Abstract
Main conclusion
The β-carotene isomerase OsDWARF27 is stereo- and double bond-specific. It converts bicyclic carotenoids with at least one unsubstituted β-ionone ring. OsDWARF27 may contribute to the formation of α-carotene-based strigolactone-like compounds.
Strigolactones (SLs) are synthesized from all-trans-β-carotene via a pathway involving the β-carotene isomerase DWARF27, the carotenoid cleavage dioxygenases 7 and 8 (CCD7, CCD8), and cytochrome P450 enzymes from the 711 clade (MAX1 in Arabidopsis). The rice enzyme DWARF27 was shown to catalyze the reversible isomerization of all-trans- into 9-cis-β-carotene in vitro. β-carotene occurs in different cis-isomeric forms, and plants accumulate other carotenoids, which may be substrates of DWARF27. Here, we investigated the stereo and substrate specificity of the rice enzyme DWARF27 in carotenoid-accumulating E. coli strains and in in vitro assays performed with heterologously expressed and purified enzyme. Our results suggest that OsDWARF27 is strictly double bond-specific, solely targeting the C9–C10 double bond. OsDWARF27 did not introduce a 9-cis-double bond in 13-cis- or 15-cis-β-carotene. Substrates isomerized by OsDWARF27 are bicyclic carotenoids, including β-, α-carotene and β,β-cryptoxanthin, that contain at least one unsubstituted β-ionone ring. Accordingly, OsDWARF27 did not produce the abscisic acid precursors 9-cis-violaxanthin or -neoxanthin from the corresponding all-trans-isomers, excluding a direct role in the formation of this carotenoid derived hormone. The conversion of all-trans-α-carotene yielded two different isomers, including 9′-cis-α-carotene that might be the precursor of strigolactones with an ε-ionone ring, such as the recently identified heliolactone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carotenoids are widely distributed pigments synthesized by all photosynthetic organisms as well as by different fungi and chemotrophic bacteria (Fraser and Bramley 2004; Walter and Strack 2011; Moise et al. 2014; Nisar et al. 2015). In plants, carotenoids fulfill vital functions in photosynthesis by protecting the photosynthetic apparatus from photo-oxidation and as antenna pigments absorbing light energy (Hirschberg 2001; Fraser and Bramley 2004). Carotenoids also contribute to the animal/plant communication and are responsible for the colors of many fruits and flowers, which accumulate these pigments in a special plastid form, the chromoplasts (Hirschberg 2001; Nisar et al. 2015). Apart from these plant-specific functions, carotenoids have a ubiquitous function as precursors of different biologically important compounds generally known as apocarotenoids (Giuliano et al. 2003; Moise et al. 2005; Bouvier et al. 2005; Auldridge et al. 2006; Walter and Strack 2011). The class of apocarotenoids includes volatiles, such as geranial and β-ionone (Ilg et al. 2009, 2014), pigments like crocetin (Frusciante et al. 2014) and neurosporaxanthin (Prado-Cabrero et al. 2007a), the fungal pheromone trisporic acid (Medina et al. 2011), the ubiquitous opsin chromophore retinal and its derivative the vertebrate morphogen retinoic acid (von Lintig 2012), the plant hormones abscisic acid (Schwartz et al. 1997) and strigolactones (Matusova et al. 2005; Alder et al. 2012). In addition, there are several hints on the presence of structurally unknown carotenoid derived signaling molecules with different functions (Kachanovsky et al. 2012; Avendano-Vazquez et al. 2014; Van Norman et al. 2014).
Carotenoids are isoprenoids with a carbon-skeleton consisting of 40 C-atoms. These pigments owe their color to an extended conjugated double bond system that usually contains 3–11 conjugated double bonds, and are divided into the oxygen free carotenes, such as β-carotene, and their hydroxylated derivatives, such as lutein and zeaxanthin, called xanthophylls (Hirschberg 2001; Fraser and Bramley 2004; Walter and Strack 2011). Plant carotenoid biosynthesis takes place in plastids and is initiated by the condensation of two molecules geranylgeranyl diphosphate with a C20 chain length, yielding the colorless 15-cis-phytoene. A series of isomerization and desaturation reactions converts 15-cis-phytoene into the red all-trans-lycopene via different intermediate cis-isomers of phytofluene, ζ-carotene, neurosporene and lycopene. The linear lycopene is the precursor of the monocyclic γ-carotene and the bicyclic β-carotene (β,β-carotene), which contains two β-ionone rings, and of the bicyclic α-carotene (β,ε-carotene) that has an ε- and a β-ionone ring. Hydroxylation of β-carotene leads via the mono-hydroxylated β-cryptoxanthin (β,β-cryptoxanthin) to zeaxanthin, while hydroxylation of α-carotene leads to lutein. Zeaxanthin is a constituent of the xanthophyll cycle and is epoxydated in reversible reactions to antheraxanthin and further to violaxanthin, the precursor of neoxanthin that represents the final product of the β-branch and a major constituent of the light-harvesting complex (Fraser and Bramley 2004; Ruiz-Sola and Rodriguez-Concepcion 2012; Moise et al. 2014).
