Abstract
Main conclusion
The Taenia solium HP6/TSOL18 antigen was produced in carrot cells, yielding an immunogenic protein that induced significant protection in an experimental murine model against T. crassiceps cysticercosis when orally administered. This result supports the potential of HP6/TSOL18-carrot as a low-cost anti-cysticercosis vaccine candidate.
Cysticercosis is a zoonosis caused by Taenia solium that can be prevented by interrupting the parasite life cycle through pig vaccination. Several injectable vaccine candidates have been reported, but the logistic difficulties and costs for its application limited its use in nationwide control programs. Oral plant-based vaccines can deal with this limitation, because of their easy administration and low cost. A stable expression of the HP6/TSOL18 anti-T. solium cysticercosis protective antigen in carrot calli transformed with an optimized transgene is herein reported. An antigen accumulation up to 14 µg g−1 of dry-weight biomass was achieved in the generated carrot lines. Mouse immunization with one of the transformed calli induced both specific IgG and IgA anti-HP6/TSOL18 antibodies. A statistically significant reduction in the expected number of T. crassiceps cysticerci was observed in mice orally immunized with carrot-made HP6/TSOL18, in a similar extent to that obtained by subcutaneous immunization with recombinant HP6/TSOL18 protein. In this study, a new oral plant-made version of the HP6/TSOL18 anti-cysticercosis vaccine is reported. The vaccine candidate should be further tested against porcine cysticercosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cysticercosis is a parasitic disease, caused by the establishment of the metacestode stage of Taenia solium in muscular or nervous tissues. It affects humans and pigs in developing countries where conditions that favor the parasite life cycle prevail (De Aluja 2008). The most severe form of the disease occurs when larvae (cysticerci) lodge in the human central nervous system (CNS), causing neurocysticercosis (NC) (Garcia et al. 2005).
With regard to vaccination, a number of highly protective, field-trial tested vaccine antigens have been reported, all of them administered by injection (Huerta et al. 2001; Morales et al. 2008; Assana et al. 2010; Jayashi et al. 2012). Research and development, as well as the approval process, demand high investments that are subsequently recovered with higher-cost vaccines. In addition, production and delivery costs also have a high impact on vaccination campaigns (Gandhi et al. 2013). All these factors limit vaccine coverage in developing countries.
A control program comprising a widespread application of the S3Pvac-phage anti-cysticercosis vaccine along with a health education program conducted for 3 years in a region of southern Mexico proved to reduce cysticercosis transmission effectively (De Aluja et al. 2014). The results of this program made clear the obstacles for the widespread application of an injectable vaccine (Larralde and Sciutto 2006). A needle-free oral vaccine could cope these difficulties, representing thus a more realistic immunization approach, avoiding costly logistics problems and suitable for nationwide vaccination programs (Hernández et al. 2014). In addition, oral immunization-elicited mucosal immunity offers a first barrier against infective agents entering the body by oral ingestion. The concomitant systemic immunity induced by oral vaccination could then damage those oncospheres that eluded mucosal immunity (Dabral et al. 2014). The viability of this technology is supported by the fact that a number of companies are pursuing the development of plant-based vaccines, with some candidates close to marketing (Yusibov et al. 2011).
Conventional vaccine production platforms require expensive reactors and extensive purification steps during production. On the other hand, plant-based vaccine production platforms offer a significant reduction in production costs that do not require sophisticated bioreactors or purification process (Batson 1998; Berman and Giffin 2004). It has been estimated that the cost of raw materials for plant systems is 10 times lower than that of the E. coli-based systems (Streatfield and Howard 2003; Gecchele et al. 2015). In conjunction with government investment, plant-based vaccines may play a key role on achieving proper vaccination coverage (Daniell 2006).
In this context, carrot is a convenient plant species, since undifferentiated carrot cells can be grown in bioreactors with low-cost culture media, yielding safe biomass that can be easily stabilized by a freeze-drying process, for instance (Streatfield and Howard 2003). Several antigens have been produced in carrot, including antigenic proteins from measles virus, the B subunit of the Escherichia coli heat-labile enterotoxin (LTB), and the F1 and V antigens from Yersinia pestis. Expression levels were sufficient to induce immune responses in mice (Rosales-Mendoza et al. 2007, 2011) or even immunoprotection against E. coli heat-labile enterotoxin (Rosales-Mendoza et al. 2008). Currently, Protalix BioTherapeutics (Carmiel, Israel) is marketing a plant-derived product named ELELYSO, a recombinant hydrolytic lysosomal glucocerebroside-specific enzyme for long-term enzyme replacement therapy (ERT) for adults with confirmed diagnosis of type-1 Gaucher’s disease. This case exemplifies the potential of the plant-made biopharmaceuticals produced in carrot cell suspensions (Aviezer et al. 2009).
