Abstract
Main conclusion
For the first time we provide a study on the physiological, ultrastructural and molecular effects of salt stress on a terrestrial symbiotic green microalga, Trebouxia sp. TR9.
Although tolerance to saline conditions has been thoroughly studied in plants and, to an extent, free-living microalgae, scientific data regarding salt stress on symbiotic lichen microalgae is scarce to non-existent. Since lichen phycobionts are capable of enduring harsh, restrictive and rapidly changing environments, it is interesting to study the metabolic machinery operating under these extreme conditions. We aim to determine the effects of prolonged exposure to high salt concentrations on the symbiotic phycobiont Trebouxia sp. TR9, isolated from the lichen Ramalina farinacea. Our results suggest that, when this alga is confronted with extreme saline conditions, the cellular structures are affected to an extent, with limited chlorophyll content loss and photosynthetic activity remaining after 72 h of exposure to 5 M NaCl. Furthermore, this organism displays a rather different molecular response compared to land plants and free-living halophile microalgae, with no noticeable increase in ABA levels and ABA-related gene expression until the external NaCl concentration is raised to 3 M NaCl. Despite this, the ABA transduction pathway seems functional, since the ABA-related genes tested are responsive to exogenous ABA. These observations could suggest that this symbiotic green alga may have developed alternative molecular pathways to cope with highly saline environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lichens are complex entities (holobionts) resulting from cyclical symbiotic association between fungi (mycobiont), cyanobacteria (cyanobiont) and/or green microalgae (phycobiont) (Margulis and Barreno 2003), bacterial consortia (Aschenbrenner et al. 2016; Biosca et al. 2016) and yeasts (Spribille et al. 2016; Duarte et al. 2016). This successful partnership has allowed them to colonise a wide range of habitats on Earth, from the polar areas to dry environments such as arid deserts (Green et al. 2012; Maphangwa et al. 2012). These symbioses involve nearly 20% of all fungi species (Kirk et al. 2001), and hundreds of cyanobacterial and green microalgal species (Leavitt et al. 2015; Moya et al. 2017).
This widespread nature relies on the ability to adapt to challenging environmental conditions, from desiccation to temperature. Examples of extreme habitats lichens have colonised are sea shores, where exposure to salinity is constant, thus prompting halotolerant adaptations to endure these conditions (Grube and Blaha 2005; Armstrong 2017).
Halotolerance in lichens has been thoroughly studied in seashore environments, where species such as Caloplaca thallincola, Hydropunctaria maura, Collemopsidium halodytes or Lichina pygmaea have developed physiological strategies to cope with haline stress (Arup 1995; Delmail et al. 2013). Lichens growing inland, away from tidal influences, show a greater sensitivity to salt exposure than their seashore counterparts (Nash and Lange 1988; Malaspina et al. 2015). However, individuals belonging to the genus Ramalina have demonstrated no intra specific variation in growth or development when exposed to high NaCl levels in both marine and non-marine species (Takahagi et al. 2002). Ramalina farinacea is a relatively abundant species in Mediterranean, subtropical and temperate areas (Casano et al. 2011). This fruticose lichen grows inland, away from any maritime influence, but displays a remarkable capability to withstand extreme desiccation (Álvarez et al. 2015).
Ramalina farinacea harbours two main phycobionts belonging to the genus Trebouxia, T. jamesii and Trebouxia sp. TR9, the latter displaying a superior performance under relatively high temperatures and irradiances (Casano et al. 2011; Álvarez et al. 2015). Trebouxia is a genus of phycobiont found in symbiosis with at least 35% of all lichens, but is seldom found free-living (Škaloud and Peksa 2010). This microalga displays a remarkable endurance to abiotic stress, from being exposed to outer space (Brandt et al. 2016) to rapid desiccation (Gasulla et al. 2009). In particular, Trebouxia sp. TR9 displays a superior performance under relatively high temperatures and irradiances compared to T. jamesii (Casano et al. 2011). All these conditions imply a sudden and prolonged water loss within the cell, akin to what is experienced in organisms exposed to haline environments. This makes Trebouxia sp. TR9 a suitable candidate to experience the effects of prolonged salt stress. Moreover, the recent availability of the annotated genome of this alga (Martínez-Alberola 2015) allows additional approaches, such as gene-expression studies, to unravel its molecular response to environmental changes.
Studies on tolerance to high saline stress conditions in microalgae have been performed mainly on individuals belonging to the halophile green microalgae genus Dunaliella (Takagi and Karseno 2006; Mishra and Jha 2009), capable of surviving extreme NaCl concentrations (5 M) for a prolonged time span; and in some species of free-living Trebouxiophyceae microalgae such as Chlorella, in both water bound (Campenni et al. 2013) and terrestrial biofilms (Gustavs et al. 2010). However, there is a lack of available data for similar studies regarding symbiotic green microalgae.
Abscisic acid (ABA) is a phytohormone which is widely acknowledged to play a fundamental role in cold, osmotic and saline stress responses in land plants (Wani et al. 2016), with an increased production of ABA under salinity conditions (Zhang et al. 2006). The presence of ABA has been detected in many organisms, from bacteria to fungi (Hauser et al. 2011). However, its role in the response against stress in other organisms apart from land plants is less understood. Regarding algae, ABA has been found in the vast majority of species tested for its presence (Hirsch et al. 1989; Jameson 1993; Lu and Xu 2015). There is limited available data on the effect of salt stress on algal ABA production; this being mainly focused on the Dunaliella species (Tietz and Kasprik 1986; Hirsch et al. 1989; Cowan et al. 1992) and Chlorella vulgaris (Maršálek et al. 1992). In every case, ABA levels increased markedly under salt stress, a behaviour similar to that observed in land plants, which could suggest the possible active involvement of this hormone in the salt stress response in algae. However, these effects have never been tested on terrestrial, symbiotic microalgae.
In this work, we aim to determine the impact of high to extreme salt concentrations for a prolonged time span on isolated phycobionts of Trebouxia sp. TR9, focusing on photosynthesis performance, pigment composition and ultrastructural changes, as well as studying the possible involvement of the phytohormone ABA in the response to salt stress in symbiotic microalgae.
Materials and methods
Sample collection
Ramalina farinacea thalli growing on the bark of Quercus rotundifolia oak trees were collected in Sierra del Toro (39°57′ 32.34″N–0°46′ 35.51″W), Castellón, Spain, at an altitude of 1000 m above sea level.
Isolation and phycobiont culture
Monoclonal cultures of Trebouxia sp. TR9 from thalli were isolated according to the protocol developed by Gasulla et al. (2010) and spread by triple-streak on a Petri dish containing axenic 3 × N Bold’s Basal Medium (3N-BBM) (Bischoff and Bold 1963). Fast molecular microalgae identification was carried out according to Molins et al. (2018).