Strigolactones (SLs) are a class of plant hormones (Gomez-Roldan et al. 2008; Umehara et al. 2008) that modulate the shape of plants in response to environmental conditions, by regulating different developmental processes, such as shoot branching and formation of lateral roots (de Saint et al. 2013; Kapulnik and Koltai 2014; Seto and Yamaguchi 2014; Waldie et al. 2014; Al-Babili and Bouwmeester 2015). SLs were first isolated from root exudates based on the capability to induce seed germination in root parasitic weeds of the genus Striga (Cook et al. 1966; Xie et al. 2010). However, SLs also induce hyphal branching of arbuscular mycorrhizal fungi, which is required to establish the mutualistic mycorrhizal symbiosis (Akiyama et al. 2005). Canonical SLs consist of a butenolide ring connected to a tricyclic lactone (ABC ring) via an enol ether bridge (Xie et al. 2010; Al-Babili and Bouwmeester 2015). They are divided into the orobanchol-like and strigol-like family, based on the stereochemistry of the B-C junction (Al-Babili and Bouwmeester 2015). SL biosynthesis (cf Fig. 5) occurs via the stereospecific cleavage of 9-cis-β-carotene that leads to the intermediate 9-cis-β-apo-10′-carotenal (Alder et al. 2012; Bruno et al. 2014). This step is catalyzed by the carotenoid cleavage dioxygenase 7 (CCD7), a member of the ubiquitous carotenoid cleavage oxygenase (CCO) enzyme family that also includes nine-cis-epoxycarotenoid-cleavage dioxygenase (NCED) involved in ABA biosynthesis (Walter and Strack 2011). In the next step CCD8, a further member of the CCO family, converts the CCD7 product 9-cis-β-apo-10′-carotenal by catalyzing a combination of yet non-understood reactions into carlactone, a SL that lacks the typical B–C-ring structure (Alder et al. 2012; Al-Babili and Bouwmeester 2015). However, CCD8 catalyzes also a common cleavage reaction by converting a different substrate, all-trans-β-apo-10′-carotenal, into all-trans-β-apo-13-carotenone (Alder et al. 2008). In Arabidopsis, carlactone is converted by a CYP450, more axillary growth 1 (MAX1), into carlactonoic acid (Abe et al. 2014). In rice, the MAX1 homolog carlactone oxidase catalyzes the formation of 4-deoxy-orobanchol from carlactone, which can be converted by a further MAX1 homolog, the orobanchol synthase, into orobanchol (Zhang et al. 2014). It is assumed that 5-deoxystrigol, the parent molecule of the second family of canonical SLs, is also formed from carlactone by a yet unidentified CYP450 enzyme (Zhang et al. 2014).
In a previous study, we showed that the rice enzyme OsDWARF27 (OsD27) catalyzes the reversible conversion of all-trans- into 9-cis-β-carotene in vitro, representing the first β-carotene isomerase reported so far (Alder et al. 2012). The OsD27 activity is required for SL biosynthesis, since it provides the CCD7 substrate 9-cis-β-carotene. However, the phenotype of the d27 mutant is not as severe as the ones of d17- (CCD7) and d10-mutants (CCD8) (Ishikawa et al. 2005), indicating that the OsD27 isomerase activity can be partially compensated by light isomerization (Jensen et al. 1982; Aman et al. 2005) or by other enzyme(s). The latter assumption is based on the presence of two OsD27-homologs in the rice genome (Waters et al. 2012), which may encode enzymes with OsD27 activity. Recently, we reported on the substrate specificity of CCD7 enzymes, and showed the cleavage of different 9-cis-configured carotenoids, including 9-cis-zeaxanthin, -lutein and cryptoxanthin (structures of the corresponding all-trans-isomers are shown in Fig. 1) (Bruno et al. 2014). The substrate range of CCD7 raised the question whether OsD27 can produce different 9-cis-carotenoids that might be precursors of different classes of SLs. To answer this question and to shed light on a possible role of OsD27 in the synthesis of other signaling molecules, which derive from 9-cis-carotenoids, we investigated the substrate specificity of OsD27.
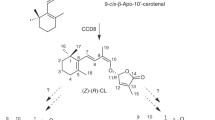
Chemical structures of substrates tested in vitro and obtained products (inset). The enzyme catalyzed reversible isomerization of all-trans-β-carotene into 9-cis-β-carotene (P1) and produced 9-cis-β,β-cryptoxanthin (P4) from the corresponding all-trans-isomer. The OsD27-mediated isomerization of all-trans-α-carotene that contains two different ionone rings (β and ε) led to two different products: 9-cis- (P2) and 9′-cis-α-carotene (P3). Other depicted substrates were not converted
Materials and methods
Cloning of pTHIO-OsD27 and pMAL-c4x-OsD27
The plasmid pCR-Blunt-OsD27, which contains the OsD27 full length cDNA, was kindly provided by Dr. Adrian Alder (Albert-Ludwigs-University, Freiburg, Germany). The plasmid pTHIO-OsD27, used for expression in carotenoid-accumulating E. coli cells, was generated by excision of the coding region for OsD27 from pCR-Blunt-OsD27 with EcoRI and ligation into the pBAD/THIO-TOPO® TA-vector (Invitrogen). The plasmid pMAL-c4X-OsD27, used for heterologous protein expression of the maltose binding protein (MBP) fusion construct, was generated by excision of the coding region for OsD27 from pThio-OsD27 with EcoRI and ligation into the pMAL-c4x-vector (New England Biolabs).
Substrate preparation
Substrates were purified using thin-layer silica gel plates from Merck (Darmstadt, Germany), according to Ruch et al. (2005). Synthetic apocarotenals were kindly provided by BASF (Ludwigshafen, Germany). Synthetic all-trans-β-carotene was obtained from Roth (Karlsruhe, Germany), β,β-cryptoxanthin from Sigma (Deisenhofen, Germany). Lutein, α-carotene, ε,ε-carotene, 9-cis/13-cis and 15-cis-β-carotene were obtained from carotenature (Lupsingen, Switzerland). Zeaxanthin, lycopene and γ-carotene were isolated from engineered carotenoid-accumulating E. coli strains. Carotenoid substrates were quantified with a photometer according to their molar extinction coefficients deduced from (Britton 1995).
Expression in carotenoid-accumulating E. coli strains
Lycopene- and β-carotene-accumulating E. coli strains were described previously (Prado-Cabrero et al. 2007b). A ζ-carotene-accumulating strain was generated by transforming TOP 10 E. coli cells with pZeta, a pPhytoene (Estrada et al. 2008) derivative equipped with phytoene desaturase from Synechocystis sp. PCC 6803. Strains were transformed either with pTHIO-OsD27 or the void plasmid that served as negative control. Three milliliters of transformed overnight culture was used to inoculate 50 ml of LB-medium with the respective antibiotics and grown at 37 °C. OsD27 expression was induced by adding 0.2 % arabinose (w/v), and cultures were incubated further at 28 °C for 6 h. The culture flasks were covered with aluminum foil during the whole growth period to prevent light induced isomerization. After harvesting, 10 ml of acetone was added to the obtained pellet, and short pulses of sonication were applied. After centrifugation for 10 min at 5000g, the supernatant containing organic compounds was dried in a rotary evaporator and re-dissolved in 100 µl CHCl3 of which 10 µl was subjected to HPLC analysis with system 1.
