Abstract
Main conclusion
In vitro conditions and benzyladenine influenced both content and composition of micropropagated Micromeria pulegium essential oils, with pulegone and menthone being the main essential oil components.
The content and chemical composition of Micromeria pulegium (Rochel) Benth. essential oils were studied in native plant material at vegetative stage and in micropropagated plants, obtained from nodal segments cultured on solid MS medium supplemented with N6–benzyladenine (BA) or kinetin at different concentrations, alone or in combination with indole-3-acetic acid. Shoot proliferation was achieved in all treatments, but the highest biomass production was obtained after treatment with 10 μM BA. Phytochemical analysis identified up to 21 compounds in the essential oils of wild-growing and in vitro cultivated plants, both showing very high percentages of total monoterpenoids dominated by oxygenated monoterpenes of the menthane type. Pulegone and menthone were the main essential oil components detected in both wild-growing plants (60.07 and 26.85 %, respectively) and micropropagated plants grown on either plant growth regulator-free medium (44.57 and 29.14 %, respectively) or BA-supplemented medium (50.77 and 14.45 %, respectively). The percentage of total sesquiterpenoids increased in vitro, particularly owing to sesquiterpene hydrocarbons that were not found in wild-growing plants. Differences in both content and the composition of the essential oils obtained from different samples indicated that in vitro culture conditions and plant growth regulators significantly influence the essential oils properties. In addition, the morphology and structure of M. pulegium glandular trichomes in relation to the secretory process were characterized for the first time using SEM and light microscopy, and their secretion was histochemically analyzed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Micromeria Benth. (Lamiaceae) comprises more than 70 herbs, subshrubs and shrubs distributed from the Himalayan region to the Macaronesia and from the Mediterranean to South Africa and Madagascar (Harley et al. 2004). Based on morphological characteristics and phylogenetic relationships, species from the genus Micromeria are grouped into four sections: Pseudomelissa Bentham, Micromeria, Cymularia Boiss. and Pineolentia P. Pérez (Harley et al. 2004). Section Pseudomelissa is one of the most complex taxa, with still unstable systematic position within Mentheae tribe of Nepetoideae subfamily. Based on molecular evidence, emphasizing the similarity of selected morphological traits, Bräuchler et al. (2006) currently include species of Micromeria, sect. Pseudomelissa in genus Clinopodium L.
In the past years, several reports have been published on the chemotaxonomical traits of Micromeria species showing the presence of biologically active phenolic compounds and the essential oils (Vladimir-Knežević et al. 2011; Kremer et al. 2012, 2014). Species belonging to the genus Micromeria contain considerable quantities (>0.5 %) of essential oils dominated by oxygenated monoterpenes of the menthane class (Slavkovska et al. 2005), which exhibit substantial antimicrobial (Duru et al. 2004; Šavikin et al. 2010) and antioxidant activities (Vladimir-Knežević et al. 2011; Tošić et al. 2015), thus protecting the plant from pathogen attacks. The essential oil composition within the genus Micromeria displays great variability among species, with significant seasonal variations (Slavkovska et al. 2005, 2013).
Micromeria pulegium (Rochel) Benth. is a perennial, medium-sized, erected plant. Green parts of the plant (especially leaves) are densely covered by small glands, rendering the plant pleasant aroma. This species inhabits screes and sheltered calcareous rocks, mainly in the gorges, at altitudes of 1000–1200 m. M. pulegium is an endangered taxon endemic to South Carpathians with scattered native area in Romania and with one single known locality in Eastern Serbia, and therefore protected by law in category of protected species in Serbia (Serbian Ministry of Environment and Physical Planning and Ministry of Agriculture, Forestry and Water Management 2010, 2011). The yield and the composition of the essential oils of M. pulegium recorded from natural populations within the territory of Eastern Serbia and from cultivated plants through different phenological stages were reported by Slavkovska et al. (2013). Both natural and cultivated populations were characterized by high amounts of the essential oils (0.8–1.4 %) at all stages of development. Chemical composition and antioxidant and antimicrobial activities of extracts of native and in vitro micropropagated M. pulegium plants have been recently reported by Tošić et al. (2015).
Because the chemical synthesis of most pharmaceutically important secondary metabolites is not economically convenient, these compounds are isolated from wild-growing or cultivated plants (Oksman-Caldentey and Inzé 2004). However, a number of publications reported on the shortage in natural resources of many medicinal and aromatic plants all over the world (Hamilton 2004). An increasing demand for plant-derived medicines confirms the importance of the establishment of an efficient plant tissue culture system with high-frequency plant regeneration for the production of pharmacologically active compounds. In addition, in vitro culture techniques can help overcome the individual variability, due to genetic and biochemical heterogeneity, which is the major difficulty in the use of the Lamiaceae species in the pharmaceutical industry (Saha et al. 2012).