Algal colonies were selected and transferred to culture media 3N-BBM supplemented with glucose (20 g l−1) and casein (10 g l−1), both in liquid and axenic media. Cultures were kept in a growth chamber at 20 ± 1 °C with light: darkness cycles of 12 h each (light intensity: 50 μmol), until an appropriate level of biomass was reached. The phycobionts were then cultured on Petri dishes with semi-solid media 3N-BBM supplemented with glucose and casein using 5 acetate discs per dish. 75 μl of suspended Trebouxia sp. TR9 were applied to each disc and then cultured in the growing chamber under the stated conditions for 21 days. Simultaneously, the same amount of phycobionts was transferred to culture tubes with liquid media 3N-BBM supplemented with glucose and casein and left to grow for 21 days under environmental conditions akin to axenic cultures.
Solid medium saline stress treatments
Acetate discs containing the grown algae (21-day-old) were transferred to several Petri dishes with semi-solid media 3 N-BBM with glucose and casein supplemented with NaCl. Five treatments were applied in function of the NaCl concentration (0, 1, 2, 3 and 5 M NaCl), 0 M NaCl serving as the control treatment. Discs were transferred to each treatment and were incubated for 72 h at 20 ± 1 °C with light: darkness cycles of 12 h each (light intensity: 50 μmol). Finally, some discs were then collected, weighted and stored at − 80 °C. Other discs were fixed and used for microscopic examinations. Some other discs were used for fluorescence analyses.
Liquid medium saline stress treatments
Culture tubes containing 21-day-old grown phycobionts were centrifuged and the supernatant was discarded. The obtained pellets were diluted with liquid media 3N-BBM with glucose and casein and supplemented with variable concentrations of NaCl depending on the treatment applied. Five treatments were applied (0, 1, 2, 3 and 5 M NaCl), 0 M NaCl serving as the control treatment. Five tubes were used per treatment and were incubated for 72 h at 20 ± 1 °C with light:darkness cycles of 12 h each (light intensity: 50 μmol). The tubes were then centrifuged and the supernatant was discarded. The pellet was collected and stored at − 80 °C.
Exogenous ABA treatments
100 µl of ABA diluted in distilled water at different concentrations (0, 1, 10 and 100 µM) were applied to three acetate discs containing the 21-day-old grown algae per treatment. Samples were incubated for 6 h at 20 ± 1 °C with light, and then collected, weighed and stored at − 80 °C
Microscopic examinations
Transmission electron microscopy (TEM) samples were prepared as described by Molins et al. (2018). In brief, samples were fixed in 2% Karnovsky fixative for 12 h at 4 °C, washed three times for 15 min with 0.01 M PBS (pH 7.4), and postfixed with 2% OsO4 in 0.01 M PBS (pH 7.4) for 2 h at room temperature. After washing in buffer, the samples were dehydrated at room temperature in a graded series of ethanol, and embedded in Spurr-resin.
Semi-thin sections were cut with a diamond knife (Diatome Histo 45°; Diatome, Hatfield, PA, USA) and an ultramicrotome (Ultratome Nova LKB Bromma) (~ 1.5 μm) and stained with 1% toluidine blue (Ruzin 2000). All observations were carried out using an Olympus Provis AX70 fluorescence microscope equipped with an infinity 2–3C Lumenera® digital camera and analysed with “Infinity Analyze” Software v.6.4.1.
Ultra-thin sections, 80 nm thick, were made with a diamond knife (Diatome ultra 458), mounted on 100 mesh copper grids, and then stained with 10% uranyl acetate and 0.1% lead citrate using the ‘Synaptek Grid-Stick Kit’. Sections were observed at 80 kV under a JEOL JEM-1010 microscope (Jeol, Peabody, MA, USA). Images were obtained using an Olympus MegaView III camera and processed by Olympus analysis getIT software (Olympus Corp., Tokyo, Japan).
Fluorescence analysis
In-vivo fluorescence was measured at room temperature with a pulse-modulated fluorometer (PAM 2000, Walz, Effeltrich, Germany). Each experimental condition was measured in triplicate, for independent samples weighing between 160 and 200 mg (FW). All the samples were kept in the dark for 30 min before measurements took place. The minimum fluorescence (F0) was determined by application of a weak measurement beam to the microalgae. The maximum fluorescence yield (Fm) was obtained with an 800 ms saturating pulse of white light in contact with the sample (SP, 8000 μmol m−2 s−1). The variable fluorescence (Fv) was calculated as Fm − F0. The maximum potential quantum efficiency of photosystem II (PSII) was estimated as Fv/Fm (Guéra et al. 2004).
Pigment quantification
Weighed samples were soaked in liquid nitrogen and ground with the help of a pestle to disrupt the cell wall. Each sample was put, separately, into 100% acetone (50 ml g−1) and homogenized in a vortex for 1 min, before being placed in a Cole Parmer Rotor-Torque for 24 h at 4 °C at maximum speed. Samples were then centrifuged at 8000g for 5 min and the supernatant transferred to a new tube.
Quantification of chlorophyll and carotenoids was performed according to the Wellburn and Lichtenthaler (1984) formulae as shown below. Absorbances were measured on a Shimadzu UV-1603 spectrophotometer. Each experimental condition was performed in triplicate.
ABA content determination
Endogenous concentrations of ABA were analysed following the protocol described by Durgbanshi et al. (2005). The samples were lyophilized and processed by extraction into 5 ml of distilled water with internal standards: [2H6]-ABA (100 ng, prepared as described in Gómez-Cadenas et al. 2002). Extracts were centrifuged at 4000g, at 4 °C for 45 min. The supernatant was collected in clean tubes and the pH adjusted to 3.0 using 30% (v/v) acetic acid solution. The acidified extracts were partitioned twice with 3 ml of ethyl ether (ACS grade, Scharlau, Barcelona, Spain). The upper organic phase was recovered in a clean vial, combining both partitions and vacuum-drying using an evaporation centrifuge coupled to a cold trap (RC 10.22 i RT 10.90, Jouan, Saint-Herblain Cedex, France). The dried residue was diluted by adding 100 μl of methanol (HPLC grade, Scharlau) to the test tube by ultrasound for 10 min. The final volume of 1 ml was completed with pure water (MilliQ). The resulting solutions were filtered using regenerated cellulose filters with a pore diameter of 0.2 μm prior to analysis.
Analysis was performed using an HPLC kit (Alliance 2860, Waters Corp., Milford, USA) coupled to a tandem mass spectrometer with an electrospray interface (Quattro LC, Micromass, Manchester, UK). 20 μl of the extract were injected and then separated by a reverse phase column (Kromasil 100, C18, 5 μm, 100 × 2.0 mm, Scharlau) using a linear gradient of methanol and ultra-pure water supplemented with acetic acid to a final concentration of 0.01% (v/v), and a flow rate of 0.3 ml min−1. The discrimination and detection of each metabolite were performed following the pattern of fragmentation and the characteristic retention time. The ionization and collision conditions for each compound were optimized by direct infusion of pure standards (approximately 5 mg l−1). The quantification of the metabolites of interest was carried out using the response factor (areaanalit/áreastd) by interpolating in a calibration curve injected alternately in the samples. Processing of the chromatograms, integration and quantification was performed using MassLynx 4.0 software.