In vitro assays
For maltose binding protein (MBP) purification, the plasmid pMAL-c4x-OsD27 was transformed into BL21 Rosetta E. coli cells. Positive clones were then grown at 37 °C in LB-medium. Three ml of overnight cultures was inoculated into 50 ml of 2YT medium containing 1.6 % (w/v) tryptone, 1 % (w/v) yeast extract, 0.5 % (w/v) NaCl and 0.2 % (w/v) glucose. Cultures were grown at 37 °C until an OD600 of 0.5, induced with 0.3 mM IPTG (isopropyl-β-d-thiogalactoside) and further grown at 28 °C for 5 h. Cells were harvested and the pellets re-suspended in column buffer (CB) (20 mM Tris–HCl, 200 mM NaCl and 1 mM EDTA). After three French press passages, the sample was centrifuged for 15 min at 12,000g, and the supernatant was loaded on the equilibrated amylose resin column (New England Biolabs). After washing with 12 column volumes of CB, the MBP-fusion protein was eluted with CB containing 10 mM maltose. Protein concentration was determined using a Bradford-based kit (Bio-Rad, Munich, Germany). In vitro assays were performed in a total volume of 200 μl incubation buffer, containing 40 µM of purified substrates, 0.2 % ethanolic Triton X-100 (Sigma), 0.4 mM FeSO4, 100 mM MOPS pH 6.4, and 2 mg/ml catalase (Sigma). Purified MBP-OsD27 was added to a final concentration of 200 ng/µl. The assays were incubated for 1 h at 28 °C, under shaking at 200 rpm in darkness. Assays were extracted by adding two volumes of acetone and three volumes of light petroleum/diethylether (2:1, v/v). After centrifugation, the epiphase was collected, dried in a vacuum concentrator and re-dissolved in 40 µl of CHCl3. 5 µl was then subjected to HPLC analysis.
HPLC analyses
For carotenoid analyses, we used a Shimadzu (Europa GmbH, Duisburg, Germany) UFLC XR separation module, equipped with an SPD-M20A photodiode array detector and the Labsolutions software. Separation was performed using an YMC-pack-C30 reversed phase column (150 × 3 mm i.d., 5 µm; YMC Europa, Schermbeck, Deutschland) kept at 35 °C, and the solvent system A: MeOH/TBME (1:1, v/v, and B: MeOH/H2O/TBME (5:1:1, by vol), and the following separation systems:
Separation system 1 (used for xanthophylls and apocarotenoids) The column was developed at a flow rate of 0.75 ml min−1 with a gradient from 100 % B to 0 % B within 20 min, maintaining the final conditions for 4 min.
Separation system 2 (used for lycopene) The column was developed at a flow rate of 0.75 ml min−1 with a gradient from 35 % B to 0 % B within 20 min, maintaining the final conditions for 2 min.
Separation system 3 (used for β-/α-carotene) The column was developed at a flow rate of 0.75 ml min−1 with a gradient from 100 % B to 45 % B within 4 min, maintaining the final conditions for 20 min.
Results
OsD27 isomerizes β-carotene in vivo
To test the substrate specificity of OsD27, we expressed the enzyme in carotenoid-accumulating E. coli strains, an in vivo system frequently used to investigate carotenoid biosynthesis and cleavage enzymes (Cunningham et al. 1993; Booker et al. 2004; Prado-Cabrero et al. 2007b). For this purpose, we expressed the enzyme as a thioredoxin-fusion encoded by the plasmid pThio-OsD27 in β-, ζ-carotene, zeaxanthin and lycopene accumulating E. coli cells. Transformed cultures were grown in the dark, induced, harvested and analyzed by HPLC. The expression of OsD27 in β-carotene-accumulating E. coli cells led to an increase in the content of 9-cis-β-carotene (P1) that was only detectable in trace amounts in the control cultures (Fig. 2a). In addition, we observed an increase in the amount of 15-cis-β-carotene. Both isomers were identified based on their chromatographic behavior and by comparison to authentic standards. In contrast to β-carotene, the expression of OsD27 did not affect the cis/trans-isomer pattern of lycopene, ζ-carotene and zeaxanthin in the corresponding E. coli cells (Fig. 2b–d). These results indicate that OsD27 does not convert linear C40-carotenoids or zeaxanthin (for chemical structures of the substrates, see Fig. 1).