Micropropagation is a valuable method for the multiplication of selected genotypes and chemotypes, leading to high regeneration rates of many medicinal and aromatic plants in vitro (Rout et al. 2000). These plants can be subsequently used for a variety of studies, thus avoiding collection from their natural habitat. In vitro propagation from wild-growing plants through axillary shoot formation reduces the possibility of the occurrence of abnormalities that exist with other methods (Kalemba and Thiem 2004). Along with somatic embryogenesis and adventitious shoot induction, micropropagation can efficiently serve to propagate clonally plants under controlled conditions, regardless of the season and to induce quantitative and qualitative modifications in the production of plant secondary metabolites by changing nutrient and hormonal composition of the growth medium, or different physical factors (Collin 2001). Several authors have shown that under certain culture conditions, the essential oil production could be increased without changing its composition (Santos-Gomes and Fernandes-Ferreira 2003; Affonso et al. 2009).
Wild-growing plants of M. pulegium are characterized by relatively high amounts of the essential oils rich in pulegone with a potential use as a bio-insecticide and a bio-pesticide (Koul et al. 2008). In view of the potential pharmacological and commercial value of this species, the present study was initiated to phytochemically examine the influence of in vitro culture on the production of the essential oils in micropropagated plants of M. pulegium. The effect of plant growth regulators on M. pulegium propagation through axillary bud induction was investigated, and the morphology and structure of M. pulegium glandular hairs in relation to the secretory process were characterized. In addition to assuring uniformity of plant material for the characterization of the essential oils, in vitro propagated plants can be subsequently used for ex situ conservation of this endemic and rare species.
Materials and methods
Plant material
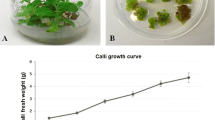
Aerial parts (shoots) of M. pulegium plants at the vegetative stage of development were randomly collected from wild-growing (natural) populations in Svrljiški Timok gorge, at latitude 43°32′23″N and longitude 22°10′16″E in August 2012 (Fig. 1a). Voucher specimen (Nº 6912) was deposited in the Herbarium collection of the Faculty of Science and Mathematics, University of Niš (HMN).
Micropropagation
Shoots dissected into one-node stem segments (~1 cm) bearing two axillary buds were used as explants to establish in vitro cultures, as described by Tošić et al. (2015). Nodal segments were surface-sterilized with 25 % commercial sodium hypochlorite solution (6 % active chlorine) containing two drops of liquid commercial detergent for 30 min and rinsed three times with sterile distilled water. Afterward, explants were placed in the solution containing 500 mg L−1 nystatin for 24 h and rinsed three times with sterile distilled water. Micropropagation was carried out on basal MS medium (Murashige and Skoog 1962) supplemented with 3 % sucrose (w/v) and 0.7 % (w/v) agar (Torlak, Belgrade) in 250-mL glass jars containing 25 mL of the medium. Ten explants were placed in each jar closed with polycarbonate cover. The pH of the media was adjusted to 5.8 prior to autoclaving at 114 °C for 25 min. Cultures were maintained in a growth chamber under a 16-h photoperiod, with a photon flux density 45 µE m−2 s−1 provided by cool white fluorescent lamps, at 25 ± 2 °C. Routine subculture was performed in 4-week intervals (Tošić et al. 2015).
Nodal segments derived from shoot cultures grown on basal medium for four weeks were transferred to MS medium supplemented with various plant growth regulators (PGRs) at different concentrations: N6–benzyladenine (BA) at 0.1, 0.3, 1, 3, 10 or 30 µM; kinetin (Kin) at 0.1, 0.3, 1, 3, 10 or 30 μM; 0.57 μM indole-3-acetic acid (IAA); 0.1–30 μM BA in combination with 0.57 μM IAA; and 0.1–30 μM Kin in combination with 0.57 μM IAA. Basal MS medium devoid of PGRs was used as a control. After 4 weeks in culture, the number of explants producing shoots, the number of shoots per explant and the shoot length, as well as explant fresh and dry weight, were recorded in order to evaluate the effect of PGRs on shoot multiplication.
For root induction, individual axillary shoots (15–20 mm) were harvested after the multiplication stage from explants grown on PGR-free MS medium and transferred to half-strength MS medium lacking growth regulators or supplemented with IAA, indole-3-butyric acid (IBA) or 1-naphthaleneacetic acid (NAA) at following concentrations: 0.57, 1.14, 1.71 and 5.71 µM for IAA; 0.49, 0.98, 1.48 and 4.9 µM for IBA; 0.54, 1.07, 1.61 and 5.37 µM for NAA. The percentage of rooted shoots and the root length were recorded after 5 weeks in culture. Well-rooted shoots were removed from the medium, washed with water to remove any adherent culture medium and transferred to plastic pots with soil mixture (60 % peat, 20 % red worm compost and 20 % sand), enclosed in a thin transparent plastic envelope to reduce water loss and maintained in the greenhouse. Plantlets were gradually acclimatized over a 4-week period.