Saline stress-related and ABA-related genes selection
The genome of Trebouxia sp. TR9 (Martínez-Alberola 2015) was screened using the TBLASN algorithm to find homologues from Arabidopsis thaliana genome (TAIR 10) of a selection of genes coding for ABA biosynthesis enzymes 9-cis-epoxycarotenoid dioxygenase (NCED3) and abscisic-aldehyde oxidase (AAO3) (Schwartz et al. 1997); salt stress response-related protein responsive to dehydration gene (RD21A) (Koizumi et al. 1993); ABA signalling-related proteins mitogen-activated protein kinases (MPK) (Danquah et al. 2014) and ADP-ribosylation factor GTPase-activating protein 9 (AGD9) (Kline et al. 2010) (Online Resource, Table S1). These genes were also selected by the presence of ABA-responsive elements (ABRE) in their promoters (Online Resource, Table S2). Additionally, a homologue to the housekeeping gene coding for polyubiquitin-10 (UBQ10) (Callis et al. 1995) was found.
Primers for the selected genes were designed with OligoCalc (Kibbe 2007). Sequences for these primers are shown in Table 1.
RNA isolation
Total RNA was isolated from three biological replicates of each treatment, using the Total RNA Plant Isolation Kit (Sigma) and following the instructions supplied by the manufacturer. Further, DNAse treatment (TURBO DNA-free Kit, Ambion) was carried out to remove genomic DNA. RNA quantification was performed by spectrophotometric analysis and its integrity was checked by denaturing agarose gel electrophoresis. The absence of genomic DNA from the RNA samples was additionally tested by the null PCR amplification of the primer pair UBQ10.
Quantitative RT-PCR
cDNAs from different samples were synthesized from 2 μg of total RNA using SuperScript® III First-Strand Synthesis System 1st (Invitrogen) with 100 pmol of random hexamers according to the manufacturer’s instructions. Quantitative real time PCR (qRT-PCR) was performed on a Thermal Cycler CFX96™ Real-Time System (BIO-RAD) using Power SYBR® Green PCR Master Mix (PE Applied Biosystems), 10 μl reactions contained 4 μl of 1:50 diluted cDNA samples, 300 nM of each primer (FW and REV) and 5 μl of SYBR Green PCR Master Mix. PCR conditions were as follows: two initial steps of 50 °C for 2 min and 95 °C for 2 min, followed by 40 cycles of 95 °C for 30 s and 60 °C for 30 s. Afterwards, the dissociation protocol was performed to identify possible unspecific products. Three technical replicates from each biological repetition per treatment were analysed by qRT-PCR.
Primers used for qRT-PCR are described in Table 1. For each transcript, the average threshold cycle (CT) was determined using Bio-Rad CFX Manager 3.1 software. The efficiency of the primers and the data were analysed according to the 2−ΔΔCT method (Livak and Schmittgen 2001). Gene coding for UBQ10 was used as the reference gene (Czechowski et al. 2005).
Statistical analyses
Data were analysed by one-way Analysis of Variance (ANOVA) followed by post hoc comparisons by Tukey’s HSD test. The value 0.05 was considered as the significance level. Calculations were performed using IBM® SPSS® Statistics v20.0 Software and R (Cornillon 2012).
Results
Salt stress affects pigment content and photosynthetic performance of Trebouxia sp. TR9
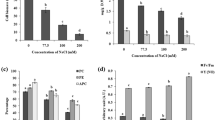
To determine the effects of salt stress, 21-day-old axenic cultures in stationary phase were exposed for 72 h to different salt concentrations (0, 1, 2, 3 and 5 M NaCl). Changes in the algae coloration were noticeable. Treated samples were dark green than their untreated counterparts, and the fresh weight was significantly lower in the microalgae treated with NaCl (Fig. 1a).
a Effect of NaCl on color, weight, photosynthesis and pigment content of Trebouxia sp. TR9. 21-day-old axenic cultures of Trebouxia sp. TR9 were grown for 72 h on media supplemented with NaCl concentrations ranging from 1 to 5 M. Changes in color and ultrastructure were apparent, as well as a reduction in fresh weight (FW). b Chlorophyll levels were quantified by spectrophotometry, chlorophyll a/b ratio (Chl a/b) was inferred from those values. c Photosystem II performance was estimated by maximum potential quantum efficiency of PSII (Fv/Fm ratio). d Total carotenoid content was also estimated by spectrophotometry. Three cultures were sampled for each condition. Graphs show the mean ± SD. Significant changes from control treatment are highlighted (*) (ANOVA, Tukey HSD test, P < 0.05)
Total chlorophyll levels in algae did not change significantly when treated with 1 M NaCl for 72 h, but decreased at higher NaCl concentrations. In comparison with the control, under 2 M NaCl, total chlorophyll levels decreased around ~ 33% and at 3 M and under 5 M NaCl there was a drop to around ~ 40% (Fig. 1b). Chlorophyll a levels decreased at 1 M NaCl (~ 35%), 2 M (~ 51%), 3 M (~ 55%) and 5 M (~ 51%), while chlorophyll b levels increased under concentrations of 1 M NaCl (~ 52%) and 2 M (~ 33%), reducing significantly the chlorophyll a/b ratio by ~ 56% at 1 and 2 M NaCl, ~ 50% at 3 M NaCl and 48% at 5 M NaCl (Fig. 1b). Total carotenoid levels were not affected at 1 M NaCl, but the levels were lower than the control in algae treated with 2 M (~ 27%), 3 M (~ 40%) and 5 M NaCl (~ 40%) (Fig. 1d).
Fv/Fm ratio was measured to estimate the PSII performance. This parameter was not altered by the 1 M NaCl treatment. However, at higher NaCl concentrations, reductions in algae Fv/Fm values of ~ 45% (3 M of NaCl) and ~ 83% (5 M NaCl) were observed (Fig. 1c).
Ultrastructural changes occur in Trebouxia sp. TR9 when exposed to high salt concentrations
After 72 h of treatment, ultrastructural TEM images reveal changes in the samples of Trebouxia sp. TR9 exposed to salt stress. The cell shape remained relatively uniform throughout all treatments, and no contents were released. Although the integrity of the cell wall was not affected by salt treatments, its morphology was modified; a diminution in cell wall thickness with the increase in salt concentration was observed, reaching its minimum width under 5 M NaCl (Fig. 2e3). At the same time, the secretion space between the cell wall and the protoplasm was enlarged and became denser when the salt concentration increased (Fig. 2a1–e1).
TEM ultrastructural analysis of phycobiont Trebouxia sp. TR9 cells under salt stress. 21-day-old Trebouxia sp. TR9 cells grown in axenic, mixotrophic cultures, under unstressed control conditions (a1–a3), and after 72 h under 1 M (b1–b3), 2 M (c1–c3), 3 M (d1–d3) and 5 M NaCl (e1–e3). CW cell wall, SS secretion space, N nucleus, Chl chloroplast, Mi mitochondria, EV electron-dense vesicles, PV peripheral vesicles, DZ degeneration zone, S starch
However, the most noticeable alteration in response to saline treatments was observed in the chloroplast, whose shape and integrity were modified (Fig. 2a2–e2). Under high salt conditions, the chloroplast size was reduced and adopted a star-like shape (Fig. 2a1–e1). As for the structure of thylakoids, under control conditions, are tightly packed, under salt exposure their structure becomes progressively disorganized, this being more evident at higher NaCl concentrations (Fig. 2a2–e2). In addition, thylakoid membranes from salt-exposed microalgae showed symptoms of degradation, especially in the central part of the organelle, where degradation products accumulated. This accumulation was more noticeable in higher salt treatments (Fig. 2a1–e1). Nonetheless, the peripheral parts of the chloroplast remained relatively unaffected (Fig. 2a3–e3). Also, the number of ribosomes within the chloroplast increased under salt exposure. Starch grains, normally accumulated between the thylakoids of the chloroplast under control conditions, were extruded to the outer part of the organelle in salt-treated algae (Fig. 2a1–e1).
