only a single gene (CaHSP60-2) had no intron, while others had multi introns. More than 10 introns were noticed in 12 (75%) of the CaHSP60 genes (Fig. 1). A total of 10 conserved motifs of CaHSP60 genes was identified through the online server MEME tool (http://meme-suite.org/tools/meme). Motifs 3, 4, 5, 6, and 7 were present in 8 (50%) of CaHSP60 (-1, -2, -3, -4, -5, 6, -7, and -8) genes, forming group I, while along with the above-mentioned motifs, additional motifs 1, 2, 8, 9, and 10 were found in CaHSP60 (-9, -10, -11, -13, -14, -15, and -16), whereas only motif 8 was absent in CaHSP60-12 (Fig. 2). Details about motifs and domains are shown in Suppl. Tables S3 and S4.
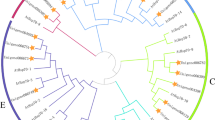
To understand the evolutionary history, similarities and differences of pepper HSP60 genes and those related genes in other crop species, an un-rooted phylogenetic tree was constructed. These sequences were from Arabidopsis thaliana, Artemisia annua, Capsicum annuum, Carica papaya, Gossypium hirsutum, Glycine max, Hordeum vulgare, Malus domestica, Nicotiana benthamiana, Oryza sativa, Sesamum indicum, Solanum lycopersicum, Solanum tubersosum, Sorghum bicolor, Triticum aestivum, and Zea mays. Analysis of the phylogeny revealed that these sequences clustered into three groups based on the sequence similarities and relatedness. Out of 16 peppers HSP60 genes, eight (50%) genes separated into group I, three (19%) into group II and five (31%) genes clustered into group III, respectively (Fig. 3). The chromosomal location revealed that CaHSP60 genes were distributed on 7 different chromosomes in pepper, where the highest number of 4 CaHSP60 was clustered on chromosome 3, followed by chromosomes 1 and 11 carrying 3 genes each (Fig. 4). In the gene-duplication event, a single tandem duplication (CaHSP60-3 and -12) and two segmental duplication events (CaHSP60-13, -9 and CaHSP60-2, -11) were recorded.
Gene ontology and cis-acting elements analysis of CaHSP60 genes
GO analysis showed that CaHSP60 genes were involved primarily in protein folding and refolding through an ATP-dependent manner. In addition, CaHSP60s were predicted to be localized in cell compartments, such as cytoplasm, mitochondrial matrix, chloroplast, and few in the nucleus (Fig. 5).
To find out the cis-regulatory elements in the promoter region of CaHSP60 genes, the upstream region of 1500 bp was analyzed in all the CaHSP60 genes through the plantCARE program. The silico analysis showed that the promoter region contained cis-acting elements related to abiotic, biotic, and hormonal stresses (Fig. 6; Table S5). Defense- and stress-related TC-rich repeats were found in all 16 CaHSP60 genes with a maximum of five in CaHSP60-6 followed by two each in CaHSP60-3, -4, -7, -9, -13, and -14, respectively, while other genes had only one. Heat shock elements (HSE) were detected in nine (56%) CaHSP60 genes, low-temperature responsive elements (LTR) in six (37%), drought responsive elements (MBS), TGAG, and TCA elements in 11 (69%) of pepper HSP60 genes, while ABRE (ABA responsive elements) were found in ten (62%) of the CaHSP60 genes. In addition, fungal elicitor P-Box and wound responsive motifs were also found in promoter region of some pepper HSP60 genes.
Expression analysis of pepper HSP60 genes under HS
To investigate the transcriptional regulation of CaHSP60 genes under high-temperature stress, pepper thermo-tolerant line R9 and thermo-sensitive line B6 were treated with 42 °C for different periods and their expression level were analyzed by qRT-PCR (Fig. 7). The pepper HSP60 genes transcriptionally responded differently in both lines at different timepoints. The expression profile revealed that 15 (93%) out of 16 genes were up-regulated under HS and only CaHSP60-3 was down-regulated in both lines. CaHSP60-4 showed a slight down-regulation, soon after HS, followed by progressive up-regulation in both the pepper lines. While CaHSP60-16 was down-regulated in the B6 line only, it was progressively up-regulated in the R9 line with maximum expression (5.22) at 8 h. CaHSP60-7 did not significantly respond to HS in line R9, whereas its expression increased in line B6 at 1 h (60.78) and 8 h (38.42), respectively. No significant response of CaHSP60- 8 and -10 occurred until 2 h, yet then the expression progressively increased in both lines. Many CaHSP60 genes during HS exhibited a gradual up-regulation, reaching to peak at 2 h HS treatment. A more prominent response was recorded in the R9 line for CaHSP60-5, -6, -11, and -15 (37.809, 45.924, 43.25, and 9.09) after 2 h exposure to HS, respectively.