HPLC analysis of OsD27 activity in vivo: HPLC analysis of -β-carotene- (a), ζ-carotene- (b), lycopene- (c) or zeaxanthin (d) accumulating cells transformed with pThio-OsD27 (Thio-OsDWARF27) and the corresponding void plasmid (Thio-control). The expression of the enzyme did not alter the isomers pattern in b–d. In β-carotene-accumulating cells (a), we observed the formation of the isomer P1, identified as 9-cis-β-carotene, and an increase in 15-cis-β-carotene (asterisk). We used HPLC system 3 for samples a and b, and HPLC systems 3 and 1 for samples c and d, respectively. UV/Vis spectra of substrates and products are summarized in Suppl. Fig. S5, for chemical structures see Fig. 1. ζ-Carotene isomers were identified according to (Breitenbach and Sandmann 2005)
Optimization of the in vitro assay conditions
The E. coli in vivo system is limited regarding the substrates that can be offered. In addition, some of the carotenoids in E. coli are not accumulated as a major all-trans-isomer, but appear as a mixture of different isomers, which impedes drawing conclusions about substrate/product identity. For instance, ζ-carotene, which is very susceptible to photo-/thermo-isomerization, is accumulated in four different cis/trans-isomers in the corresponding E. coli strain, even when grown in the dark (see Fig. 2b). Therefore, we further explored the substrate specificity of the enzyme in vitro, using affinity-purified MBP-OsD27, as described in the methods section (for monitoring the purification, see Supplementary Fig. S1). To check the substrate specificity in vitro, we first identified optimal assay conditions by testing different buffers, detergents, reducing agents and metal-cofactors, and using all-trans-β-carotene as a substrate. After extraction, we analyzed the assays by HPLC and estimated the activity by comparing the peak area of the product 9-cis-β-carotene with that of the substrate all-trans-β-carotene. The activities were then compared with that obtained under published conditions (Alder et al. 2012), but in the absence of additional iron. Among the detergents tested, Triton X-100 yielded the best activity, whereas the usage of β-octyl glucopyranoside led to the lowest activity. OsD27 is an iron-binding enzyme that binds this co-factor during expression in E. coli (Lin et al. 2009). Nevertheless, adding iron to the assays increased the activity, likely because part of the protein is produced without iron or loses it during purification. Addition of other metals at 1 mM final concentrations did not alter the product formation. Similarly, the addition of reducing agents such as NAD(P)H or TCEP, had no effect on the enzyme activity. We also tested the activity of the enzymes in a range between pH 5.9 and pH 7.8 and in different buffers. Best results were obtained with pH 6.4 in MOPS buffer. The results of the in vitro assay optimization are summarized in Table 1.
OsD27 is double bond-specific
Using optimized in vitro assay conditions and a cis-/trans-isomers HPLC separation system (Separation system 3), we assayed the enzymatic activity of MBP-OsD27 with all-trans-β-carotene as substrate (Fig. 3a). The enzyme converted all-trans-β-carotene into one distinct product, 9-cis-β-carotene, identified by comparison to an authentic standard. Unlike the in vivo assays, we did not observe any formation of 15-cis-β-carotene. To further explore the stereo-specificity of the isomerization reaction catalyzed by OsD27, we incubated the enzyme with 9-cis, 13-cis and 15-cis-β-carotene isomers (for chemical structures, see Fig. 1). As reported previously (Alder et al. 2012), the enzyme catalyzes the reversible isomerization of C9–C10 double bond and, accordingly, it converted 9-cis-β-carotene into the all-trans-β-carotene isomer, leading to an equilibrium between both β-carotene isomers (Fig. 3b). The ratio of the product (9-cis-β-carotene)/substrate (all-trans-) in these assays was much higher than that observed upon using all-trans-β-carotene as a substrate (Fig. 3a), and, in both cases, the equilibrium was in favor of all-trans-β-carotene, indicating that the conversion of 9-cis- into all-trans-β-carotene is the preferred reaction. In contrast to 9-cis-β-carotene, the enzyme did not isomerize 13-cis- or 15-cis-β-carotene (Fig. 3c, d), suggesting a very strict specificity for the C9–C10 double bond.
HPLC analysis of OsD27 in vitro activity with different β-carotene isomers: Affinity-purified MBP-OsD27 fusion protein yielded 9-cis-β-carotene when all-trans-β-carotene was used as substrate (a). The isomerase performed the reverse reaction when 9-cis-β-carotene was offered (b). We did not observe any activity with the 13-cis- (c) and 15-cis (d) isomers of β-carotene. UV/Vis spectra of substrates and products are summarized in Suppl. Fig. S5. HPLC analysis was performed using system 3
OsD27 does not isomerize all-trans-apocarotenals
Since 9-cis-β-apo-10′-carotenal, the CCD7 cleavage product derived from 9-cis-β-carotene, is an essential intermediate in the biosynthetic pathway leading to carlactone (Alder et al. 2012; Bruno et al. 2014), we investigated whether apocarotenals are suitable substrates for OsD27. Such an activity would indicate that OsD27 can contribute to SL biosynthesis at two different levels, i.e., by providing the cis-substrate of both CCD7 and CCD8. To answer this question, we incubated the affinity-purified enzyme with all-trans-β-apo-8′-carotenal (C30) and all-trans-β-apo-10′-carotenal (C27) and analyzed the in vitro assays by HPLC (for chemical structures, see Fig. 1). However, we did not detect any activity with these substrates (Supplementary Fig. S2).
OsD27 isomerizes carotenoids containing one unmodified β-ionone ring
We further explored the substrate specificity of OsD27 by incubating the enzyme with all-trans-γ-carotene, -lycopene, α-carotene (β,ε-carotene), ε-carotene (ε,ε-carotene) and the xanthophylls all-trans-β,β-cryptoxanthin, -zeaxanthin, -lutein, -violaxanthin, -and neoxanthin (for chemical structures, see Fig. 1). As shown in Supplementary Fig. S3a, the enzyme did not convert the monocyclic γ-carotene. During testing the activity of the enzyme with acyclic lycopene, we observed that this substrate is spontaneously isomerized upon solubilization in Triton X-100 micelles. Therefore, we used liposomes containing this substrate to test the OsD27 activity. However, we did not detect any conversion activity (Supplementary Fig. S3b), confirming the results obtained from the E. coli in vivo system.
The incubation with α-carotene (β,ε-carotene) yielded two distinct products (Fig. 4a; P2 and P3) tentatively identified based on literature as 9-cis-α-carotene (P2) and 9′-cis-α-carotene (P3) of α-carotene (Emenhiser et al. 1996). We observed that the amount of 9-cis-α- is higher than that of 9′-cis-α-carotene, indicating a preference for isomerizing the 9-cis-double bond that occurs next to the β-ionone ring moiety (for chemical structures, see Fig. 1). The Incubation with all-trans-β,β-cryptoxanthin led to the formation of product P4 (Fig. 4c), which was identified based on literature (Melendez-Martinez et al. 2013) as 9-cis-β,β-cryptoxanthin (cis-double bond located in the vicinity of the unsubstituted β-ionone, for structures, see Fig. 1). In contrast to α-carotene (β,ε-carotene), the enzyme did not convert ε-carotene (ε,ε-carotene) (Fig. 4b). We also did not detect any conversion of lutein, zeaxanthin, violaxanthin and neoxanthin (Supplementary Fig. S4). These data suggest that the presence of one unmodified β-ionone ring in the bicyclic substrates is mandatory for the activity of the enzyme.