A plant-based anti-cysticercosis vaccine was developed in our research group, using transgenic embryogenic papaya cells. S3Pvac-papaya induced high protection levels against T. crassiceps and T. pisiformis cysticercosis when parenterally or orally administered (Hernández et al. 2007; Betancourt et al. 2012). This vaccine promoted an exacerbated specific cellular immunity that could mediate the observed protection. On the other hand, the HP6/TSOL18 vaccine candidate antigen, initially identified in T. ovis (131 aa) and T. saginata (131 aa) (Benitez et al. 1996; Harrison et al. 1996), and subsequently found in T. solium (130 aa) (Gauci et al. 1998), induced protection thorough an exacerbated humoral immunity. (Flisser et al. 2004). Its effective expression in a delivery system adequate for its oral administration will offer two feasible oral vaccine candidates that may be used in complementary schedules to favor the two main branches of immune response. Thus, the potential of the carrot-made HP6/TSOL18 antigen in terms of expression levels, immunogenicity, and protection efficiency is herein investigated.
Materials and methods
Gene design and vector construction
The sequence of the hp6/tsol18 gene, based on the previously described T. solium genome (Parkhouse et al. 2008), was optimized for codon usage in carrot cells, and flanking restriction sites SmaI and SacI were inserted to facilitate cloning. The hp6/tsol18 gene was produced by Genscript (Piscataway, NJ, USA). Then, hp6/tsol18 was cloned into the pBI121 binary vector downstream of the CaMV 35S promoter following conventional cloning procedures, to yield the expression vector named pBin-HP6/TSOL18. After transforming chemically competent E. coli cells, a positive clone was selected by restriction analysis and used for plasmid isolation. After confirming sequence integrity by standard sequencing, the pBin-HP6/TSOL18 construct was mobilized into the Agrobacterium tumefaciens GV3101 strain by electroporation, and this recombinant strain was used for explant carrot transformation.
Genetic transformation of carrot stems
Carrot transformation was carried out following a previously described method (Rosales-Mendoza et al. 2007). Briefly, carrot (Daucus carota L.) seeds (Eden, Lower Beechmont, QLD, Australia) were surface-sterilized with 50 % commercial Clorox bleach for 15 min and washed five times in sterile distilled water (5 min per rinse). Seeds were then treated with HCl 0.01 M in sterilized distilled water for 15 min, washed five times with sterile distilled water, and then germinated in 15 × 100 mm glass jars containing Murashige and Skoog (MS) (1962) medium. Cultures were maintained under a 16-h photoperiod (100 µmol m−2 s−1) at 25 °C for 4–5 weeks. Stems from carrot seedlings were cut into 7–10-mm segments on wet sterile filter paper. Explants were incubated in an overnight-grown culture of Agrobacterium (OD600nm = 0.5) for 15 min, transferred onto the co-cultivation medium [MS basal medium supplemented with 1.33 µM 6-benzyladenine (BA) and 4.52 µM 2,4-dichlorophenoxyacetic acid (2,4-D)], and co-cultivated in the dark for 48 h. Explants were thoroughly washed with sterilized-deionized water and cultured on selection medium (MS basal medium supplemented with 1.33 µM BA and 4.52 µM 2,4-D along with 500 mg/l cefotaxime and 50 mg/l kanamycin). All cultures were grown under a 16-h photoperiod (100 µmol m−2 s−1) at 25 °C. Explants were subcultured once every 2 weeks onto fresh selection medium. Kanamycin-resistant calli were propagated in selective media over a period of 4 months to obtain enough amounts for further analysis. Biomass from candidate lines was collected, freeze-dried overnight, and milled in an analytical grinder.