Expression pattern HSP60 genes of pepper thermo-tolerant line R9 and thermo-sensitive line B6 under HS condition. 6–8 true leaves of R9 and B6 seedlings were used to check gene expression levels at different timepoints (0, 0.5, 1, 2, 4 and 8 h) with HS treatment (42 °C). Mean values ± SD for three replicates and small letters (a–f) represent significant differences at (P < 0.05)
Expression profile of pepper HSP60 genes in response to other abiotic stresses
To investigate the transcriptional regulation of pepper HSP60 genes in response to other abiotic stresses, 9 candidate CaHSP60s (1, 3, 5, 6, 9, 10, 11, 13, and 14) in the R9 line were selected as representatives of the pepper HSP60 family on the basis of cis-acting elements and the expression profile to HS. These genes were subjected to cold, salt, and osmotic stresses (Fig. 8). The results revealed that almost all the candidate genes showed a differential up-regulation to these abiotic stresses except CaHSP60-3, which showed down-regulation under cold and NaCl stresses. The expression of CaHSP60-1 gradually increased and reached the maximum at 12 hpt under cold and drought (5.389 and 13.072) and at 24 hpt under NaCl (9.16) treatment. Pepper HSP60-3 was down-regulated under cold and NaCl stresses, while no response occurred to mannitol stress until 6 hpt, and afterwards, it was gradually up-regulated. For other candidate pepper HSP60 genes, a steady and gradual increase in expression was recorded for NaCl stresses with the maximum fold transcription recorded at 24 hpt. CaHSP60-5, -6, and -14 responded in a similar fashion to cold stress. However, the expression peaked at 6 hpt (23.3, 34.9, and 10.58), respectively. The same genes were maximally expressed at 12 hpt under mannitol stress. In response to cold stress, CaHSP60-13 showed no significant response, a gradual increase was recorded for NaCl, reaching a maximum of 10 folds at 24 hpt, whereas the same gene showed a concomitant up/down expression response for mannitol stress. CaHSP60-11 was significantly up-regulated (14-, 14-, and 15-folds) at 3, 6, and 12 hpt under cold treatment and abruptly down-regulated at 24 hpt.
Expression profile of CaHSP60s in response to hormonal treatments
The above selected 9 candidate pepper HSP60 genes were also treated with exogenous SA, ABA, and MeJA treatment to study the effect of these treatments on the expression level of these genes. Many of the studied genes responded to these hormonal treatments (Fig. 9). The pepper HSP60 genes showed a gradual and steady expression pattern to MeJA treatment, where maximum expression folds were recorded at 24 hpt except for CaHSP60-3, which showed no response to MeJA at all the timepoints. Maximum expression of CaHSP60-14 was observed when exposed to SA treatment at 1 h (12.188), to ABA (15.18) at 6 hpt and to MeJA (4.50) at 24 hpt. In case of SA treatments, CaHSP60-1, -5, and -6 were up-regulated (5, 23, and 15 folds, respectively) at 6 hpt; CaHSP60-9 and -11 expressions peaked (67 and 14 folds) at 12 hpt, while CaHSP60-3 and -13 showed no response to SA treatment. After ABA treatment, CaHSP60-1, -10, and -11 showed a maximal expression (9, 28, and 13 folds) at 1 hpt and then smoothly declined. Expression of CaHSP60-5, -6, and -14 suddenly increased (50.84, 48.32, and 7.12) at 1 hpt and then steadily increased over time; the expression was at peak (55.24, 53.4, and 15.18) at 6 hpt and then declined. CaHSP60-9 and -11 were maximally expressed (10 and 24 folds) at 12 h post-ABA treatments.
Expression analysis of CaHSP60 genes in different tissues of pepper
To further explore the role of CaHSP60 genes in growth and development of pepper, we conducted in silico tissue-specific analysis in vegetative (roots, stems, and leaves) and reproductive parts (seven different developmental stages of pericarp and placenta) through publically available RNA-Seq data (Kim et al. 2014). It can be seen in the heat map (Fig. 10a), pepper HSP60 genes exhibited a differential expression in various tissues and developmental stages. CaHSP60-12 and -14 showed the lowest expression, while CaHSP60-1,-5 and -16 showed a higher expression in almost all the tested tissues and stages. Since the HSP60 gene family is stress-responsive and the in silico transcriptomic analysis were conducted at normal condition, R9 pepper plants were also exposed to 2 h heat treatment at 42 °C. As shown in Fig. 10b, most of the genes showed higher expression in many of the tested tissues. Flower buds exhibited the highest CaHSP60 genes expression, followed by stems and leaves, while roots had the lowest expression level. In addition, CaHSP60-3 showed the lowest genes expression in almost all the tested tissues, while CaHSP60-16 was also down-regulated in leaves. In roots, CaHSP60-3, -8, -9, -10, and -14 showed the lowest expression, while maximum expression was recorded in leaves for CaHSP60-5, -6, and -11.
a In silico expression pattern of CaHSP60 genes in different tissues as derived from the database of pepper (CM334). The results were log2 transformed before generating heat maps in leaf, root, stem, 6, 16, and 25 day post-anthesis (6DPA, 16DPA, and 25DPA), mature green (MG), breaker (B), 5- and 10-day post-breaker (B5 and B10) of pericarp (PC) and placenta (PL). b Tissue-specific expression of CaHSP60 genes in the R9 pepper plant. The samples were collected after 2 h heat treatment at 42 °C from different parts root (R), stem (S), leaf (L), and flower (F) and were analyzed by qRT-PCR; the results were log2 transformed before generating heat maps
CaHSP60-6 knockdown impact on resistance to HS
To study the loss of function of CaHSP60-6 in pepper, VIGS was employed in pepper cultivar R9. To witness the success of the VIGS phenotypically, TRV2:CaPDS vector (positive control) was used for the silencing of the pepper PDS gene, which resulted in typical white color leaves considered as markers of the photo-bleached phenotypes. TRV2:00 (empty vector) was used as a negative control. After 6 weeks of infiltration, the CaPDS-treated plants showed a photo-bleached phenotype demonstrating the success of the VIGS (Fig. 11a). At that time, silencing efficiency was measured through qRT-PCR which confirmed that transcript level of TRV2:CaHSP60-6 was almost 72% lower than TRV2:00 (Fig. 11b). HS (42 °C) was applied to CaHSP60-6 silenced and control pepper plants and the transcript level was recorded after different HS exposure times (0, 0.5, 1, 2, 4, and 8 h) in the pepperline R9. The highest transcript level (20 folds) was recorded for HS on 2 h in the TRV2:CaHSP60-6 knock-downed plants as compared to (42 folds) in the control (empty vector TRV2:00) which corresponds to (52%) a lowered level in the silenced plants compared to control plants.