HPLC analysis of OsD27 in vitro activity with α-, ε-carotene and β,β-cryptoxanthin: Incubations of purified MBP-OsD27 with all-trans-α-carotene (a) yielded the two distinct products, P2 and P3, which were identified as 9-cis- and 9′-cis-α-carotene, respectively. (b) We did not observe any conversion of all-trans-ε-carotene (ε,ε). The incubation with all-trans-β,β-cryptoxanthin (c) yielded product P4 which was identified as the 9-cis-isomer of β,β-cryptoxanthin. UV/Vis spectra of substrates and products are summarized in Suppl. Fig. S5, for chemical structures see Fig. 1. For analysis, we used HPLC system 3 for a and b, and system 1 for c
Discussion
The rice enzyme D27 was originally identified as an iron-containing enzyme required for SL biosynthesis, based on the SL deficiency of the corresponding mutant (Lin et al. 2009). However, its enzymatic activity and role in SL biosynthesis was elusive due to the absence of significant homology to enzymes with known functions. Previously, the stereo-specificity of CCD7 and its selectivity for 9-cis-β-carotene as the sole isomer (Alder et al. 2012) guided us to the hypothesis that OsD27 may be a 9-cis/all-trans-β-carotene isomerase required to deliver the CCD7 substrate. Indeed, using purified enzyme, we showed that OsD27 exerts the presumed isomerase activity. We also observed that the enzyme catalyzes the isomerization in both directions, forming 9-cis-β- from all-trans-β-carotene and vice versa (Alder et al. 2012). The position of D27 upstream of other known SL biosynthesis enzymes, i.e., CCD7, CCD8 and MAX1, was confirmed by studies on the corresponding Arabidopsis mutant (Waters et al. 2012). The capability of CCD7 in cleaving 9-cis-configured carotenoids other than 9-cis-β-carotene, such as 9-cis-zeaxanthin and 9-cis-lutein (Bruno et al. 2014), raised the question whether these isomers are also produced by D27 activity. In addition, the 9-cis/all-trans isomerase activity of D27 led also to the question whether this enzyme is involved in ABA biosynthesis (Schwartz et al. 1997), by providing the precursor 9-cis-violaxanthin from the corresponding all-trans-isomer, or in the formation of proposed, yet unidentified signaling molecules that derive from linear, cis-configured C40-carotenoids. Such signals are assumed to be involved in chloroplast development (Avendano-Vazquez et al. 2014) or in the regulation of carotenoid biosynthesis (Kachanovsky et al. 2012). To answer these questions, we investigated the substrate specificity of OsD27, using an in vivo and an in vitro test system. Our results demonstrate that the substrates of OsD27 are bicyclic C40-carotenoids with at least one unsubstituted β-ionone ring. This substrate range includes β-carotene, α-carotene and β,β-cryptoxanthin. Other substrates like all-trans-ε-carotene, -zeaxanthin, -lutein, -violaxanthin or -neoxanthin were not converted. Similarly, the enzyme did not isomerize all-trans-apocarotenals. These results suggest narrow substrate specificity and make a direct involvement of OsD27 in the formation of ABA unlikely. It might be also speculated that 9-cis-zeaxanthin, 9-cis-, and 9′-cis-lutein, which are cleaved by CCD7, are formed by other enzymes, such as the D27-homologs that occur in rice and Arabidopsis (Waters et al. 2012) and which might have different substrate specificity. In addition, it appears reasonable that the other carotene isomerases, such as ZISO and CrtISO, which act on ζ-carotene and lycopene isomers, respectively (Isaacson et al. 2004; Park 2002), rather than OsD27, may be involved in the metabolism of signaling molecules that derive from acyclic intermediates of the carotenoid biosynthesis. The absence of all-trans-β-apocrotenals isomerization activity indicates that OsD27 cannot direct all-trans-β-apo-10′-carotenal produced by CCD4 (Bruno et al. 2015), a further member of the plant CCD family, into the SL pathway.
Investigation of the OsD27 activity in β-carotene-accumulating E. coli strain revealed the expected increase in 9-cis/all-trans-β-carotene ratio. However, we also observed an increase in the 15-cis-/all-trans-β-carotene ratio, indicating that OsD27 may also isomerize the central C15–C15′ double bond. To account for this possibility, we incubated the enzyme with all-trans- and 15-cis-β-carotene. In addition, we also tested the conversion of 13-cis-β-carotene. Subsequent HPLC analysis excluded the conversion of 13-cis- and 15-cis-β-carotene into all-trans-β carotene. This result suggests the stereo-specificity of OsD27, i.e., it does not introduce a C9–C10-double bond in 13-cis- or 15-cis-β-carotene substrates, which would lead to di-cis-products. In addition, this result shows strict double bond-specificity, i.e., the enzyme targets only the C9–C10 double bond and does not convert 13-cis- or 15-cis-β-carotene into all-trans-β-carotene. Therefore, it can be speculated that the increase in 15-cis-β-carotene observed in the E. coli system is due to non-enzymatic, secondary isomerization of 9-cis-β-carotene that was produced by OsD27. As can be deduced from the HPLC analysis (Fig. 3b), the enzyme established an equilibrium between 9-cis- and all-trans-β-carotene, in which all-trans-β-carotene is the predominant β-carotene form, regardless which isomer was offered as a substrate. This result indicates that the conversion of 9-cis- into all-trans-β-carotene is the preferred reaction and is in line with a very recent study about carlactone-forming enzymes (Harrison et al. 2015) that shows a higher binding affinity for 9-cis-β-carotene.