Transgene analysis
To confirm the presence of the transgene in candidate carrot lines, a PCR assay was performed. Total genomic DNA was isolated from putatively transformed calli lines and wild-type calli, according to Dellaporta et al. (1983). A 25-µl reaction mixture containing 100 ng of DNA, 1.5 mM magnesium chloride, 2.5 U Taq DNA polymerase, 1 mM dNTPs, and 1 µM primers (Sigma, http://www.sigmaaldrich.com) was prepared. Two primer sets were used to detect the selectable marker nptII gene (forward, 5′TATTCGGCTATGACTGGGCA3′; reverse, 5′GCCAACGCTATGTCCTGAT3′) or the TEV-UTR sequence (forward 5′TCTAGAAACACAACATATACAAAACAAACGA3′; reverse 5′CCCGGGCTATCGTTCGTAAATGGTGAAAA3′). Amplification protocol comprised an initial denaturation at 95 °C for 3 min and 35 cycles including incubations at 95 °C for 30 s, 55 °C for 60 s, and 72 °C for 90 s, followed by a final extension at 72 °C for 5 min. PCR products were analyzed by electrophoresis in 1 % agarose gels.
Determination of transgene copy number by real-time PCR
To determine the number of transgene copies in the transformed carrot lines, a real-time PCR (q-PCR) protocol was performed following the method reported by Weng et al. (2004) with some modifications. Two primer sets were used to amplify the gene nptII, which is present in the T-DNA region (sense 5′ATGCCTGCTTGCCGAATA3′; antisense 5′AGCGTGAGGAGTCTTGTTATTC3′), and the DcPRP1 gene, which codes for a wound-inducible, proline-rich cell wall protein, serving as single-copy gene control (sense 5′CAACCACCGAAGACTGAGAAG3′; antisense 5′GGAGGATTAGGCTTGTGTGTAG3′). Primers were designed using the IDT PrimerQuest tool (www.idtdna.com). PCR was carried out in 48-well reaction plates (Applied Biosystems, Waltham, MA, USA) using the SUPER SYBR Mix (Applied Biosystems) with primers at a final concentration of 200 nM and a 10-µl final volume. PCR reactions were performed in a StepOneTM Real-Time PCR system (Applied Biosystems) under the following cycling conditions: 95 °C for 10 min (initial denaturation), 40 cycles at 95 °C for 15 s for denaturation, and 60 °C for 60 s for annealing and extension. PCR specificity was confirmed by melting-curve analysis to ensure the absence of non-specific amplicons and primer dimers. To construct standard curves, target or endogenous genes were amplified by duplicate from transgenic line 3, and 7 genomic DNA concentrations randomly chosen, ranging from 1.56 to 100 ng/µl (set by twofold serial dilutions). To determine the number of transgene copies, PCR reactions were performed by triplicate with 15 ng of DNA from transgenic lines 3, 4, and 5, or the WT carrot line. Ct values for the transgene and endogenous genes were determined under the same auto baseline and threshold. Results were analyzed using the StepOneTM Software v2.1.
Transgene copy number was calculated using the following equation:
where R 0 is the initial amount of reference copies, X 0 is the initial amount of copies of the target gene, IX and IR are intercepts of the relative standard curves of target and reference genes, respectively, SX and SR are the slopes for both genes, and Ct,X and Ct,R are the Ct values of the target and the reference gene, respectively. The equation was applied on the basis that if the copy number of the reference gene (R 0) is known, the copy number of the target gene (X 0) can be deduced from the Ct,X, Ct,R, SX, SR, IX, and IR values obtained from the standard curve.
Protein analysis
HP6/TSOL18 expression was assessed by a Western blot assay in the candidate carrot calli lines. Protein extracts were obtained by resuspending 50 mg of freeze-dried callus tissue in 50 µl of 1X reducing loading buffer. E. coli-produced GST-HP6/TSOL18 recombinant protein was used as positive control. Samples were denatured by boiling for 5 min at 95 °C, debris were eliminated by centrifugation at 16,000g for 15 min at 4 °C, and SDS-PAGE was performed in 4–12 % acrylamide gels under denaturing conditions. The gel was blotted onto BioTrace PVDF membranes (Pall Corporation, http://www.pall.com). After blocking with PBS-Tween 0.1 % plus 5 % fat-free milk (Carnation, Nestle, http://www.nestle.com), blots were incubated overnight with a polyclonal rabbit antibody against the recombinant protein GST-HP6/TSOL18 (1:800 diluted in saline). Thereafter, blots were incubated with a goat anti-rabbit antibody horseradish peroxidase-conjugate at a 1:2000 dilution (Sigma) for 2 h at room temperature to visualize the specific antibody bound. Antibody binding was detected with the SuperSignal West Dura Extended Duration Substrate solution, following the manufacturer’s instructions (Thermo Scientific, http://www.thermoscientific.com) and by means of an X-ray film, following standard procedures.