Phenotypes and of loss of function of CaHSP60-6 in the pepper cultivar R9. a Phenotypes of TRV2:00, TRV2:CaPDS, and TRV2:CaHSP60-6. b Relative expression of CaHSP60-6-silenced and control (TRV2:00) plants. c Phenotypes of CaHSP60-6 silenced and control plants before and after HS. d Relative expression of CaHSP60-6 in silenced and control plants was analyzed under HS (42 °C). Data represent mean ± SD of three biological replicates; letters a–f stands for the significant difference (P < 0.05)
Influence of CaHSP60-6 silencing on the physiological indices in response to HS
To see the effect of CaHSP60-6 knockdown in pepper plants, we measured the leaves water loss rate to the fresh weight, membrane integrity, chlorophyll contents, reactive oxygen species (ROS), and antioxidant enzymes in the leaves after HS. Water loss assay indicated a higher water loss (30%) in silenced plants compared to (22%) control pepper plants after 8 h of HS (Fig. 12a). A gradual increase in the relative electrolyte leakage was noted after HS, where excessive damage (> 22%) was recorded at 72 h in the silenced pepper plants leaves as compared to control plants. As photosynthetic efficiency is related to chlorophyll content (Dai et al. 2009), the chlorophyll contents were measured in CaHSP60-6 silenced and control pepper plants. In control pepper plants, a slight decrease in chlorophyll contents was noted until 24 h post-treatment, then a significant decline was recorded, whereas in the CaHSP60-6 silenced pepper plants, HS caused a significant decline in the chlorophyll content. A substantial reduction (almost 65%) in the chlorophyll contents at 24 h post-heat treatment in the silenced pepper plants was recorded as compared to control pepper plants (Fig. 12b, c). Malondialdehyde (MDA) and H2O2 contents both followed a similar trend of increase after HS in both the silenced and control pepper plants. MDA contents at 72 h after HS were significantly higher in the silenced plants compared to control pepper plants (Fig. 12d).
HS increased the accumulation of ROS as estimated by histochemical NBT and DAB staining; this accumulation was more apparent in the leaves of CaHSP60-6 silenced plants as compared to control pepper plants (Fig. 13a, b). Similarly, the measured H2O2 contents at 72 h were also significantly higher (7 folds) in the knockdown plants as compared to (5 folds) in the empty vector (control) pepper plants (Fig. 13c). Pepper plants activated the antioxidant enzyme system in response to stress to mitigate the ROS-associated damage. HS significantly induced the activity of superoxide dismutase; however, this increase in control plants was significantly higher than in silenced pepper plants. A similar trend was recorded for peroxidase (POD) activity which also increased significantly up to 48 h post-stress in both the silenced and control pepper plants and then decreased in activity at 72 h. However, the control pepper plants had a significantly higher POD activity as compared to CaHSP60-6 silenced plants (Fig. 13d, e).
Accumulation of ROS and comparison of enzymatic activity under HS in TRV2:CaHSP60-6 silenced and TRV2:00 pepper plants. a NBT leaves staining which showed the accumulation of O2. b DAB staining which shows H2O2 accumulation. c H2O2 contents. d Superoxide dismutase (SOD) activities. e Peroxidase (POD) activities. Error bars stand for SDs and a–f are representing significant variation (P < 0.05)
Discussion
Heat shock proteins genes are important stress-related genes that not only prevent protein aggregation and maintain non-native protein functional conformation and cell homeostasis under HS but also in various biotic and abiotic stresses. Heat shock proteins (HSPs) are grouped into different classes based on their molecular weight, such as HSP100, HSP90, HSP70s, HSP60s, and the small HSPs including HSP40, HSP20, and HSP10, respectively (Wang et al. 2004; Mittler 2006; Kotak et al. 2007).
Heat shock proteins (HSP60), also called chaperonins, cpn60, HSD1, or CCT with approximately 60 kDa of molecular weight, along with HSP70, are the most significant molecular chaperones that assist in folding and refolding of proteins under HS (Hartl et al. 2011; Balchin et al. 2016). Chaperonins have been little studied in plants; however, no studies have been conducted on identification, transcriptional regulation, and characterization of HSP60 gene family in pepper. Thus, the current study described the identification and analysis of 16 putative HSP60 genes in the pepper genome (Table 1). The investigation of their structure, evolutionary relationship, chromosomal organization, tissue-specific and dynamic expression profile under heat and other abiotic stresses in pepper provides a basis for further functional characterization of HSP60 genes in solanaceous and other crop species.