Scanning electron microscopy
For scanning electron microscopy (SEM), fresh leaves isolated from shoots cultured on MS without PGRs were used. Leaf samples were coated with a thin layer of gold and palladium in a BAL-TEC SCD 005 sputter coater. Both adaxial and abaxial surfaces were examined with a JEOL JSM-6390 LV (JEOL, Tokyo, Japan) scanning electron microscope operated at 15 kV.
Light microscopy
Leaf specimens were fixed in 3 % glutaraldehyde in 0.1 M phosphate buffer (pH 7.2) for 24 h at 4 °C. Following a 2-h wash in the same buffer, material was postfixed in 1 % osmium tetroxide in phosphate buffer at 4 °C overnight. Fixed material was washed, dehydrated and embedded in Araldite resin CY 212 (Agar Scientific Ltd. England), as described by Glauert and Glauert (1958). Semithin cross sections (1–1.5 µm thick) were cut on LKB III ultramicrotome, stained with methylene blue and examined under a Zeiss Axiovert light microscope (Carl Zeiss GmbH, Göttingen, Germany).
Histochemical characterization
Histochemical analyses were performed on hand-sections of fresh leaves, in order to detect the presence of lipophilic substances in the actively secreting trichomes. The following histochemical tests were used: Sudan Black B and Sudan IV for total lipids (Jensen 1962); Nile Blue A for neutral and acidic lipids (Cain 1947); and Nadi reagent for terpenes (David and Carde 1964). For all the histochemical methods used, control tests were carried out following the suggestions of the respective authors. Sections were examined and photographed using a Zeiss Axiovert light microscope (Carl Zeiss GmbH, Göttingen, Germany).
Isolation and analysis of the essential oils
Wild-growing and micropropagated plants at the vegetative stage of development were used for the essential oil analysis. The essential oils from the aerial parts (shoots) of the plants dried at room temperature were obtained by hydrodistillation for 3 h in a Clevenger-type apparatus according to the procedure given in The European Pharmacopoeia (2011). The essential oils were dissolved using n-hexane, dried over anhydrous sodium sulfate and kept at −40 °C until analysis. The oil yield was expressed in mL per 100 g of dry weight of the plant material.
Volatile constituents were determined by GC and GC–MS analyses. GC and GC–MS analyses were performed on an Agilent 6890 N GC system equipped with 5975 MSD and FID, using a HP-5 MS column (30 m × 0.32 mm × 0.25 μm). Injection volume was 1 μL and injector temperature was 200 °C with a 10:1 split ratio. Helium was a carrier gas and its flow rate was 1.0 mL min−1 (constant flow mode). Column temperature was linearly programmed in the range from 60 to 280 °C at a rate of 3 °C min−1 and held at 280 °C for 5 min. The transfer line was heated at 250 °C. The FID detector temperature was 300 °C. EI mass spectra (70 eV) were acquired in the m/z range 35–550. The retention indices were experimentally determined using n-alkanes (C8–C20 and C21–C40) injected after the essential oil, under the same chromatographic conditions.
The identification of the compounds was based on the comparison of their retention indices (RI), their retention times and mass spectra with those obtained from authentic samples and/or the NIST AMDIS (Automated Mass Spectral Deconvolution and Identification System) software, Wiley libraries, Adams database and literature (Adams 2001). Relative percentages of the identified compounds were computed from the GC–FID peak area.
Statistical analysis
The efficiency of different PGR treatments on growth rate was determined by comparing biomass increase and in vitro proliferation rates, using different methods. Biomass increase was calculated on both fresh and dry weight basis. Proliferation rate was assessed by counting the number of shoots at subculture and following 4-week PGR treatment under previously defined conditions. The data were obtained from two repeated experiments, with sixty explants per each medium (ten explants/jar). Percentage data were subjected to angular transformation prior to analysis. The data were averaged and statistically analyzed, and differences were tested for significance using ANOVA multiple range test at the significance level of P ≤ 0.05.
Results and discussion
Shoot multiplication
The nodal explants of M. pulegium cultured on MS medium without plant growth regulators produced shoots (Fig. 1b). This contradicts the results obtained for different species of Lamiaceae family grown in vitro, where shoot proliferation required application of cytokinins (usually Kin or BA), alone or with auxin at low concentration (Saha et al. 2012; Bakhtiar et al. 2014). On media supplemented with cytokinins, the percentage of explants developing shoots was high for both BA and kinetin (Table 1).
Maximum axillary bud proliferation was obtained at 3 µM BA, whereas Kin did not increase shoot proliferation (Table 1). However, shoots cultured on medium supplemented with Kin had somewhat healthier appearance since hyperhydricity frequently occurred on BA-supplemented media. One of the most important factors known to induce vitrification is the excess of cytokinins in the culture medium (Leshem et al. 1988; Zuzarte et al. 2010). In combination with IAA neither of the applied cytokinins significantly affected axillary shoot induction (Table 1). However, IAA slightly increased shoot elongation (up to 10.58 ± 0.51 mm) when applied with BA. In contrast, Santoro et al. (2013) showed that supplementation with BA alone resulted in the highest values for shoot and root length.