The incapability of OsD27 to convert xanthophylls like zeaxanthin, viola- and neoxanthin demonstrates that OsD27 does not directly provide the ABA precursors 9-cis-violaxanthin/9′-cis-neoxanthin. However, it can be speculated that 9-cis-β-carotene produced by OsD27 is converted by a series of hydroxylation and epoxidation reactions into 9-cis-violaxanthin, via a 9-cis-route similar to the established all-trans-pathway that leads from all-trans-β-carotene to all-trans-violaxanthin (Fig. 5). This speculation is based on our observation that modulating the OsD27 activity in rice leads to changes in ABA levels (data not shown). We are currently investigating the role of OsD27 in abiotic stress response.
SL and ABA biosynthesis. OsD27 catalyzes the formation of 9-cis-β-carotene that is converted by CCD7 and CCD8 into carlactone. In rice, the canonical SL, orobanhol, is synthesized from carlactone by the sequential action of carlactone oxidase (CO) and orobanchol synthase (OS) (Zhang et al. 2014). In Arabidopsis, carlactone is converted by MAX1 (more axillary growth 1) into carlactonoic acid which is methylated by an unknown enzyme, yielding methylcarlactonate (Abe et al. 2014). OsD27 catalyzes also the isomerization of all-trans-α-carotene into the corresponding 9′-cis-isomer. It can be speculated that 9′-cis-α-carotene is the precursor of an ε-ionone ring containing carlactone and that this ε-carlactone is converted into heliolactone via a route similar to that leading from carlactone to methylcarlaconate. The carotenoid pathway leads from all-trans-β-carotene to all-trans-violaxanthin via zeaxanthin. 9-cis-Violaxanthin is formed by a yet unidentified isomerase from the corresponding all-trans-isomer. The enzyme 9-cis-epoxycarotenoid dioxygenase (NCED) cleaves 9-cis-violaxanthin into the ABA precursor xanthoxin. Dashed arrows indicate hypothetical reactions
The conversion of α-carotene demonstrates that OsD27 acts on the α- and the β-branch of the plant carotenoid biosynthetic pathway. It can be speculated that 9-cis-α-carotene produced by OsD27 is a precursor of α-carotene-derived SLs/SL-like compounds (Fig. 5). This assumption is based on the conversion of the α-carotene-derived xanthophyll 9-cis-lutein by CCD7 and on the presence of heliolactone, a SL-like compound isolated from sunflower, which carries a ε-ionone ring instead of β-ionone ring (Ueno et al. 2014). We are currently investigating whether CCD7/CCD8 combined activity can lead to α-carotene derived, ε-ionone ring containing carlactone that may be the precursor of heliolactone and similar compounds.
Author contribution statement
Salim Al-Babili designed the research. Mark Bruno performed the experiments. The manuscript was written by Salim Al-Babili and Mark Bruno.
Abbreviations
- CCD:
-
Carotenoid cleavage dioxygenase
- MBP:
-
Maltose binding protein
- SL:
-
Strigolactone
References
Abe A, Sado A, Tanaka K, Kisugi T, Asami K, Ota S, Kim HI, Yoneyama K, Xie X, Ohnishi T, Seto Y, Yamaguchi S, Akiyama K, Yoneyama K, Nomura T (2014) Carlactone is converted to carlactonoic acid by MAX1 in Arabidopsis and its methyl ester can directly interact with AtD14 in vitro. Proc Natl Acad Sci USA 111:18084–18089
Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435:824–827
Al-Babili S, Bouwmeester HJ (2015) Strigolactones, a novel carotenoid-derived plant hormone. Annu Rev Plant Biol 66:161–186
Alder A, Holdermann I, Beyer P, Al-Babili S (2008) Carotenoid oxygenases involved in plant branching catalyse a highly specific conserved apocarotenoid cleavage reaction. Biochem J 416:289–296
Alder A, Jamil M, Marzorati M, Bruno M, Vermathen M, Bigler P, Ghisla S, Bouwmeester H, Beyer P, Al-Babili S (2012) The path from beta-carotene to carlactone, a strigolactone-like plant hormone. Science 335:1348–1351
Aman R, Schieber A, Carle R (2005) Effects of heating and illumination on trans-cis isomerization and degradation of beta-carotene and lutein in isolated spinach chloroplasts. J Agric Food Chem 53:9512–9518
Auldridge ME, McCarty DR, Klee HJ (2006) Plant carotenoid cleavage oxygenases and their apocarotenoid products. Curr Opin Plant Biol 9:315–321
Avendano-Vazquez AO, Cordoba E, Llamas E, San Roman C, Nisar N, De la Torre S, Ramos-Vega M, Gutierrez-Nava MD, Cazzonelli CI, Pogson BJ, Leon P (2014) An uncharacterized apocarotenoid-derived signal generated in zeta-carotene desaturase mutants regulates leaf development and the expression of chloroplast and nuclear genes in Arabidopsis. Plant Cell 26:2524–2537
Booker J, Auldridge M, Wills S, McCarty D, Klee H, Leyser O (2004) MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Curr Biol 14:1232–1238
Bouvier F, Isner JC, Dogbo O, Camara B (2005) Oxidative tailoring of carotenoids: a prospect towards novel functions in plants. Trends Plant Sci 10:187–194
Breitenbach J, Sandmann G (2005) zeta-Carotene cis isomers as products and substrates in the plant poly-cis carotenoid biosynthetic pathway to lycopene. Planta 220:785–793
Britton G (1995) Carotenoids, vol 1b: Spectroscopy. Birkhäuser Verlag, Basel
Bruno M, Hofmann M, Vermathen M, Alder A, Beyer P, Al-Babili S (2014) On the substrate- and stereospecificity of the plant carotenoid cleavage dioxygenase 7. FEBS Lett 588:1802–1807