Structural analysis demonstrated that only a single gene had no intron, while more than 75% of genes had more than 10 introns (Fig. 1). ORF analysis of CaHSP60 genes revealed that the protein sequences were in the range from 502 (CaHSP60-1) to 640 (CaHSP60-16) amino acids, respectively. The phylogeny analysis (Fig. 3) showed that these sequences clustered into three groups based on sequence similarities and relatedness. Out of 16 peppers HSP60 genes, 8 genes were separated into group I, which constitute the T-complex polypeptide (TCP-1) group of the HSP60 gene family. This group is formed by motifs 3, 4, 6, 7, and 9 out of the 10 conserved motifs found. Group II forms the mitochondrial GroEL-like chaperonin60 group, while group III forms the chloroplast Rubisco large subunit-binding protein group of the HSP60 gene family. Group II and III contain all the 10 putative conserved motifs except for CaHSP60-12 which lacks the motif 8. Our classification of CaHSP60 genes into 3 groups in the phylogenetic tree and other physiochemical properties are consistent with the previous studies in foxtail millet (Singh et al. 2016), where the protein lengths of SiHSP60 proteins showed that SiHSP60-13 was the smallest (525 amino acids; 57.4 kDa), whereas SiHSP60-10 was the largest (655 amino acids; 70.94 kDa). Thus, possibly exists a similarity in structure and function due to the classification based on relatedness and homology of sequences and the presence of a core conserved Cpn60_TCP1 domain. However, the difference in gene structure and sequence length showed an evolutionary relationship among the CaHSP60 genes which employ that these genes share common ancestors and similar biological functions (Ishida et al. 2018). In the duplication events, we obtained a single (CaHSP60-3 and -12) tandem duplication and two (CaHSP60-13, -9 and CaHSP60-2, -11) segmental duplications. As compared to tandem, segmental duplication occurred more frequently due to polyploidy in most of the plants (Kim et al. 2007). Gene duplication plays an important role in the expansion of the gene family members and the evolutionary mechanism of the genome (Vision et al. 2000). Tandem duplication occurred mainly in genes encoding membrane and stress-related proteins (Cannon et al. 2004; Guo et al. 2015).
To know the possible role of HSP60 genes in pepper growth and development, we analyzed the tissue-specific expression pattern in different vegetative and reproductive tissues. First, we analyzed the publically available transcriptomic data (Kim et al. 2014), where most of the CaHSP60 genes showed no or low expression level in almost all the tested tissues except for CaHSP60-1 and -16 which showed a higher expression throughout the tested vegetative and reproductive tissues (Fig. 10a). As HSPs are stress-related genes, we tested the R9 plants with HS of 42 °C for 2 h and checked the transcriptional level through qRT-PCR (Guo et al. 2015, 2016). The results revealed that CaHSP60 genes were strongly induced by HS and were constitutively expressed in roots, stems, leaves, and flowers (Fig. 10b). Among the 16 tested genes, CaHSP60-5 and CaHSP60-6 possess more promising expression in all the tested tissues, whereas CaHSP60-3 did not respond to HS in all the tested tissues. Therefore, it was assumed that this gene possibly lacks a chaperone activity and could have some specific housekeeping activity. The same pattern was reported earlier (Sung et al. 2001; Guo et al. 2016), where some cytosolic HSP70 genes in Arabidopsis were responsible for housekeeping, but this needs to be more evidenced and studied. In all the tested tissues, a higher expression was recorded in flowers as compared to other vegetative parts, which is in agreement with Duck et al. (1989), who also recorded a tenfold higher expression for HSP70 in tomato flowers than in leaves. The same trend was also reported in the previous studies, where reproductive parts were more responsive than vegetative parts (Sung et al. 2001; Guo et al. 2015, 2016). Tissue-specific expression corresponds to characteristic-specific biological functions (Passarinho et al. 2001), and different transcriptional levels from in silico could be due to different cultivars and possibly some changed environmental conditions. The tissue-specific expression pattern of pepper HSP60 genes suggests their possible role in different biological processes along with a role as chaperones.
In current studies, we investigated and analyzed the dynamic expression profile of all the 16 HSP60 genes in pepper, in response to HS, in thermo-sensitive B6 and thermo-tolerant R9 lines. Almost all genes were induced by HS to varying levels of stresses in both pepper lines, indicating that CaHSP60 genes effectively reduced the damage from HS. It is established that the expression of downstream HSPs is regulated by binding of heat shock factors (HSF) to the heat shock elements (HSE) in the promoter region (Miller and Ron 2006). Cis-regulatory elements analysis showed that CaHSP60s contain various stress-related cis-acting elements along with HSE (Fig. 6). Many of the HSP60 genes were instantly induced on HS (0.5 h) and their expression levels reached to maximum at 2 h after HS and then lowered at 8 h, indicating that CaHSP60s may interact with co-chaperones HSP10 which help to mitigate aggregation of stress-denatured proteins and to refolding of non-native proteins functional conformation as an HS response (Guo et al. 2015). Intriguingly, the expression pattern of CaHSP60 genes was genotype specific; the thermo-tolerant line started a thermal response quicker at 0.5 h HS exposure as compared to the thermo-sensitive pepper line. The same trend was also reported in other HSPs such as HSP70, HSP20 (Guo et al. 2015, 2016), and HSP40 (Fan et al. 2017). The increased expression under HS was also noted in the genes which do not have the HSE, because the heat shock response (HSR) is a complex process, which is also regulated by DREB 2A (Sakuma et al. 2006). HSP gene expression is also associated with small HSPs, i.e., HSP10, in case of chaperonin family, which post-transcriptionally binds to many proteins that have been transported to many of cell organelles (Wang et al. 2004; Hartl et al. 2011; Balchin et al. 2016; Ishida et al. 2018).
Based on gene ontology (GO) analysis, which revealed that this gene family is also responsive in ATP-dependent manner to protein folding and refolding along with response to heat and other abiotic stresses (Fig. 5), we extended our studies to investigate the expression of nine representative CaHSP60 genes to other abiotic and hormonal stresses. The results revealed that almost all the candidate genes were induced by these stresses except CaHSP60-3 which was down-regulated in response to cold and NaCl stresses. The response of CaHSP60 genes to multiple abiotic and hormonal stresses could be attributed to various stress-related cis-acting elements such as LTR, MBS, TCA elements, ABRE, and TC-rich repeats in the promoter region of the genes (Fig. 6). Few earlier studies also reported the role of HSP60 in different abiotic stress responses, such as Arabidopsis chloroplast HSP60 not only responded in normal condition but also under high temperature and drought situation (Xu and Huang 2010). Likewise, HSP60 and HSP21 in sunflower were reported to undergo down-regulation upon cold stress (Balbuena et al. 2011); a similar trend was also reported in winter wheat, where Rubisco stability was associated with down-regulation of HSP60 and 21 (Rinalducci et al. 2011). HSP40 in rice (Wang et al. 2018) and poplar HSP100 (-21, -75), HSP90 (-9, -12), HSP60 (-31, -33, -38, -49), HSP40 (-113,-117), and HSP21 were also up-regulated under salt stress (Yer et al. 2018). Similarly, soybean proteomics studies showed a differential expression for (HSP90, chloroplast HSP70, HSP60, and HSP20) under salt stress (Komatsu et al. 2011).