Compared to medium without PGRs, neither of the cytokinins at lower concentrations increased the biomass production. BA at higher concentrations (10–30 µM BA) led to an increase in both fresh and dry weight per explant, regardless of the presence of auxin in the medium. Maximum biomass production was achieved on medium supplemented with 10 µM BA (Table 1). This contradicts the findings of Santoro et al. (2013), who showed that supplementation with BA alone resulted in the lowest values for shoot fresh weight in Mentha piperita, whereas the highest shoot fresh weight was obtained on medium supplemented with auxin (IBA), alone or in combination with BA. The effect of Kin on both fresh and dry biomass production was strongest when applied at 10 µM, in combination with 0.57 µM IAA. In general, Kin was more effective in increasing the biomass production when applied in combination with auxin (Table 1).
Rooting and acclimatization
Spontaneous rooting of micropropagated shoots was frequently observed in a number of Lamiaceae species such as Lavandula pedunculata (Zuzarte et al. 2010), Salvia dolomitica (Bassolino et al. 2015) and others, where roots were induced on PGR-free medium, or Origanum vulgare, where rooting did not require auxin supplementation but occurred at the end of the subculture cycle on BA-supplemented medium (Morone-Fortunato and Avato 2008). Spontaneous rooting that avoids the use of auxins known to induce callus and anomalous vascular connections between shoots and adventitious roots is advantageous for in vitro propagation. However, in M. pulegium only about 28 % of the shoots rooted spontaneously on the PGR–free medium; hence, the auxin treatment was used to promote rooting. Of all the auxins tested, IAA was the most efficient in promoting root induction (54 % at 5.71 µM IAA) as well as in subsequent root growth (Fig. 1c). Neither of the tested IBA concentrations stimulated rooting, whereas NAA induced few short roots only when applied at higher concentrations (data not shown).
Seventy-eight percent of the rooted plantlets were successfully acclimatized 4 weeks upon transfer to pots in the greenhouse. The survival of plants during acclimatization did not depend on the type or concentration of the auxin pretreatment. Acclimatized plants appeared healthy, without exhibiting any morphological abnormality or variation (Fig. 1d).
Morphoanatomical analysis
SEM and histochemical analysis revealed that leaf indumentum of in vitro grown M. pulegium, like in other members of the Lamiaceae, consisted of non-glandular and glandular trichomes distributed over both adaxial and abaxial surface (Fig. 2a). Single, uniseriate non-glandular trichomes were sharply pointed, with tendency to lean toward the leaf apex, and consisted of one to three cells. The main types of glandular trichomes observed in M. pulegium leaves were peltate (Fig. 2b–e) and capitate (Fig. 2f–l), which were also found in the wild-growing plants (data not shown). The presence of these two glandular trichome types is a characteristic feature of Lamiaceae species (Ascensão et al. 1999).
SEM and light micrographs of in vitro leaf trichomes of Micromeria pulegium. a The abaxial surface of leaf lamina showing both non-glandular (ng) and two types of glandular trichomes, peltate (p) and capitate (c), stained with NADI reagent. b Upper view of glandular head of fully developed peltate trichome, with eight secretory cells arranged in a disk. c Glandular head of immature peltate trichome with the cuticular cap attached to the head cells. d Glandular head of peltate trichome with developing subcuticular space during maturation. e Longitudinal section of mature peltate trichome with broad vacuolated basal cell (b) and short unicellular stalk (s) subtending the multicellular head (h). Note osmiophilic secretion in the large subcuticular space. f Upper view of capitate trichome type I. g Capitate trichome type I, with a short unicellular stalk (s) subtending unicellular glandular head (h) stained with Sudan IV. h Longitudinal section of the secretory-stage capitate trichome type I, showing the basal cell (b), unicellular stalk (s) and unicellular head (h). i Capitate trichome type II with elongated basal cell (b), short stalk (s) and glandular head with round tip (h). j Capitate trichome type II stained with Sudan Black B, showing vacuolated pyramidal basal cell (b) with thick cell wall (arrow) subtending cylindrical unicellular stalk (s) and glandular head (h), both of approximately same diameter. k Longitudinal section of capitate trichome type II, showing more vacuolated stalk cell with large nucleus, and apical secretory cell rich in cytoplasm with large subcuticular space (asterisk) filled with osmiophilic secretion. l Longitudinal section of capitate trichome type II, following cuticle rupture and the release of accumulated secretory product
In SEM micrographs, the surface of immature peltate glands appeared wrinkled, indicative of the close attachment of the cuticle to the secretory upper cell walls (Fig. 2c). During maturation, the gland surface becomes smoother as the secretory products accumulate within developing subcuticular space formed by detachment of the cuticle (Fig. 2d). Light micrographs showed that peltate trichomes consisted of a broad basal cell embedded in the epidermis, a short single-celled stalk with cutinized lateral walls, and a large round multicellular head (Fig. 2e). The glandular head of fully developed peltate trichome usually comprised eight secretory cells, arranged in a single disk (Fig. 2b). Such eight-celled glandular heads of peltate trichomes are similar to those reported for Salvia aurea (Serrato-Valenti et al. 1997), Plectranthus ornatus (Ascensão et al. 1999) or Mentha × piperita (Turner et al. 2000).