Likewise, mitochondria were also affected. At 1 and 2 M NaCl concentrations, mitochondrial membranes started to disassemble from the inner part, acquiring a granulated texture. The presence of these organelles was less apparent at 3 and 5 M NaCl (Fig. 2d1–e1). Furthermore, under control conditions, Trebouxia sp. TR9 displayed large, dark electron-dense vesicles (Ev) and smaller, non-electron-dense (electron-lucent) peripheral vesicles located in the outlying areas of the cytoplasm (Fig. 2a1–e1). Under salt stress, Ev become less electron-dense, and the number of non-electron-dense vesicles increased along with the NaCl concentration of the treatment, occupying a sizeable portion of the cytoplasmic space at 5 M NaCl (Fig. 2e1).
ABA levels are increased in Trebouxia sp. TR9 only when exposed to higher salt concentrations
After salt treatments, internal ABA levels did not change significantly when exposed to 1 and 2 M NaCl (Fig. 3) with levels ranging around 8.4–9.8 ng ABA g FW−1. On the other hand, ABA levels changed in algae treated with 3 and 5 M NaCl, reaching 13.3–14.2 ng ABA g FW−1.
Effect of NaCl treatment on ABA levels of axenic cultures of Trebouxia sp. TR9. 21-day-old axenic cultures of Trebouxia sp. TR9 were transferred and grown for 3 days on media supplemented with 1, 2, 3 or 5 M NaCl as well as on control plates without the amendment of NaCl. Three cultures were sampled for each condition, and ABA levels were quantified. Graph shows the mean ± SD. Significant differences between treatments are indicated with letters (ANOVA, Tukey HSD test, P < 0.05)
Additionally, the expression of genes coding for homologues of ABA biosynthesis enzymes 9-cis-epoxycarotenoid dioxygenase (NCED3) and abscisic-aldehyde oxidase (AAO3) (Schwartz et al. 1997) was checked by qRT-PCR (Fig. 4a). NCED3 was slightly repressed under 1 and 2 M, and only showed a relatively small induction (less than twofold) under 5 M NaCl when compared to the control. On the other hand, AAO3 relative expression increased more than twofold compared to the control at 1 M NaCl, but its mRNA levels remained similar to the control at higher salt concentrations (Fig. 4a). Expression of these genes was also checked in liquid cultures. However, a different expression pattern was observed: NCED3 mRNA numbers increased to 3.42-fold under 1 M NaCl. Meanwhile, at higher NaCl concentrations, NCED3 relative expression remained around 1.55–2.20-fold higher than the control (Fig. 5a). AAO3 mRNA levels were also increased by salt treatment, but to a lower extent than was observed for NCED3, with the exception of 2 M NaCl, where AAO3 expression was similar to control conditions.
Effect of NaCl treatment on the expression of ABA biosynthesis (a) and stress-related and ABA response genes (b) on axenic-grown cultures of Trebouxia sp. TR9. 21-day-old axenic cultures of Trebouxia sp. TR9 were transferred and grown for 3 days on media supplemented with 1, 2, 3 or 5 M NaCl as well as on control plates without the amendment of NaCl. Expression levels were determined by qRT-PCR. For each gene, data are expressed as fold change relative to the level measured in control conditions (2−ΔΔCT). Graph show the mean of three biological replicates ± SD. Significant differences between treatments are indicated with letters (ANOVA, Tukey HSD test, P < 0.05)
Effect of NaCl treatment on the expression of ABA biosynthesis (a) and stress-related and ABA response genes (b) on liquid-grown cultures of Trebouxia sp. TR9. 21-day-old liquid cultures of Trebouxia sp. TR9 were grown for 3 days without (control) or with the addition of NaCl until a final concentration of 1, 2, 3 or 5 M. Expression levels were determined by qRT-PCR. For each gene, data are expressed as fold change relative to the level measured in control conditions (2−ΔΔCT). Graph show the mean of three biological replicates ± SD. Significant differences between treatments are indicated with letters (ANOVA, Tukey HSD test, P < 0.05)
ABA pathway response in Trebouxia sp. TR9 is functional but is not triggered when exposed to extreme salt conditions
The effect of NaCl exposure in the expression of a set of ABA-related and saline stress response-related genes was also checked in axenic (Fig. 4b) and liquid cultures (Fig. 5b). RD21A encodes for a cysteine proteinase precursor-like protein that is induced by dehydration and high-salt conditions in Arabidopsis (Koizumi et al. 1993). Regarding axenic growth conditions, RD21A mRNA levels were not significantly altered at 1 M NaCl, but the relative expression decreased significantly at 2 and 3 M NaCl, while at 5 M NaCl it remained similar to the control (Fig. 4b).
Moreover, the expression patterns of two MPK kinases homologues to several Arabidopsis MPK genes were also checked. The majority of these Arabidopsis MPKs have been described as part of response mechanisms to several stress conditions, most of them being members of ABA-related signalling pathways (Online Resource, Table S1 and references within). Moreover, both genes present putative ABRE elements in their promoter sequences (Online Resource, Table S2). In axenic cultures, the expression pattern of Trebouxia sp. TR9 MPK5 showed no change at 1 M NaCl and lower mRNA levels at higher salt concentrations relative to the control (Fig. 4b). Also, the Trebouxia sp. TR9 MPK9 gene showed a slight induction at 1 M NaCl, but again mRNA levels were lower than the control at 2 and 5 M NaCl (Fig. 4b).
Finally, the salt response expression profile of a homologue of the AGD9 gene was also checked. AGD9 has been described as one of the phosphorylation targets of the Arabidopsis ABA transduction pathway (Kline et al. 2010). The Trebouxia sp. TR9 AGD9 gene showed an expression pattern similar to MPK5, although the repression compared to the control observed at NaCl treatments higher than 1 M was observed at a lower extent (Fig. 4b).
In contrast, a different pattern in response to NaCl exposure was observed in liquid cultures for some of the stress-related and ABA-related genes (Fig. 5b). RD21A again showed no significant changes in its mRNA level relative to the control at 1 and 5 M NaCl. There was a slight decrease in RD21A expression at 2 M NaCl, and a 1.84-fold increase at 3 M NaCl. On the other hand, MPK5 expression remained similar to the control at 1 and 2 M NaCl but increased at 3 and 5 M NaCl (Fig. 5b). Additionally, MPK9 expression rose slightly but significantly, with NaCl exposure, with expression changes ranging from 1.32 to 2.21-fold compared to the control. A similar expression pattern was also observed for the AGD9 gene, with broader changes in response to NaCl exposure (1.79–3.46-fold higher than the control) (Fig. 5b).