To affirm the functional role of CaHSP60-6, this gene was successfully knockdown through VIGS, which was confirmed through photo-bleached phenotype in TRV2: CaPDS-treated pepper plants and through gene expression in TRV2:CaHSP60-6 silenced and control plants. Expression analysis of CaHSP60-6 after HS showed a substantially lowered expression in the silenced plants as compared to control plants (Fig. 11b, d), while expression of all other HSP60 genes after VIGS at control and HS showed no significant variation in the expression pattern, which showed that only the target gene was silenced (Fig. S1). Water losses in the CaHSP60-6 silenced pepper excised leaves were significantly higher than in control plants (Fig. 12a). Plants will tend to transpire more water through stomata, to cope with the HS, but at the same time will close stomata in ABA-dependent way to conserve more water. The ability of a plant to respond to stress situations depends on its ability to restrict water loss through the leaf epidermis after stomata attain a minimum aperture. The increased water loss in the silenced pepper leaves could be a non-stomatally controlled water loss through the leaf epidermis; epidermal or residual transpiration also share substantial water loss in stress situations (Augustine et al. 2015). Earlier studies on alfalfa also reported that MsHSP70 was induced by ABA and conferred drought tolerance (Li et al. 2017). Cho and Hong (2006) reported that NtHSP70 overexpression in tobacco contributed to drought stress and maintained leaf water potential by up-regulation of dehydration-related genes such as ERD and DHNs. Similarly, a significantly higher membrane damage was observed in the CaHSP60-6 silenced pepper plants than in control plants. HSP60 genes are reported to be involved in membrane stabilization as lipochaperonin that prevented the irreversible thermal aggregation and assisted the refolding of membrane proteins. Similarly, water-soluble proteins were safeguarded by lipochaperonins under stress situations (Török et al. 1997). The cell membrane is the first cellular structure which acts as the first line of defense encountering the external stresses and transducing it into signaling pathways (Nakamoto and Vigh 2007). The membrane integrity and electrolyte leakage assay were used to estimate thermo-tolerance in potato (Savic et al. 2012). Small HSPs played a vital role in maintaining the membrane integrity by functioning as part of the multi-chaperone network as well as a membrane stabilizing factor being part of the lipid (Nakamoto and Vigh 2007).
Chlorophyll contents (Dai et al. 2009) were significantly decreased in the knockdown pepper plants as compared to control plants. Lowered chlorophyll contents after HS could be attributed to chlorophyll degradation enzymes such as chlorophyllase (Schelbert et al. 2009) and chlorophyll degrading peroxidases (Yamauchi et al. 2004) which degrade chlorophyll in the presence of H2O2 and phenolic compounds. HSP100 in Arabidopsis has been reported to be involved in chloroplast development and chlorophyll accumulation (Lee et al. 2007).
In our study, H2O2 and malondialdehyde contents were significantly higher in CaHSP60-6 silenced plants as compared to control pepper plants. ROS were estimated in pepper leaves by NBT and DAB staining and H2O2 was chemically quantified (Fig. 13a–c), which indicated that pepper plants exhibited low resilience to HS after CaHSP60-6 silencing. ROS include free radicals such as superoxide anion (O ·−2 ), hydroxyl radical (·OH), and non-radicals such as H2O2 and singlet oxygen (1O2) which were produced under normal growth and development in various cell organelles (Choudhury et al. 2017). Multilevel of interaction between HSP and ROS exists; plants are wise enough to use a low concentration of ROS as a signal molecule to produce HSP and other stress-related proteins (Lavania et al. 2015). However, a higher concentration of ROS, as a result of stress conditions, causes oxidation of proteins, peroxidation of lipids, and damage to nuclear materials which ultimately leads to cell death (Uzilday et al. 2012). Thus, plants maintain an equilibrium between the ROS production and elimination (Miller et al. 2007).
Plants have evolved the antioxidant defense system to detoxify ROS which consists of non-enzymatic and antioxidant enzymes such as superoxide dismutase (SOD) and peroxidase (POD) which detoxify ROS through scavenging and protect plants from oxidative stress (Choudhury et al. 2017). Among enzymes, superoxide radical (O2−) is dismutated by SOD into H2O2, which is further scavenged by POD through converting into H2O (Alam et al. 2018). Antioxidant enzyme activities were significantly lowered in the TRV2:CaHSP60-6 silenced plants as compared to TRV2:00 pepper plants after HS, which could be attributed not only to HSP60 genes induce antioxidant enzymes synthesis, but also maintained their function through protecting their structure as chaperone (Qi et al. 2019).
Previous studies also reported a similar trend of reduced resistance to abiotic stresses after knockdown of stress-related genes in pepper (Ali et al. 2019; Feng et al. 2019). Taken together, these results suggest that CaHSP60-6 might act as a positive regulator in the defense of pepper against heat and other abiotic stresses. This study will provide further insights in the functional analysis of HSP60 genes in solanaceous and other crop species for adaptability to various stress conditions.