Unlike morphologically rather similar peltate trichomes, capitate trichomes differ in their morphological characteristics in the way that reflects different secretory processes within trichomes and probably distinct functions (Ascensão et al. 1999). Two types of capitate trichomes, differing in size and structure, could be distinguished in M. pulegium. Type I capitate trichomes were positioned at an angle to the leaf surface and more abundant on the abaxial side (Fig. 2f). They were composed of one basal epidermal cell, short cutinized stalk and unicellular ellipsoidal head (Fig. 2g, h). In M. pulegium type I capitate trichomes, cuticle elevation was not observed and the secretory product accumulated within the secretory cell (Fig. 2g, h). Although type I capitate trichomes are known to be present in only a few Lamiaceae species, a comparable trichome type was also observed in Micromeria croatica (Kremer et al. 2012) and Micromeria longipedunculata (Kremer et al. 2014). Type II capitate trichomes displayed similar density on both sides. They were comprised of one conical, usually elongated basal cell, unicellular stalk and one apical secretory cell (Fig. 2i, j). Type II capitate trichomes in M. pulegium correspond to those described by Werker et al. (1985) for the Lamiaceae, and are very similar to those found in Salvia officinalis (Corsi and Bottega 1999) or L. pedunculata (Zuzarte et al. 2010). In mature trichomes, apical cell had well-developed round subcuticular space (Fig. 2k). Following rupture, the cuticle remained firmly attached to the cell wall in the basal part of the secretory cell, with cuticle detachment occurring only in its upper region (Fig. 2l).
Histochemical analysis
The results of our histochemical analysis showed that the secretion of all types of M. pulegium glandular trichomes contained lipophilic substances. Although peltate trichomes have been generally assumed to contain the bulk of the essential oils produced by Lamiaceae, capitate trichomes of M. pulegium seemed to produce significant amounts of the essential oils.
In peltate trichomes, light-blue staining with Sudan Black B revealed the presence of lipids within the subcuticular space (Fig. 3a). The glandular secretion stained for neutral and acidic lipids with Nile Blue A gave positive reaction in the subcuticular space (pink staining) and head cells (intense blue staining, Fig. 3b). NADI reaction resulted in an intense dark-violet staining of the secretory product within subcuticular space, thus confirming the presence of terpenoid compounds in this trichome type (Fig. 3c). Terpenoid secretion was shown to be restricted to peltate glandular trichomes in Isodon rubescens (Lamiaceae), which accumulate their secretory products in a large subcuticular space (Liu et al. 2012).The accumulation of the secretory product between cuticular sheath and the head cells has been described in many aromatic Lamiaceae (Werker et al. 1985; Serrato-Valenti et al. 1997; Zuzarte et al. 2010).
Histochemistry of in vitro leaf glandular trichomes of Micromeria pulegium. a–c Peltate trichomes. a Secretion stained light blue with Sudan Black B. b Positive Nile Blue A reaction in the subcuticular space (pink droplet + weak violet staining) and head cells (intense blue staining). c Secretion in the subcuticular space stained positively with NADI reagent. d-f Capitate type I trichomes. d Secretion in the head cell showing faint staining with Sudan Black B. Note intense staining of the stalk cell lateral walls. e Secretion stained with Nile Blue A. f Positive NADI reaction (dark violet) in the head and stalk cell. g–l Capitate type II trichomes. Secretion stained dark blue in the head cell (g) and light blue in the subcuticular space (h) with Sudan Black B. Note intense staining of the head cell in g. Essential oils stained with Nile Blue A (pink droplets) accumulated in the subcuticular space of the early (i)- and late (j)-secretory-stage trichome. Terpenoids stained with NADI reagent (dark violet), accumulated in the subcuticular space of the head cell and in the stalk cell of the early (k)- and late (l)-secretory-stage trichome
In type I capitate trichomes, head cell contained small amount of lipids (Fig. 3d), both neutral and acidic as suggested by positive reaction with Nile Blue A (Fig. 3e). Staining with NADI reagent confirmed the presence of essential oils in head and stalk cell (Fig. 3f). Lipophilic secretion of capitate trichome type II observed within the head cell (Fig. 3g) and the subcuticular space (Fig. 3h) stained blue with Sudan Black B. Secretory product within subcuticular space stained red with Nile Blue A, indicating the presence of neutral lipids (Fig. 3i, j). The NADI reaction resulted in an intense violet staining of type II capitate trichome, indicative of the presence of terpenoids (essential oils) in head and stalk cell (Fig. 3k), that became even more abundant in mature trichomes, as evidenced by dark-violet staining of the secretion accumulated in their subcuticular space (Fig. 3l). Zuzarte et al. (2010) showed that type II capitate trichomes of L. pedunculata were rich in lipidic and terpenic compounds, in contrast to type I capitate trichomes that were not involved in the production of the essential oils. Significant amounts of the essential oils were also found in the secretions of long-stalked capitate trichomes of P. ornatus, which also contained non-cellulosic polysaccharides (Ascensão et al. 1999).