Bruno M, Beyer P, Al-Babili S (2015) The potato carotenoid cleavage dioxygenase 4 catalyzes a single cleavage of beta-ionone ring-containing carotenes and non-epoxidated xanthophylls. Arch Biochem Biophys 572:126–133
Cook CE, Whichard LP, Turner B, Wall ME, Egley GH (1966) Germination of witchweed (Striga lutea Lour.): isolation and properties of a potent stimulant. Science 154:1189–1190
Cunningham FX, Chamovitz D, Misawa N, Gantt E, Hirschberg J (1993) Cloning and functional expression in Escherichia coli of a cyanobacterial gene for lycopene cyclase, the enzyme that catalyzes the biosynthesis of β-carotene. FEBS Lett 328:130–138
de Saint Germain A, Bonhomme S, Boyer FD, Rameau C (2013) Novel insights into strigolactone distribution and signalling. Curr Opin Plant Biol 16:583–589
Emenhiser C, Englert G, Sander LC, Ludwig B, Schwartz SV (1996) Isolation and structural elucidation of the predominant geometrical isomers of alpha-carotene. J Chromatogr A 719:333–343
Estrada AF, Maier D, Scherzinger D, Avalos J, Al-Babili S (2008) Novel apocarotenoid intermediates in Neurospora crassa mutants imply a new biosynthetic reaction sequence leading to neurosporaxanthin formation. Fungal Genet Biol 45:1497–1505
Fraser PD, Bramley PM (2004) The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res 43:228–265
Frusciante S, Diretto G, Bruno M, Ferrante P, Pietrella M, Prado-Cabrero A, Rubio-Moraga A, Beyer P, Gomez-Gomez L, Al-Babili S, Giuliano G (2014) Novel carotenoid cleavage dioxygenase catalyzes the first dedicated step in saffron crocin biosynthesis. Proc Natl Acad Sci USA 111:12246–12251
Giuliano G, Al-Babili S, Von Lintig J (2003) Carotenoid oxygenases: cleave it or leave it. Trends Plant Sci 8:145–149
Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pages V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, Bouwmeester H, Becard G, Beveridge CA, Rameau C, Rochange SF (2008) Strigolactone inhibition of shoot branching. Nature 455:189–194
Harrison PJ, Newgas SA, Descombes F, Shepherd SA, Thompson AJ, Bugg TD (2015) Biochemical characterization and selective inhibition of beta-carotene cis-trans isomerase D27 and carotenoid cleavage dioxygenase CCD8 on the strigolactone biosynthetic pathway. FEBS J 282:3986–4000
Hirschberg J (2001) Carotenoid biosynthesis in flowering plants. Curr Opin Plant Biol 4:210–218
Ilg A, Beyer P, Al-Babili S (2009) Characterization of the rice carotenoid cleavage dioxygenase 1 reveals a novel route for geranial biosynthesis. FEBS J 276:736–747
Ilg A, Bruno M, Beyer P, Al-Babili S (2014) Tomato carotenoid cleavage dioxygenases 1A and 1B: relaxed double bond specificity leads to a plenitude of dialdehydes, mono-apocarotenoids and isoprenoid volatiles. FEBS Open Biol 4:584–593
Isaacson T, Ohad I, Beyer P, Hirschberg J (2004) Analysis in vitro of the enzyme CRTISO establishes a poly-cis-carotenoid biosynthesis pathway in plants. Plant Physiol 136:4246–4255
Ishikawa S, Maekawa M, Arite T, Onishi K, Takamure I, Kyozuka J (2005) Suppression of tiller bud activity in tillering dwarf mutants of rice. Plant Cell Physiol 46:79–86
Jensen NH, Nielsen AB, Wilbrandt R (1982) Chlorophyll a-sensitized trans-cis photoisomerization of all-trans-beta-carotene. J Am Chem Soc 104:6117–6119
Kachanovsky DE, Filler S, Isaacson T, Hirschberg J (2012) Epistasis in tomato color mutations involves regulation of phytoene synthase 1 expression by cis-carotenoids. Proc Natl Acad Sci USA 109:19021–19026
Kapulnik Y, Koltai H (2014) Strigolactone involvement in root development, response to abiotic stress, and interactions with the biotic soil environment. Plant Physiol 166:560–569
Lin H, Wang R, Qian Q, Yan M, Meng X, Fu Z, Yan C, Jiang B, Su Z, Li J, Wang Y (2009) DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell 21:1512–1525
Matusova R, Rani K, Verstappen FWA, Franssen MCR, Beale MH, Bouwmeester HJ (2005) The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiol 139:920–934
Medina HR, Cerda-Olmedo E, Al-Babili S (2011) Cleavage oxygenases for the biosynthesis of trisporoids and other apocarotenoids in Phycomyces. Mol Microbiol 82:199–208
Melendez-Martinez AJ, Stinco CM, Liu C, Wang XD (2013) A simple HPLC method for the comprehensive analysis of cis/trans (Z/E) geometrical isomers of carotenoids for nutritional studies. Food Chem 138:1341–1350
Moise AR, von Lintig J, Palczewski K (2005) Related enzymes solve evolutionarily recurrent problems in the metabolism of carotenoids. Trends Plant Sci 10:178–186
Moise AR, Al-Babili S, Wurtzel ET (2014) Mechanistic aspects of carotenoid biosynthesis. Chem Rev 114:164–193
Nisar N, Li L, Lu S, Khin NC, Pogson BJ (2015) Carotenoid metabolism in plants. Mol Plant 8:68–82
Park H (2002) Identification of the carotenoid isomerase provides insight into carotenoid biosynthesis, prolamellar body formation, and photomorphogenesis. Plant Cell 14:321–332
Prado-Cabrero A, Estrada AF, Al-Babili S, Avalos J (2007a) Identification and biochemical characterization of a novel carotenoid oxygenase: elucidation of the cleavage step in the Fusarium carotenoid pathway. Mol Microbiol 64:448–460
Prado-Cabrero A, Scherzinger D, Avalos J, Al-Babili S (2007b) Retinal biosynthesis in fungi: characterization of the carotenoid oxygenase CarX from Fusarium fujikuroi. Eukaryot Cell 6:650–657