Author contribution statement
SuH and Z-HG conceived the study. SuH and AK collected the data and performed the experiment. MA performed the data analysis. W-XG performed the supplementary experiment during revision. SuH drafted the manuscript. H-XZ, Q-HY and S-BY reviewed and edited the manuscript. A-MW and Z-HG provided reagents, materials and analysis tools. All authors critically reviewed, read and approved the final manuscript.
Abbreviations
- DAB:
-
Diaminobenzidine
- HS:
-
Heat stress
- HSP:
-
Heat shock protein
- MeJA:
-
Methyl jasmonate
- NBT:
-
Nitro-blue tetrazolium
- PDS:
-
Phytoene desaturase
- ROS:
-
Reactive oxygen species
- SA:
-
Salicylic acid
- TRV:
-
Tobacco rattle virus
- VIGS:
-
Virus-induced gene silencing
References
Ahuja I, de Vos RCH, Bones AM, Hall RD (2010) Plant molecular stress responses face climate change. Trends Plant Sci 15:664–674
Alam MN, Zhang L, Yang L et al (2018) Transcriptomic profiling of tall fescue in response to heat stress and improved thermotolerance by melatonin and 24-epibrassinolide. BMC Genom 19:224. https://doi.org/10.1186/s12864-018-4588
Ali M, Luo D-X, Khan A et al (2018) Classification and genome-wide analysis of chitin-binding proteins gene family in pepper (Capsicum annuum L.) and transcriptional regulation to phytophthora capsici, abiotic stresses and hormonal applications. Int J Mol Sci 19:2216. https://doi.org/10.3390/ijms19082216
Ali M, Gai W-X, Khattak AM et al (2019) Knockdown of the chitin-binding protein family gene CaChiIV1 increased sensitivity to Phytophthora capsici and drought stress in pepper plants. Mol Genet Genom. https://doi.org/10.1007/s00438-019-01583-7
Al-whaibi MH (2011) Plant heat-shock proteins: a mini review. J King Saud Univ Sci 23:139–150. https://doi.org/10.1016/j.jksus.2010.06.022
Augustine SM, Cherian AV, Syamaladevi DP, Subramonian N (2015) Erianthus arundinaceus HSP70 (EaHSP70) acts as a key regulator in the formation of anisotropic interdigitation in sugarcane (Saccharum spp. hybrid) in response to drought stress. Plant Cell Physiol 56:2368–2380
Balbuena TS, Salas JJ, Martínez-Force E et al (2011) Proteome analysis of cold acclimation in sunflower. J Proteome Res 10:2330–2346
Balchin D, Hayer-Hartl M, Hartl FU (2016) In vivo aspects of protein folding and quality control. Science 353:aac4354. https://doi.org/10.1126/science.aac4354
Campos PS, Quartin V, Ramalho JC, Nunes MA (2003) Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. plants. J Plant Physiol 160:283–292. https://doi.org/10.1078/0176-1617-00833
Cannon SB, Mitra A, Baumgarten A et al (2004) The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol 4:10
Cheng L, Zou Y, Ding S et al (2009) Polyamine accumulation in transgenic tomato enhances the tolerance to high temperature stress. J Integr Plant Biol 51:489–499
Cho EK, Hong CB (2006) Over-expression of tobacco NtHSP70-1 contributes to drought-stress tolerance in plants. Plant Cell Rep 25:349–358. https://doi.org/10.1007/s00299-005-0093-2
Choudhury FK, Rivero RM, Blumwald E, Mittler R (2017) Reactive oxygen species, abiotic stress and stress combination. Plant J 90:856–867. https://doi.org/10.1111/tpj.13299
Dai Y, Shen Z, Liu Y et al (2009) Effects of shade treatments on the photosynthetic capacity, chlorophyll fluorescence, and chlorophyll content of Tetrastigma hemsleyanum Diels et Gilg. Environ Exp Bot 65:177–182. https://doi.org/10.1016/j.envexpbot.2008.12.008
Deng W, Wang Y, Liu Z et al (2014) HemI: a toolkit for illustrating heatmaps. PLoS One 9:e111988. https://doi.org/10.1371/journal.pone.0111988
Duck N, McCormick S, Winter J (1989) Heat shock protein 70 cognate expression in vegetative and reproductive organs of Lycopersicon esculentum. Proc Natl Acad Sci USA 86:3674–3678
Fan F, Kang Y, Yang X et al (2017) The DnaJ gene family in pepper (Capsicum annuum L.): comprehensive identification, characterization and expression profiles. Front Plant Sci 8:1–11. https://doi.org/10.3389/fpls.2017.00689
Feng X, Zhang H, Ali M et al (2019) A small heat shock protein CaHsp25.9 positively regulates heat, salt, and drought stress tolerance in pepper (Capsicum annuum L.). Plant Physiol Biochem 142:151–162. https://doi.org/10.1016/j.plaphy.2019.07.001
Gasteiger E, Gattiker A, Hoogland C et al (2003) ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res 31:3784–3788
Gu Z, Cavalcanti A, Chen F-C et al (2002) Extent of gene duplication in the genomes of Drosophila, nematode, and yeast. Mol Biol Evol 19:256–262
Guo WL, Chen RG, Gong ZH et al (2012) Exogenous abscisic acid increases antioxidant enzymes and related gene expression in pepper (Capsicum annuum) leaves subjected to chilling stress. Genet Mol Res 11:4063–4080
Guo M, Liu J-H, Lu J-P et al (2015) Genome-wide analysis of the CaHsp20 gene family in pepper: comprehensive sequence and expression profile analysis under heat stress. Front Plant Sci 6:806
Guo M, Liu JH, Ma X et al (2016) Genome-wide analysis of the Hsp70 family genes in pepper (Capsicum annuum L.) and functional identification of CaHsp70-2 involvement in heat stress. Plant Sci 252:246–256. https://doi.org/10.1016/j.plantsci.2016.07.001