Stalk cells of both type I (Fig. 3d) and type II (Fig. 3g, h) capitate trichomes of M. pulegium had thick cutinized lateral walls. In most glandular trichomes, the stalk cells contain lipophilic substances (Ascensão et al. 1999; Corsi and Bottega 1999). The thickenings of the cuticle on the lateral walls of the stalk cell, besides providing structural support, probably prevent the backflow of the secreted products into mesophyll tissue and regulate directional transport of metabolites to the glandular cells above (Ascensão et al. 1999; Gersbach 2002).
Content and essential oil composition
In Lamiaceae, plant extracts secreted from glandular trichomes are a valuable source of biologically active compounds (Bassolino et al. 2015). Phytochemical analysis of wild-growing and micropropagated plants of M. pulegium revealed differences in both content and the composition of the essential oils obtained from different samples (Table 2). Micropropagated plants had lower yield of the essential oils than their parent plants, which yielded 0.47 % (v/w). The essential oil content in micropropagated plants ranged from 0.24 % (v/w) for plants grown on medium supplemented with 10 µM BA, to 0.38 % (v/w) for plants grown on PGR-free medium. The essential oil content was shown to depend on environmental factors, harvesting season and phenological stage (Hassiotis et al. 2014). Samples originating from natural and cultivated populations of M. pulegium were characterized by high amounts of the essential oils (0.8–1.4 %, v/w) at all stages of development (Slavkovska et al. 2013).
Differences in the essential oils composition were both quantitative and qualitative. In total, 12 compounds belonging to different terpenoid fractions were identified in the essential oils of the wild-growing plants, whereas in the essential oils of micropropagated plant material grown without PGRs or supplemented with BA 16 and 17 compounds were identified, respectively. The monoterpene fraction was dominant in all the analyzed samples (85.53–96.48 %), with the prevalence of oxygenated monoterpenes of the menthane type (Table 2). The main constituent was pulegone (60.07 % in wild-growing plants, 44.57 % in micropropagated plants grown on PGR-free medium, 50.77 % in micropropagated plants grown on BA-supplemented medium), followed by menthone (26.85 % in wild-growing plants, 29.14 % in micropropagated plants grown on PGR-free medium, 14.45 % in micropropagated plants grown on BA-supplemented medium). Some of the volatiles belonging to monoterpenoids were found only in plant material obtained by micropropagation but not in wild-growing plants. These include sabinene and myrcene among monoterpene hydrocarbons, and geranial, piperitone epoxide and piperitenone oxide belonging to oxygenated monoterpenes. Predominance of monoterpene hydrocarbons and oxygenated monoterpenes in the essential oils of in vitro cultivated plants, in comparison with in vivo grown plants, was reported for S. dolomitica (Bassolino et al. 2015). The authors argued that the variation in the composition of the essential oils was the consequence of different ontological stage, where in vitro cultured plants are by definition considered as a juvenile-stage plant, with monoterpene accumulation being restricted to young leaf tissues (Croteau et al. 1981).
In our sample, the percentage of total sesquiterpenoids in micropropagated plants ranged from 5.62 % (PGR-free medium) to 10.60 % (BA-supplemented medium), and was considerably higher than in wild-growing plants (1.47 %). Sesquiterpene hydrocarbons in micropropagated plants accounted for the majority of detected sesquiterpenoids and were dominated by germacrene D, but were absent from the essential oils of wild-growing plants (Table 2). This contradicts the findings of Bassolino et al. (2015) for micropropagated S. dolomitica, where the percentage of total sesquiterpenoids strongly decreased in plantlets grown in vitro.
Monoterpene-to-sesquiterpene ratio was shown to be a stable character of M. pulegium essential oils and largely independent of environmental conditions (Slavkovska et al. 2013). Our study showed that sesquiterpene fraction was higher (lower monoterpene-to-sesquiterpene ratio) in micropropagated plants, especially those treated with BA, due to the presence of sesquiterpene hydrocarbons that were not found in parent plants from natural populations (Table 2).
In contrast to L. pedunculata where no qualitative changes in the volatile profile of micropropagated plants were observed (Zuzarte et al. 2010), a number of authors have reported on quali-quantitative differences between wild-growing plants and those grown in vitro. In addition, secondary metabolite production can be drastically altered in response to different growth regulators in the culture medium (Affonso et al. 2009; Grzegorczyk-Karolak et al. 2015). Our results demonstrate that M. pulegium, both that gathered from natural populations and cultivated in vitro, produces essential oil of relatively stable composition, yet at the same time suggest that the careful selection of the culture conditions could increase the accumulation of biomass and production of secondary metabolites, which could be employed to obtain essential oils for commercial use.