The functionality of the ABA response pathway in Trebouxia sp. TR9 was also tested by exposing 21-old axenic Trebouxia sp. TR9 cultures to ABA treatments (Fig. 6). In this case, expression of the ABA key biosynthesis gene NCED3 and the same set of stress-related and ABA-responsive genes (RD21A, MPK5, MPK9 and AGD9) was evaluated after 6 h of exposure to increasing concentrations of ABA (1–100 µM). NCED3 and RD21A relative expression increased significantly with 10 and 100 µM external ABA, while in the case of Trebouxia sp. TR9 MPK5, MPK9 and AGD9 this significant induction is also observed at 1 µM ABA (Fig. 6). Moreover, increases in ABA concentration resulted in greater increases in the expression of these genes, up to levels ranging from 5 to 17-fold higher than the control at 100 µM ABA exposure (Fig. 6).
Effect of external ABA treatment on the expression of ABA biosynthesis genes (NCED3) and stress-related and ABA response genes (RD21A, MPK5, MPK9 and AGD9) on axenic-grown cultures of Trebouxia sp. TR9. 21-day-old axenic cultures of Trebouxia sp. TR9 were treated with several ABA solutions (0–100 µM) and collected after 6 h. Expression levels were determined by qRT-PCR. For each gene, data are expressed as fold change relative to the level measured in 0 M conditions (2−ΔΔCT). Graph show the mean of three biological replicates ± SD. Significant differences between treatments are indicated with letters (ANOVA, Tukey HSD test, P < 0.05)
Discussion
Trebouxia sp. TR9 displays an outstanding resistance to high salt stress
Despite the increasing amount of literature on the effects of salinity and other osmotic stresses in plants and, to some extent, free-living microalgae, little is known about these responses in symbiotic microalgae. Trebouxia is a phycobiont found in at least 35% of all lichen species (Škaloud and Peksa 2010), with a widespread distribution occupying different environments. Some of them, such as polar regions or dry deserts, suppose extreme conditions the phycobionts must adapt to to survive (Green et al. 2012; Maphangwa et al. 2012). This resistant nature makes them suitable candidates to study adaptations to extreme environments, such as cold, desiccation or salinity. Even though Trebouxia sp. TR9 is found lichenised in salt-free environments, away from the influence of the sea, it appears to display remarkable resistance to a broad range of salinity, from high (1 M) to extreme NaCl concentrations (5 M, near saturation point). The maximum quantum yield of PSII photochemistry (Fv/Fm) is frequently used as an indicator of the photoinhibition or stress on the PSII (Krause and Weis 1991). In our conditions, Fv/Fm ratio, remains unaltered at 1 M NaCl, although at higher concentrations this parameter is negatively affected, indicating that 3 and 5 M NaCl modified the photochemistry of PSII (Lan et al. 2010). Despite this, it retains more than 50% of the Fv/Fm ratio at 3 M after 72 h, and nearly 20% at 5 M (Fig. 1c), a concentration so extreme that only a few halophilic organisms, such as Dunaliella, can withstand it (Cowan et al. 1992). Likewise, chlorophyll content (Fig. 1b) is not affected at 1 M NaCl after 72 h. It falls at higher concentrations, but not below 60% of its content under unstressed conditions, unlike its land counterparts, such as Arabidopsis or Triticum aestivum, which experience massive chlorophyll drops under much lower NaCl concentrations (Negrão et al. 2017). Chlorophyll a experiences a decrease under 1 M NaCl, but this is compensated with an increase of chlorophyll b content, so total chlorophyll is not altered. The decrease in the chlorophyll a/b ratio has also been reported in land plants under salt stress (Mane et al. 2010), lichens under metal exposure (Cu, Zn and Pb) (Chettri et al. 1998) and light stress (Balarinová et al. 2014). These changes could be due to a higher sensitivity of Chl a to salt stress.
In summary, Trebouxia sp. TR9 chlorophyll content and PSII activity estimated by maximum potential quantum efficiency do not seem to be significantly affected under 1 M NaCl after 72 h of exposure, but they are negatively altered at higher salt concentrations. This contrasts with most land plants, where photosynthesis is affected at much lower salt exposure. Even salt-resistant mutants of Arabidopsis find their photosystems inhibited at 0.4 M (Hayashi et al. 1997), and halophile plants such as Thellungiella halophila start to show signs of cellular damage at concentrations above 0.5 M NaCl (Stepien and Johnson 2009). Moreover, freshwater microalgae such as C. vulgaris (Hiremath and Mathad 2010) or Chlamydomonas reinhardtii (Neale and Melis 1989) experience a large reduction in chlorophyll content at much lower salt concentrations (0.4 M).
The response of Trebouxia sp. TR9 to salt stress includes chloroplast and cell wall reorganization as well as vesicle accumulation
Ultrastructural studies of Trebouxia sp. TR9 reveal that the shape and composition of the chloroplast is noticeably altered by salt stress. While damage is not excessive under 1 M NaCl (Fig. 2b1–b3), it is more apparent at higher salt concentrations (Fig. 2c–e). Degradation happens mostly at the centre of the organelle, with thylakoid membranes disintegrating and forming accumulations of degradation products (Fig. 2b–e). Apparently, the structural integrity of the thylakoids is still maintained in the peripheral areas even at 5 M NaCl (Fig. 2e1), which may account for the maximum quantum potential of the PSII measured at this concentration (Fig. 1c). Ribosomes within the chloroplast grow in number when exposed to salinity, an indication that the microalga seems to be modifying its protein synthesis as a response to salt stress. Starch grain deposition in the chloroplast is also altered by salt exposure, being extruded to the outer parts of the organelle (Fig. 2a1–e1). In addition, starch granules number increases when the microalga is exposed to salinity. Starch is the primary photosynthetic carbon sink in several species of microalgae (Lan et al. 2010) and tends to accumulate under conditions of stress, such as nitrogen and nutrient limitation, in Chlorella (Brányiková et al. 2011; Dragone et al. 2011). Trebouxia sp. TR9 seems to be developing a similar response under salt stress.
Although a reduction of fresh weight is observed in Trebouxia sp. TR9 treated with NaCl (Fig. 1a), probably due to cell water loss caused by a low water potential situation in the culture medium, the shape of this microalga appears to be unaltered under all salt concentrations. Meaning that, no changes in cell wall structural coherence are observed and no cell contents are released (Fig. 2a1–e1). In contrast, other free-living Trebouxiophyceae microalgae, for example Chlorella, display changes in morphology such as deformation and rupture of the cell wall as a result of osmotic stress derived from salinity (Qiao et al. 2015). On the other hand, cell wall reorganization seems to be part of the Trebouxia sp. TR9 response to salt exposure, as suggested by the changes observed in cell wall thickness, and by the enlargement and modification of the secretion zone under salt stress (Fig. 2b1–e1), which may suggest that the alga is devoting large resources to maintain cell wall integrity. A similar reduction in cell wall thickness under salt stress has also been observed in microalgae from the genus Nannochloropsis (Borges et al. 2016). Similar changes in shape and coloration of the secretion space are also observed, although at much lower salt concentrations, in Trebouxiophyceae free-living algae retrieved from alkali soils (Qiao et al. 2015).