Hartl FU, Bracher A, Hayer-Hartl M (2011) Molecular c haperones in protein folding and proteostasis. Nature 475:324–332. https://doi.org/10.1038/nature10317
Horton P, Park K-J, Obayashi T et al (2007) WoLF PSORT: protein localization predictor. Nucleic Acids Res 35:W585–W587
Huang L, Cheng G, Khan A et al (2018) CaHSP16.4, a small heat shock protein gene in pepper, is involved in heat and drought tolerance. Protoplasma 256:39. https://doi.org/10.1007/s00709-018-1280-7
Ishida R, Okamoto T, Motojima F et al (2018) Physicochemical properties of the mammalian molecular chaperone HSP60. Int J Mol Sci 19:e489. https://doi.org/10.3390/ijms19020489
Jungkunz I, Link K, Vogel F et al (2011) AtHsp70-15-deficient Arabidopsis plants are characterized by reduced growth, a constitutive cytosolic protein response and enhanced resistance to TuMV. Plant J 66:983–995
Kang W-H, Kim S, Lee H-A et al (2016) Genome-wide analysis of Dof transcription factors reveals functional characteristics during development and response to biotic stresses in pepper. Sci Rep 6:33332
Khan A, Li R-J, Sun J-T et al (2018) Genome-wide analysis of dirigent gene family in pepper (Capsicum annuum L.) and characterization of CaDIR7 in biotic and abiotic stresses. Sci Rep 8:5500. https://doi.org/10.1038/s41598-018-23761-0
Kim H-J, Hwang NR, Lee K-J (2007) Heat shock responses for understanding diseases of protein denaturation. Mol Cells (Springer Sci Bus Media BV) 23:123–131
Kim S, Park M, Yeom SI et al (2014) Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum sp. Nat Genet 46:270–278. https://doi.org/10.1038/ng.2877
Komatsu S, Yamamoto A, Nakamura T et al (2011) Comprehensive analysis of mitochondria in roots and hypocotyls of soybean under flooding stress using proteomics and metabolomics techniques. J Proteome Res 10:3993–4004
Kotak S, Larkindale J, Lee U et al (2007) Complexity of the heat stress response in plants. Curr Opin Plant Biol 10:310–316
Lavania D, Dhingra A, Siddiqui MH et al (2015) Current status of the production of high temperature tolerant transgenic crops for cultivation in warmer climates. Plant Physiol Biochem 86:100–108
Lee U, Rioflorido I, Hong S et al (2007) The Arabidopsis ClpB/Hsp100 family of proteins: chaperones for stress and chloroplast development. Plant J 49:115–127
Lescot M, Déhais P, Thijs G et al (2002) PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res 30:325–327
Li Z, Long R, Zhang T et al (2017) Molecular cloning and functional analysis of the drought tolerance gene MsHSP70 from alfalfa (Medicago sativa L.). J Plant Res 130:387–396
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592. https://doi.org/10.1042/bst0110591
Liu RH, Meng JL (2003) MapDraw: a microsoft excel macro for drawing genetic linkage maps based on given genetic linkage data. Yi Chuan Hered 25:317–321
Liu Z, Liu Y, Shi L et al (2016) SGT1 is required in PcINF1/SRC2-1 induced pepper defense response by interacting with SRC2-1. Sci Rep 6:21651
Miller G, Ron Mittler (2006) Could heat shock transcription factors function as hydrogen peroxide sensors in plants? Ann Bot 98:279–288. https://doi.org/10.1093/aob/mcl107
Miller G, Suzuki N, Rizhsky L et al (2007) Double mutants deficient in cytosolic and thylakoid ascorbate peroxidase reveal a complex mode of interaction between reactive oxygen species, plant development, and response to abiotic stresses. Plant Physiol 144:1777–1785. https://doi.org/10.1104/pp.107.101436
Mittler R (2006) Abiotic stress, the field environment and stress combination. Trends Plant Sci 11:15–19
Nakamoto H, Vigh L (2007) The small heat shock proteins and their clients. Cell Mol Life Sci 64:294–306
Passarinho PA, Van Hengel AJ, Fransz PF, de Vries SC (2001) Expression pattern of the Arabidopsis thaliana AtEP3/AtchitIV endochitinase gene. Planta 212:556–567
Prasad TK, Hack E, Hallberg RL (1990) Function of the maize mitochondrial chaperonin hsp60: specific association between hsp60 and newly synthesized F1-ATPase alpha subunits. Mol Cell Biol 10:3979–3986
Qi C, Lin X, Li S et al (2019) SoHSC70 positively regulates thermotolerance by alleviating cell membrane damage, reducing ROS accumulation, and improving activities of antioxidant enzymes. Plant Sci 283:385–395. https://doi.org/10.1016/j.plantsci.2019.03.003
Qin C, Yu C, Shen Y et al (2014) Whole-genome sequencing of cultivated and wild peppers provides insights into Capsicum domestication and specialization. Proc Natl Acad Sci USA 111:5135–5140
Rinalducci S, Egidi MG, Mahfoozi S et al (2011) The influence of temperature on plant development in a vernalization-requiring winter wheat: a 2-DE based proteomic investigation. J Proteom 74:643–659
Saibil HR, Fenton WA, Clare DK, Horwich AL (2013) Structure and allostery of the chaperonin GroEL. J Mol Biol 425:1476–1487. https://doi.org/10.1016/j.jmb.2012.11.028
Sakuma Y, Maruyama K, Qin F et al (2006) Dual function of an Arabidopsis transcription factor DREB2A in water-stress-responsive and heat-stress-responsive gene expression. Proc Natl Acad Sci USA 103:18822–18827