Author contribution statement
DS designed and supervised the whole study and performed statistical analysis of the data. DS and ST performed tissue culture experiments. VS performed chemical analysis. BZ provided starting plant material and performed its botanical identification. DJ and SB performed microscopic analysis. BU wrote the manuscript and helped with data analysis and interpretation. All authors read and approved the manuscript.
Abbreviations
- BA:
-
N6–benzyladenine
- GC–MS:
-
Gas chromatography–mass spectrometry
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- Kin:
-
Kinetin
- MS:
-
Murashige and Skoog’s medium
- NAA:
-
1-naphthaleneacetic acid
- PGR:
-
Plant growth regulator
- SEM:
-
Scanning electron microscopy
References
Adams RP (2001) Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. Allured Publishing Corporation, Illinois
Affonso VR, Bizzo HR, Salguiero Lage CL, Sato A (2009) Influence of growth regulators in biomass production and volatile profile of in vitro plantlets of Thymus vulgaris L. J Agr Food Chem 57:6392–6395
Ascensão L, Mota L, Castro M De M (1999) Glandular trichomes on the leaves and flowers of Plectranthus ornatus: morphology, distribution and histochemistry. Ann Bot 84:437–447
Bakhtiar Z, Mirjalili MH, Sonboli A, Moridi Farimani M, Ayyari M (2014) In vitro propagation, genetic and phytochemical assessment of Thymus persicus—a medicinally important source of pentacyclic triterpenoids. Biologia 69:594–603
Bassolino L, Giacomelli E, Giovanelli S, Pistelli L, Casetti A, Damonte G, Bisio A, Ruffoni B (2015) Tissue culture and aromatic profile in Salvia dolomitica Codd. Plant Cell Tiss Organ Cult 121:83–95
Bräuchler C, Meimberg H, Heubl G (2006) New names in Old World Clinopodium—the transfer of the species of Micromeria sect. Pseudomelissa to Clinopodium. Taxon 55:977–981
Cain AJ (1947) The use of Nile blue in the examination of lipids. Q J Microsc Sci 88:383–392
Collin HA (2001) Secondary product formation in plant tissue cultures. Plant Growth Regul 34:119–134
Corsi G, Bottega S (1999) Glandular hairs of Salvia officinalis: new data on morphology, localization and histochemistry in relation to function. Ann Bot 84:657–664
Croteau R, Felton M, Karp F, Kjonaas R (1981) Relationship of camphor biosynthesis to leaf development in sage (Salvia officinalis). Plant Physiol 67:820–824
David R, Carde JP (1964) Coloration différentelle des inclusions lipidiques et terpéniques des pseudophylles du pin maritime au moyen du réactif Nadi. Comptes Rendus de l`Académie des Sciences. Paris 258:1338–1340
Duru ME, Öztürk M, Uğur A, Ceylan Ö (2004) The constituents of essential oil and in vitro antimicrobial activity of Micromeria cilicica from Turkey. J Ethnopharmacol 94:43–48
Gersbach PV (2002) The essential oil secretory structures of Prostanthera ovalifolia (Lamiaceae). Ann Bot 89:255–260
Glauert AM, Glauert RH (1958) Araldite as an embedding medium for electron microscopy. J Biophys Biochem Cytol 4:191–194
Grzegorczyk-Karolak I, Kuźma Ł, Wysokińska H (2015) The effect of cytokinins on shoot proliferation, secondary metabolite production and antioxidant potential in shoot cultures of Scutellaria alpina. Plant Cell Tiss Organ Cult 122:699–708
Hamilton AC (2004) Medicinal plants, conservation and livelihoods. Biodivers Conserv 13:1477–1517
Harley RM, Atkins S, Budantsev AL, Cantino PD, Conn BJ, Grayer R, Harley MM, de Kok R, Krestovskaja T, Morales R, Paton AJ, Ryding O, Upson T (2004) Labiatae. In: Kadereit JW, Kubitzki K (eds) The families and genera of vascular plants VII. Flowering plants. Dicotyledons: Lamiales (except Acanthaceae including Avicenniaceae). Springer, Berlin, pp 167–275
Hassiotis CN, Ntana F, Lazari DM, Poulios S, Vlachonasios KE (2014) Environmental and developmental factors affect essential oil production and quality of Lavandula angustifolia during flowering period. Ind Crops Prod 62:359–366
Jensen WA (1962) Botanical histochemistry: principles and practice. San Francisco, Freeman
Kalemba D, Thiem B (2004) Constituents of the essential oils of four micropropagated Solidago species. Flavour Frag J 19:40–43
Koul O, Walia S, Dhaliwal GS (2008) Essential oils as green pesticides: potential and constraints. Biopestic Int 4:63–84
Kremer D, Stabentheiner E, Dunkić V, Dragojević Müller I, Vujić L, Kosalec I, Ballian D, Bogunić F, Bezić N (2012) Micromorphological and chemotaxonomical traits of Micromeria croatica (Pers.) Schott. Chem Biodivers 9:755–768