Ruch S, Beyer P, Ernst H, Al-Babili S (2005) Retinal biosynthesis in Eubacteria: in vitro characterization of a novel carotenoid oxygenase from Synechocystis sp. PCC 6803. Mol Microbiol 55(4):1015–1024. doi:10.1111/j.1365-2958.2004.04460.x
Ruiz-Sola MA, Rodriguez-Concepcion M (2012) Carotenoid biosynthesis in Arabidopsis: a colorful pathway. Arabidopsis Book 10:e0158
Schwartz SH, Tan BC, Gage DA, Zeevaart JA, McCarty DR (1997) Specific oxidative cleavage of carotenoids by VP14 of maize. Science 276:1872–1874
Seto Y, Yamaguchi S (2014) Strigolactone biosynthesis and perception. Curr Opin Plant Biol 21C:1–6
Ueno K, Furumoto T, Umeda S, Mizutani M, Takikawa H, Batchvarova R, Sugimoto Y (2014) Heliolactone, a non-sesquiterpene lactone germination stimulant for root parasitic weeds from sunflower. Phytochemistry 108:122–128
Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, Kyozuka J, Yamaguchi S (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455:195–200
Van Norman JM, Zhang J, Cazzonelli CI, Pogson BJ, Harrison PJ, Bugg TD, Chan KX, Thompson AJ, Benfey PN (2014) Periodic root branching in Arabidopsis requires synthesis of an uncharacterized carotenoid derivative. Proc Natl Acad Sci USA 111:E1300–E1309
von Lintig J (2012) Metabolism of carotenoids and retinoids related to vision. J Biol Chem 287:1627–1634
Waldie T, McCulloch H, Leyser O (2014) Strigolactones and the control of plant development: lessons from shoot branching. Plant J 79:607–622
Walter MH, Strack D (2011) Carotenoids and their cleavage products: biosynthesis and functions. Nat Prod Rep 28:663–692
Waters MT, Brewer PB, Bussell JD, Smith SM, Beveridge CA (2012) The Arabidopsis ortholog of rice DWARF27 acts upstream of MAX1 in the control of plant development by strigolactones. Plant Physiol 159:1073–1085
Xie X, Yoneyama K, Yoneyama K (2010) The strigolactone story. Annu Rev Phytopathol 48:93–117
Zhang Y, van DijK ADJ, Scaffidi A, Flematti GR, Hofmann M, Charnikhova T, Verstappen F, Hepworth J, van der Krol S, Leyser O, Smith SM, Zwanenburg B, Al-Babili S, Ruyter-Spira C, Bouwmeester H (2014) Rice cytochrome P450 MAX1 homologs catalyze distinct steps in strigolactone biosynthesis. Nat Chem Biol 10:1028–1033
Acknowledgments
We thank Dr. Peter Beyer, University of Freiburg, Germany, for valuable discussions and Dr. Hansgeorg Ernst, BASF, Germany, for providing the synthetic apocarotenoid substrates. The research reported in this publication was supported by funding from King Abdullah University of Science and Technology (KAUST) and the EU (METAPRO; FP7 KBBE-2009-3-1-01).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
425_2016_2487_MOESM1_ESM.pptx
Suppl. Fig. S1 SDS PAGE of maltose binding protein (MBP) purified D27-fusion protein. Lanes represent: M, molecular marker (size in kDa on the left). a Solubilized total lysate of control cells expressing MBP after French press. b Total lysate of MBP-OsD27 producing cells. c Supernatant from (b) after centrifugation. d Flow through obtained after (C) binding to amylose resin. e Eluate of control, (2) indicating the expressed MBP. f Eluate from amylose resin of OsD27-fusion protein; (1) pMAL-OsD27 fusion protein (PPTX 99 kb)
425_2016_2487_MOESM2_ESM.pptx
Suppl. Fig. S2 HPLC analysis of in vitro assays with apocarotenoids: We did not observe any isomerization of all-trans β-apo-8′-carotenal (a) or for all-trans β-apo-10′-carotenal (b). UV/Vis spectra of substrates are summarized in Suppl. Fig. S5, for chemical structures see Fig. 1. HPLC system 1 was employed for separation (PPTX 67 kb)
425_2016_2487_MOESM3_ESM.pptx
Suppl. Fig. S3 In vitro activity of MBP-OsD27 with linear and monocyclic carotenes: No conversion was observed when purified MBP-fusion protein was incubated with the linear all-trans-lycopene (a) or the monocyclic γ-carotene (b). For separation, we used systems 2 (a) and 1 (b). UV/Vis spectra of substrates are summarized in Suppl. Fig. S5, for chemical structures see Fig. 1 (PPTX 68 kb)
425_2016_2487_MOESM4_ESM.pptx
Suppl. Fig. S4 In vitro activity of MBP-OsD27 with xanthophylls: The MBP-OsD27 did not convert the dihydroxylated all-trans zeaxanthin (a) and all-trans-lutein (b), nor the epoxydized all-trans-viola- (c) and neoxanthin (d). UV/Vis spectra of substrates are summarized in Suppl. Fig. S5, for chemical structures see Fig. 1. We used HPLC system 1 for analysis (PPTX 106 kb)
425_2016_2487_MOESM5_ESM.pptx
Suppl. Fig. S5 UV/Vis spectra of substrates and products: (I) all-trans-β-carotene, (II) 9-cis-β-carotene, (III) 13-cis-β-carotene, (IV) 15-cis-β-carotene, (V) all-trans-α-carotene, (VI) 9-cis-α-carotene, (VII) 9′-cis-α-carotene, (VIII) all-trans-ε,ε-carotene, (IX) all-trans-β,β-cryptoxanthin, (X) 9-cis-β,β-cryptoxanthin, (XI) all-trans-zeaxanthin, (XII) all-trans-lutein, (XIII) all-trans-violaxanthin, (XIV) all-trans-neoxanthin, (XV) all-trans-lycopene, (XVI) all-trans-γ-carotene, (XVII) all-trans-β-apo-8′-carotenal, (XVIII) β-apo-10′-carotenal (PPTX 92 kb)
Rights and permissions
About this article
Cite this article
Bruno, M., Al-Babili, S. On the substrate specificity of the rice strigolactone biosynthesis enzyme DWARF27. Planta 243, 1429–1440 (2016). https://doi.org/10.1007/s00425-016-2487-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-016-2487-5