Savić J, Dragićević I, Pantelić D et al (2012) Expression of small heat shock proteins and heat tolerance in potato (Solanum tuberosum L.). Arch Biol Sci (Belgrade) 64:135–144. https://doi.org/10.2298/abs1201135s
Schelbert S, Aubry S, Burla B et al (2009) Pheophytin pheophorbide hydrolase (pheophytinase) is involved in chlorophyll breakdown during leaf senescence in Arabidopsis. Plant Cell 21:767–785. https://doi.org/10.1105/tpc.108.064089
Sergiev I, Alexieva V, Karanov E (1997) Effect of spermine, atrazine and combination between them on some endogenous protective systems and stress markers in plants. Proc Bulg Acad Sci 51:121–124
Singh RK, Jaishankar J, Muthamilarasan M et al (2016) Genome-wide analysis of heat shock proteins in C 4 model, foxtail millet identifies potential candidates for crop improvement under abiotic stress. Sci Rep 6:32641
Su Y, Xu L, Fu Z et al (2014) ScChi, encoding an acidic class III chitinase of sugarcane, confers positive responses to biotic and abiotic stresses in sugarcane. Int J Mol Sci 15:2738–2760
Sung D, Kaplan F, Guy CL (2001) Plant Hsp70 molecular chaperones: protein structure, gene family, expression and function. Physiol Plant 113:443–451
Tan W, Wei Meng Q, Brestic M et al (2011) Photosynthesis is improved by exogenous calcium in heat-stressed tobacco plants. J Plant Physiol 168:2063–2071
Török Z, Horváth I, Goloubinoff P et al (1997) Evidence for a lipochaperonin: association of active protein-folding GroESL oligomers with lipids can stabilize membranes under heat shock conditions. Proc Natl Acad Sci USA 94:2192–2197. https://doi.org/10.1073/pnas.94.6.2192
Tubiello FN, Soussana J-F, Howden SM (2007) Crop and pasture response to climate change. Proc Natl Acad Sci USA 104:19686–19690
Uzilday B, Turkan I, Sekmen AH et al (2012) Comparison of ROS formation and antioxidant enzymes in Cleome gynandra (C4) and Cleome spinosa (C3) under drought stress. Plant Sci 182:59–70. https://doi.org/10.1016/j.plantsci.2011.03.015
Vision TJ, Brown DG, Tanksley SD (2000) The origins of genomic duplications in Arabidopsis. Science 290:2114–2117
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223. https://doi.org/10.1016/j.envexpbot.2007.05.011
Wang L-J, Li S-H (2006) Salicylic acid-induced heat or cold tolerance in relation to Ca2+ homeostasis and antioxidant systems in young grape plants. Plant Sci 170:685–694
Wang W, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci 9:244–252
Wang Y, Lin S, Song Q et al (2014) Genome-wide identification of heat shock proteins (Hsps) and Hsp interactors in rice: Hsp70s as a case study. BMC Genom 15:344
Wang X, Zhang H, Shao L-Y et al (2018) Expression and function analysis of a rice OsHSP40 gene under salt stress. Genes Genom 11:1–8. https://doi.org/10.1007/s1325
Xu C, Huang B (2010) Comparative analysis of drought responsive proteins in Kentucky bluegrass cultivars contrasting in drought tolerance. Crop Sci 50:2543–2552
Yamauchi N, Funamoto Y, Shigyo M (2004) Peroxidase-mediated chlorophyll degradation in horticultural crops. Phytochem Rev 3:221–228. https://doi.org/10.1023/B:PHYT.0000047796.98784.06
Yer EN, Baloglu MC, Ayan S (2018) Identification and expression profiling of all Hsp family member genes under salinity stress in different poplar clones. Gene 678:324–336
Yin Y-X, Guo W-L, Zhang Y-L et al (2014) Cloning and characterisation of a pepper aquaporin, CaAQP, which reduces chilling stress in transgenic tobacco plants. Plant Cell Tissue Organ Cult 118:431–444
Young LW, Wilen RW, Bonham-Smith PC (2004) High temperature stress of Brassica napus during flowering reduces micro-and megagametophyte fertility, induces fruit abortion, and disrupts seed production. J Exp Bot 55:485–495
Zhang H-X, Jin J-H, He Y-M et al (2016) Genome-wide identification and analysis of the SBP-box family genes under Phytophthora capsici stress in pepper (Capsicum annuum L.). Front Plant Sci 7:504
Funding
This work was supported through funding from the National Natural Science Foundation of China (no. U1603102) and National Key R&D Program of China (no. 2016YFD0101900)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Expression pattern of pepper HSP60 genes under HS treatment (42 °C) at 0, 2, and 8 h in the TRV2:CaHSP60-6 and TRV2:00 pepper R9 plants after VIGS to check the silencing specificity of CaHSP60-6 knockdown. Mean values and SDs are for three replicates, while letters (a–c) represent the significant differences at P < 0.05 (TIFF 80574 kb)
Table S1
Primers for qRT-PCR for CaHSP60 genes (DOCX 15 kb)
Table S2
Primers for gene sequencing and confirmation (DOCX 15 kb)
Table S3
Ten conserved motifs found in pepper HSP60 proteins (DOCX 504 kb)
Table S4
List of Cpn60_TCP1 domain, e value, formulas and total number of atoms of HSP60 genes in pepper (DOCX 16 kb)
Table S5
Detail of the cis-acting elements found in the promoter region of CaHSP60s (DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Haq, S.u., Khan, A., Ali, M. et al. Knockdown of CaHSP60-6 confers enhanced sensitivity to heat stress in pepper (Capsicum annuum L.). Planta 250, 2127–2145 (2019). https://doi.org/10.1007/s00425-019-03290-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-019-03290-4