Kremer D, Dunkić V, Stešević D, Kosalec I, Ballian D, Bogunić F, Bezić N, Stabentheiner E (2014) Micromorphological traits and essential oil of Micromeria longipedunculata Bräuchler (Lamiaceae). Centr Eur J Biol 9:559–568
Leshem B, Werker E, Shalev DP (1988) The effect of cytokinins on vitrification in melon and carnation. Ann Bot 62:271–276
Liu MQ, Liu ZW, Zhou J (2012) Morphology and histochemistry of the glandular trichomes of Isodon rubescens (Hemsley) H. Hara [Lamiaceae]: a promising medicinal plant of China. J Med Plants Res 6:1455–1460
Morone-Fortunato I, Avato P (2008) Plant development and synthesis of essential oils in micropropagated and mycorrhiza inoculated plants of Origanum vulgare L. ssp. hirtum (Link) Ietswaart. Plant Cell Tiss Organ Cult 93:139–149
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Oksman-Caldentey K-M, Inzé D (2004) Plant cell factories in the post-genomic era: new ways to produce designer secondary metabolites. Trends Plant Sci 9:433–440
Rout GR, Samantaray S, Das P (2000) In vitro manipulation and propagation of medicinal plants. Biotechnol Adv 18:91–120
Saha S, Kader A, Sengupta C, Ghosh P (2012) In vitro propagation of Ocimum gratissimum L. (Lamiaceae) and its evaluation of genetic fidelity using RAPD marker. Am J Plant Sci 3:64–74
Santoro MV, Nievas F, Zygadlo J, Giordano W, Banchio E (2013) Effects of growth regulators on biomass and the production of secondary metabolites in peppermint (Mentha piperita) micropropagated in vitro. Am J Plant Sci 4:49–55
Santos-Gomes PC, Fernandes-Ferreira M (2003) Essential oils produced by in vitro shoots of sage (Salvia officinalis). J Agric Food Chem 51:2260–2266
Šavikin PK, Menković RN, Zdunić MG, Tasić RS, Ristić SM, Stević RT, Dajić-Stevanović PZ (2010) Chemical composition and antimicrobial activity of the essential oils of Micromeria thymifolia (Scop.) Fritsch., M. dalmatica Benth., and Satureja cuneifolia Ten. and its secretory elements. J Essent Oil Res 22:91–96
Serbian Ministry of Environment and Physical Planning and Ministry of Agriculture, Forestry and Water Management (2010, 2011) Rule book about proclamation and protection of strictly protected and protected wild species of plants, animals and fungi, Beograd, JP “Službeni glasnik”, 5/10, 47/11 [Pravilnik o proglašenju i zaštiti strogo zaštićenih i zaštićenih divljih vrsta biljaka, životinja i gljiva]
Serrato-Valenti G, Bisio A, Cornara L, Ciarallo G (1997) Structural and histochemical investigation of the glandular trichomes of Salvia aurea L. leaves, and chemical analysis of the essential oil. Ann Bot 79:329–336
Slavkovska V, Couladis M, Bojović S, Tzakou O, Pavlović M, Lakušić B, Jančić R (2005) Essential oil and its systematic significance in species of Micromeria Bentham from Serbia and Montenegro. Plant Syst Evol 255:1–15
Slavkovska V, Zlatković B, Bräuchler C, Stojanović D, Tzakou O, Couladis M (2013) Variations of essential oil characteristics of Clinopodium pulegium (Lamiaceae) depending on phenological stage. Bot Serb 37:97–104
The European Pharmacopoeia (2011) The European Pharmacopoeia, 7th edn. Council of Europe, Strasbourg
Tošić S, Stojičić D, Stankov-Jovanović V, Mitić V, Mihajilov-Krstev T, Zlatković B (2015) Chemical composition, antioxidant and antimicrobial activities of micropropagated and native Micromeria pulegium (Lamiaceae) extracts. Oxid Commun 38:55–66
Turner GW, Gershenzon J, Croteau RB (2000) Distribution of peltate glandular trichomes on developing leaves of peppermint. Plant Physiol 124:655–664
Vladimir-Knežević S, Blažeković B, Bival Štefan M, Alegro A, Kőszegi T, Petrik J (2011) Antioxidant activities and polyphenolic contents of three selected Micromeria species from Croatia. Molecules 16:1454–1470
Werker E, Ravid U, Putievsky E (1985) Structure of glandular hairs and identification of the main components of their secreted material in some species of the Labiatae. Isr J Bot 34:31–45
Zuzarte MR, Dinis AM, Cavaleiro C, Salgueiro LR, Canhoto JM (2010) Trichomes, essential oils and in vitro propagation of Lavandula pedunculata (Lamiaceae). Ind Crop Prod 32:580–587
Acknowledgments
This research was financially supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia, Grant Nos. 173015, 173021 and 173030.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Stojičić, D., Tošić, S., Slavkovska, V. et al. Glandular trichomes and essential oil characteristics of in vitro propagated Micromeria pulegium (Rochel) Benth. (Lamiaceae). Planta 244, 393–404 (2016). https://doi.org/10.1007/s00425-016-2513-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-016-2513-7