Furthermore, accumulation of vesicles is also apparent under all salt treatments (Fig. 2a1–e1), with the higher salt concentrations displaying a greater number of these structures. Electron-dense vesicles display a variation in density under salt stress, suggesting a variation of their contents. An increase in the number of vesicles has been observed in Dunaliella cells when exposed to extreme salt concentrations, the functionality of which has been tied to active maintenance of the cell membrane (Einspahr et al. 1988). However, unlike Trebouxia, Dunaliella cells lack cell wall. The composition and function of these vesicles remain to be determined.
Abscisic acid does not seem to be a key signal in the response of Trebouxia sp. TR9 to salinity
The role of ABA in the response against salt stress in microalgae is not yet fully understood (Lu and Xu 2015). It is known to play a significant role in species such as free-living Trebouxiophyceae C. vulgaris, where cell content increases more than 300% after 4 days of exposure to 0.1 M NaCl (Maršálek et al. 1992) and in the halophile green microalga Dunaliella parva, it increases by 225% after 8 days of exposure to 2.5 M NaCl (Tietz and Kasprik 1986). These results may hint that ABA indeed plays a pivotal role in the response against haline stress in green microalgae. However, Trebouxia sp. TR9 does not seem to behave in a similar way, with no significant changes in the phytohormone cell content below 3 M NaCl after 72 h of exposure. It does display, however, a mild increase (< 60%) at higher salinity ranges (Fig. 3). This is consistent with the expression of ABA biosynthesis pathway genes in axenic cultures (Fig. 4a) where, with the exception of ABA3 at 1 M NaCl, no increase in gene expression is observed at concentrations below 5 M NaCl, and even at this extreme, changes in expression are far below those in land plants at much lower salinity levels (Zhang et al. 2006). Likewise, the majority of ABA-signalling related genes tested on axenic cultures show that they are repressed at NaCl concentrations higher than 1 M (Fig. 4b). This behaviour contrasts with the response of Trebouxia sp. TR9 treated in liquid medium, where expression of the ABA-related genes (Fig. 5) is induced, albeit mildly, and far from the induction levels observed with ABA treatment (Fig. 6). This phycobiont is a terrestrial organism not found naturally in water, although it can be cultured in liquid medium, but growth is slower under these conditions (Archibald 1977). Whether the added stress of being submerged is responsible for this change in gene expression remains to be determined.
These results suggest that ABA may play a different role in the mechanisms of the salt stress signalling response of Trebouxia sp. TR9 axenic cultures. Unlike water-bound closely related taxa, such as Chlorella or Dunaliella, where ABA production seems to be part of the response mechanism against salinity (Hirsch et al. 1989; Maršálek et al. 1992; Cowan et al. 1992), Trebouxia sp. TR9 is a terrestrial, lichenised alga that might have developed alternative ways to cope with this kind of abiotic stress. Nonetheless, ABA perception and response pathways seem to be functional, because the observed induction of the ABA-related genes checked in response to the addition of exogenous ABA (Fig. 6), which can be explained by the presence of ABRE elements in their promoter sequences (Online resource, Table S2). Interestingly, this upregulation by ABA includes the Trebouxia sp. TR9 RD21A gene, in contrast with Arabidopsis, where this gene is not induced by ABA (Koizumi et al. 1993). Further research is needed to determine the role of ABA within this phycobiont.
In summary, Trebouxia sp. TR9 does not seem to be significantly affected by saline stress below 2 M NaCl after a period of 72 h, displaying only limited ultrastructural damage and maintaining PSII activity and chlorophyll levels similar to the control. In addition, ABA levels and ABA-related gene expression are not altered. Ultrastructural reorganizations and ABA production start to appear only at the higher concentrations assayed. These effects are comparatively less severe than those experienced by other Trebouxiophyceae microalgae evaluated to date, and far less severe than in land plants. Further research is needed to determine the molecular bases of this tolerance and its potential for biotechnological applications.
Author contribution statement
FM, PC and EB conceived the experimental design. EH-V, FM and FE conducted the experiments. EB, FG-B and JR-A conducted microscopy analysis. EH-V, FM and FM-A conducted gene selection and primer design. EH-V, FM, PC and EB wrote the manuscript.
References
Álvarez R, del Hoyo A, Díaz-Rodríguez C et al (2015) Lichen rehydration in heavy metal-polluted environments: Pb modulates the oxidative response of both Ramalina farinacea thalli and its isolated microalgae. Microb Ecol 69:698–709. https://doi.org/10.1007/s00248-014-0524-0
Archibald PA (1977) Physiological characteristics of Trebouxia (Chlorophyceae, Chlorococcales) and Pseudotrebouxia (Chlorophyceae, Chlorosarcinales). Phycologia 16:295–300. https://doi.org/10.2216/i0031-8884-16-3-295.1
Armstrong RA (2017) Adaptation of lichens to extreme conditions. In: Kumar V, Shukla S, Kumar N (eds) Plant adaptation strategies in changing environment. Springer Singapore, Singapore, pp 1–27
Arup U (1995) Littoral species of Caloplaca in North America: a summary and a key. Bryologist 98:129–140. https://doi.org/10.2307/3243649
Aschenbrenner IA, Cernava T, Berg G, Grube M (2016) Understanding microbial multi-species symbioses. Front Microbiol 7:180. https://doi.org/10.3389/fmicb.2016.00180
Balarinová K, Barták M, Hazdrová J, Hájek J, Jílková J (2014) Changes in photosynthesis, pigment composition and glutathione contents in two Antarctic lichens during a light stress and recovery. Photosynthetica 52:538–547. https://doi.org/10.1007/s11099-014-0060-7
Biosca EG, Flores R, Santander RD, Díez-Gil JL, Barreno E (2016) Innovative approaches using lichen enriched media to improve isolation and culturability of lichen associated bacteria. PLoS One 11:e0160328. https://doi.org/10.1371/journal.pone.0160328
Bischoff HW, Bold HC (1963) Some soil algae from Enchanted Rock and related algal species. Phycol Stud 44(1):1–95
Borges L, Caldas S, Montes D’Oca MG, Abreu PC (2016) Effect of harvesting processes on the lipid yield and fatty acid profile of the marine microalga Nannochloropsis oculata. Aquac Rep 4:164–168. https://doi.org/10.1016/j.aqrep.2016.10.004
Brandt A, Posthoff E, de Vera J-P, Onofri S, Ott S (2016) Characterisation of growth and ultrastructural effects of the Xanthoria elegans photobiont after 1.5 years of space exposure on the International Space Station. Orig Life Evol Biosph 46:311–321. https://doi.org/10.1007/s11084-015-9470-1
Brányiková I, Maršálková B, Doucha J et al (2011) Microalgae—novel highly efficient starch producers. Biotechnol Bioeng 108:766–776. https://doi.org/10.1002/bit.23016
Callis J, Carpenter T, Sun CW, Vierstra RD (1995) Structure and evolution of genes encoding polyubiquitin and ubiquitin-like proteins in Arabidopsis thaliana ecotype Columbia. Genetics 139:921–939
Campenni L, Nobre BP, Santos CA et al (2013) Carotenoid and lipid production by the autotrophic microalga Chlorella protothecoides under nutritional, salinity, and luminosity stress conditions. Appl Microbiol Biotechnol 97:1383–1393. https://doi.org/10.1007/s00253-012-4570-6
Casano LM, del Campo EM, García-Breijo FJ et al (2011) Two Trebouxia algae with different physiological performances are ever-present in lichen thalli of Ramalina farinacea. Coexistence versus competition? Environ Microbiol 13:806–818. https://doi.org/10.1111/j.1462-2920.2010.02386.x
Chettri M, Cook C, Vardaka E, Sawidis T, Lanaras L (1998) The effect of Cu, Zn and Pb on the chlorophyll content of the lichens Cladonia convoluta and Cladonia rangiformis. Environ Exp Bot 39:1–10. https://doi.org/10.1016/S0098-8472(97)00024-5
Cornillon P-A (2012) R for statistics. CRC Press, Boca Raton
Cowan AK, Rose PD, Horne LG (1992) Dunaliella salina: a model system for studying the response of plant cells to stress. J Exp Bot 43:1535–1547. https://doi.org/10.1093/jxb/43.12.1535
Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139:5–17. https://doi.org/10.1104/pp.105.063743
Danquah A, de Zelicourt A, Colcombet J, Hirt H (2014) The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol Adv 32:40–52. https://doi.org/10.1016/j.biotechadv.2013.09.006
Delmail D, Labrousse P, Hourdin P et al (2013) Micropropagation of Myriophyllum alterniflorum (Haloragaceae) for stream rehabilitation: first in vitro culture and reintroduction assays of a heavy-metal hyperaccumulator immersed macrophyte. Int J Phytoremediation 15:647–662. https://doi.org/10.1080/15226514.2012.723068
Dragone G, Fernandes BD, Abreu AP, Vicente AA, Teixeira JA (2011) Nutrient limitation as a strategy for increasing starch accumulation in microalgae. Appl Energy 88:3331–3335. https://doi.org/10.1016/j.apenergy.2011.03.012
Duarte AWF, Passarini MRZ, Delforno TP et al (2016) Yeasts from macroalgae and lichens that inhabit the South Shetland Islands, Antarctica. Environ Microbiol Rep 8:874–885. https://doi.org/10.1111/1758-2229.12452
Durgbanshi A, Arbona V, Pozo O et al (2005) Simultaneous determination of multiple phytohormones in plant extracts by liquid chromatography–electrospray tandem mass spectrometry. J Agric Food Chem 53:8437–8442. https://doi.org/10.1021/JF050884B
Einspahr KJ, Maeda M, Thompson GA (1988) Concurrent changes in Dunaliella salina ultrastructure and membrane phospholipid metabolism after hyperosmotic shock. J Cell Biol 107:529–538. https://doi.org/10.1083/JCB.107.2.529
Gasulla F, de Nova PG, Esteban-Carrasco A et al (2009) Dehydration rate and time of desiccation affect recovery of the lichenic algae Trebouxia erici: alternative and classical protective mechanisms. Planta 231:195–208. https://doi.org/10.1007/s00425-009-1019-y
Gasulla F, Guéra A, Barreno E (2010) A simple and rapid method for isolating lichen photobionts. Symbiosis 51:175–179. https://doi.org/10.1007/s13199-010-0064-4
Gómez-Cadenas A, Arbona V, Jacas J, Primo-Millo E, Talon M (2002) Abscisic acid reduces leaf abscission and increases salt tolerance in citrus plants. J Plant Growth Regul 21:234–240. https://doi.org/10.1007/s00344-002-0013-4
Green TGA, Brabyn L, Beard C, Sancho LG (2012) Extremely low lichen growth rates in Taylor Valley, Dry Valleys, continental Antarctica. Polar Biol 35:535–541. https://doi.org/10.1007/s00300-011-1098-7
Grube M, Blaha J (2005) Halotolerance and lichen symbioses. In: Gunde-Cimerman N, Oren A, Plemenitaš A (eds) Adaptation to life at high salt concentrations in Archaea, Bacteria, and Eukarya. Springer, Berlin, pp 471–488
Guéra A, Calatayud A, Sabater B, Barreno E (2004) Involvement of the thylakoidal NADH-plastoquinone-oxidoreductase complex in the early responses to ozone exposure of barley (Hordeum vulgare L.) seedlings. J Exp Bot 56:205–218. https://doi.org/10.1093/jxb/eri024
Gustavs L, Eggert A, Michalik D, Karsten U (2010) Physiological and biochemical responses of green microalgae from different habitats to osmotic and matric stress. Protoplasma 243:3–14. https://doi.org/10.1007/s00709-009-0060-9
Hauser F, Rainer W, Schroeder JI (2011) Evolution of abscisic acid synthesis and signaling mechanisms. Curr Biol 21:346–355. https://doi.org/10.1016/j.cub.2011.03.015.Hauser
Hayashi H, Alia L, Mustardy L, Ida M, Murata N (1997) Transformation of Arabidopsis thaliana with the codA gene for choline oxidase; accumulation of glycine betaine and enhanced tolerance to salt and cold stress. Plant J 12:133–142. https://doi.org/10.1046/j.1365-313X.1997.12010133.x
Hiremath S, Mathad P (2010) Impact of salinity on the physiological and biochemical traits of Chlorella vulgaris Beijerinck. J Algal Biomass Util 1:51–59
Hirsch R, Hartung W, Gimmler H (1989) Abscisic acid content of algae under stress. Bot Acta 102:326–334. https://doi.org/10.1111/j.1438-8677.1989.tb00113.x
Jameson P (1993) Plant hormones in the algae. Prog Phycol Res 9:239–279
Kibbe WA (2007) OligoCalc: an online oligonucleotide properties calculator. Nucleic Acids Res 35:W43–W46. https://doi.org/10.1093/nar/gkm234
Kirk PM, Cannon PF, David JC, Stalpers JA (2001) Ainsworth and Bisby’s dictionary of the fungi, 9th edn. CABI Publishing, Wallingford, UK
Kline KG, Barrett-Wilt GA, Sussman MR (2010) In planta changes in protein phosphorylation induced by the plant hormone abscisic acid. Proc Natl Acad Sci USA 107:15986–15991. https://doi.org/10.1073/pnas.1007879107
Koizumi M, Yamaguchi-Shinozaki K, Tsuji H, Shinozaki K (1993) Structure and expression of two genes that encode distinct drought-inducible cysteine proteinases in Arabidopsis thaliana. Gene 129:175–182. https://doi.org/10.1016/0378-1119(93)90266-6
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol 42:313–349. https://doi.org/10.1146/annurev.pp.42.060191.001525
Lan SB, Wu L, Zhang DL, Hu CX, Liu YD (2010) Effects of drought and salt stresses on man-made cyanobacterial crusts. Eur J Soil Biol 46:381–386. https://doi.org/10.1016/j.ejsobi.2010.08.002
Leavitt SD, Kraichak E, Nelsen MP et al (2015) Fungal specificity and selectivity for algae play a major role in determining lichen partnerships across diverse ecogeographic regions in the lichen-forming family Parmeliaceae (Ascomycota). Mol Ecol 24:3779–3797. https://doi.org/10.1111/mec.13271
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/METH.2001.1262
Lu Y, Xu J (2015) Phytohormones in microalgae: a new opportunity for microalgal biotechnology? Trends Plant Sci 20:273–282. https://doi.org/10.1016/j.tplants.2015.01.006
Malaspina P, Giordani P, Pastorino G, Modenesi P, Mariotti MG (2015) Interaction of sea salt and atmospheric pollution alters the OJIP fluorescence transient in the lichen Pseudevernia furfuracea (L.) Zopf. Ecol Indic 50:251–257. https://doi.org/10.1016/j.ecolind.2014.11.015
Mane AV, Karadge BA, Samant JS (2010) Salt stress induced alteration in photosynthetic pigments and polyphenols of Pennisetum alopecuroides (L.). J Ecophysiol Occup Health 10:177–182. https://doi.org/10.18311/jeoh/2010/18339
Maphangwa KW, Musil CF, Raitt L, Zedda L (2012) Experimental climate warming decreases photosynthetic efficiency of lichens in an arid South African ecosystem. Oecologia 169:257–268. https://doi.org/10.1007/s00442-011-2184-9
Margulis L, Barreno E (2003) Looking at lichens. Bioscience 53:776–778. https://doi.org/10.1641/0006-3568(2003)053%5b0776:lal%5d2.0.co;2
Maršálek B, Zahradníčková H, Hronková M (1992) Extracellular abscisic acid produced by cyanobacteria under salt stress. J Plant Physiol 139:506–508. https://doi.org/10.1016/S0176-1617(11)80503-1
Martínez-Alberola F (2015) Genome characterization of the symbiotic microalga Trebouxia sp. TR9 isolated from the lichen Ramalina farinacea (L.) Ach. by means of NGS techniques. PhD Dissertation. Universitat de València. http://roderic.uv.es/handle/10550/48824
Mishra A, Jha B (2009) Isolation and characterization of extracellular polymeric substances from micro-algae Dunaliella salina under salt stress. Bioresour Technol 100:3382–3386. https://doi.org/10.1016/j.biortech.2009.02.006
Molins A, Moya P, García-Breijo FJ, Reig-Arminana J, Barreno E (2018) A multi-tool approach to assess microalgal diversity in lichens: isolation, Sanger sequencing, HTS and ultrastructural correlations. Lichenologist 50:123–138. https://doi.org/10.1017/S0024282917000664
Moya P, Molins A, Martínez-Alberola F, Muggia L, Barreno E (2017) Unexpected associated microalgal diversity in the lichen Ramalina farinacea is uncovered by pyrosequencing analyses. PLoS One 12:e0175091. https://doi.org/10.1371/journal.pone.0175091
Nash TH III, Lange OL (1988) Responses of lichens to salinity: concentration and time-course relationships and variability among Californian species. New Phytol 109:361–367. https://doi.org/10.1111/j.1469-8137.1988.tb04206.x
Neale PJ, Melis A (1989) Salinity-stress enhances photoinhibition of photosynthesis in Chlamydomonas reinhardtii. J Plant Physiol 134:619–622. https://doi.org/10.1016/S0176-1617(89)80158-0
Negrão S, Schmöckel SM, Tester M (2017) Evaluating physiological responses of plants to salinity stress. Ann Bot 119:1–11. https://doi.org/10.1093/aob/mcw191
Qiao K, Takano T, Liu S (2015) Discovery of two novel highly tolerant NaHCO3 Trebouxiophytes: identification and characterization of microalgae from extreme saline–alkali soil. Algal Res 9:245–253. https://doi.org/10.1016/j.algal.2015.03.023
Ruzin SE (2000) Plant microtechnique and microscopy. New Phytol 148:57–58
Schwartz SH, Tan BC, Gage DA, Zeevaart JAD, McCarty DR (1997) Specific oxidative cleavage of carotenoids by VP14 of maize. Science 276:1872–1874. https://doi.org/10.1126/science.276.5320.1872
Škaloud P, Peksa O (2010) Evolutionary inferences based on ITS rDNA and actin sequences reveal extensive diversity of the common lichen alga Asterochloris (Trebouxiophyceae, Chlorophyta). Mol Phylogenet Evol 54:36–46. https://doi.org/10.1016/J.YMPEV.2009.09.035
Spribille T, Tuovinen V, Resl P et al (2016) Basidiomycete yeasts in the cortex of ascomycete macrolichens. Science 353:488–492. https://doi.org/10.1126/science.aaf8287
Stepien P, Johnson GN (2009) Contrasting responses of photosynthesis to salt stress in the glycophyte Arabidopsis and the halophyte Thellungiella: role of the plastid terminal oxidase as an alternative electron sink. Plant Physiol 149:1154–1165. https://doi.org/10.1104/pp.108.132407
Takagi M, Karseno YT (2006) Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J Biosci Bioeng 101:223–226. https://doi.org/10.1263/jbb.101.223
Takahagi T, Yamamoto Y, Kinoshita Y, Takeshita S, Yamada T (2002) Inhibitory effects of sodium chloride on induction of tissue cultures of lichens of Ramalina species. Plant Biotechnol 19:53–55. https://doi.org/10.5511/plantbiotechnology.19.53
Tietz A, Kasprik W (1986) Identification of abscisic acid in a green alga. Biochem Physiol Pflanz 181:269–274. https://doi.org/10.1016/S0015-3796(86)80093-2
Wani SH, Kumar V, Shriram V, Sah SK (2016) Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J 4:162–176. https://doi.org/10.1016/j.cj.2016.01.010
Wellburn AR, Lichtenthaler H (1984) Formulae and program to determine total carotenoids and chlorophylls A and B of leaf extracts in different solvents. In: Sybesma C (ed) Advances in photosynthesis research. Springer, Dordrecht, pp 9–12
Zhang J, Jia W, Yang J, Ismail AM (2006) Role of ABA in integrating plant responses to drought and salt stresses. Field Crop Res 97:111–119. https://doi.org/10.1016/j.fcr.2005.08.018
Acknowledgements
Supported by the Ministerio de Economía y Competitividad (MINECO, Spain) and FEDER (CGL2016-79158-P), and the PROMETEO Excellence in Research Program (Generalitat Valenciana, Spain) (PROMETEO/2017/039). Funding for Ernesto Hinojosa-Vidal was also provided by MINECO (BES-2013-065511). We want to thank the technicians (Mª Teresa Mínguez and Nuria Cebrián) from the Servicio de Microscopía Electrónica (SCSIE) and Jardí Botànic (Universitat de Valencia) who helped us to perform the TEM process; Dr. Ángeles Calatayud (IVIA, Valencia) for her valuable remarks on fluorescence analysis and Guillermo Salvá who performed the earliest experiments and fluorescence measurements. Daniel Sheerin revised the English manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hinojosa-Vidal, E., Marco, F., Martínez-Alberola, F. et al. Characterization of the responses to saline stress in the symbiotic green microalga Trebouxia sp. TR9. Planta 248, 1473–1486 (2018). https://doi.org/10.1007/s00425-018-2993-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-018-2993-8