To estimate the amount of protein produced in carrot cell lines, 50 mg of lyophilized callus tissue was grinded and suspended in 500 µl of protein extraction buffer (50 mM Tris pH = 8, 40 mM NaCl, 0.1 % Tween 20, 0.05 % PMSF). Samples were centrifuged at 16,000g for 15 min at 4 °C. ELISA assay plates were then coated overnight at 4 °C with protein extracts diluted 1:2 in carbonate buffer (0.2 M, pH = 9.6). Plates were washed three times with 0.1 % PBST and blocked with 5 % fat-free dry milk in PBS for 2 h at room temperature. After washing, polyclonal rabbit anti-GST-HP6/TSOL18 diluted 1:800 in PBS was added and plates were incubated overnight at 4 °C. A goat anti-rabbit antibody horseradish peroxidase-conjugated diluted 1:2000 (Sigma) was added and incubated for 2 h at 25 °C. After washing, a substrate solution of 0.3 mg/ml 2-20-azino-bis-3 2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) acid (ABTS; Sigma) and 0.1 mM H2O2 was added for 30 min at 25 °C. Optical density (OD) was read in an iMark™ microplate reader (Bio-Rad, Hercules, CA, USA) at 410 nm. GST-HP6/TSOL18 recombinant protein was used to make a standard curve to determine HP6/TSOL18 level expression in each carrot cell line and was expressed as µg of HP6/TSOL18 per gram of dried biomass (µg g−1 DW).
Mouse immunization
All experiments herein reported were conducted in accordance with the principles set forth in the Guide for the Use of Laboratory Animals, Institute of Laboratory Animal Resources, National Research Council, Washington, DC, USA, 1996. All experimental protocols were approved by the Animal Care Committee of the University and followed the Guide for Care and Use of Experimental Animals.
Groups of ten 8–11-week-old female BALB/cAnN mice were subcutaneously immunized at the back of the neck three times at days 1, 7, and 14 with 10 mg of freeze-dried calli re-suspended in 100 µl of PBS. To improve the antibody response, 2 weeks after the last s.c. immunization, mice received an oral boost of 50 mg of freeze-dried transgenic calli material (carrot line 5) (containing approximately 700 ng of recombinant HP6/TSOL18) or the wild-type line suspended in 300 µl of PBS. Two weeks later, mice were sacrificed, and serum samples as well as intestinal washes were collected for antibody detection. The latter were obtained by resecting the intestines and flushing through them 1 ml of cold PBS supplemented with 1 mM PMSF. The material was collected and samples were centrifuged at 16 000g at 4 °C for 10 min. Supernatants were stored at −70 °C.
Anti-HP6/TSOL18 antibody detection
Antibody levels were assessed by ELISA using microtiter plates (Costar, Corning Incorporated, Corning, NY, USA) coated with 300 ng of recombinant GST-HP6/TSOL18 in 100 µl of carbonate buffer 0.2 M, pH 9.6, and incubated overnight at 4 °C. Plates were washed with 0.05 % PBST and blocked for 2 h at room temperature with 5 % fat-free dry milk dissolved in PBS. To determine serum antibody levels, plates were incubated with 1:20 diluted sera and incubated overnight at 4 °C. Either horseradish peroxidase-conjugated goat anti-mouse IgG or horseradish peroxidase-conjugated goat anti-mouse IgA (1:2000 dilution; Sigma) was added and incubated for additional 2 h at room temperature. After washing with 0.05 % PBST, 100 µl of a substrate solution of 0.3 mg l−1 ABTS (Sigma) and 0.1 mM H2O2 was added and incubated for additional 20–30 min at room temperature. OD at 410 nm was recorded in an iMark™ microplate reader (Bio-Rad).
HP6/TSOL18 histological immunolocalization
Taenia crassiceps cysticerci recovered from the peritoneal cavity of BALB/cAnN female mice were treated using the procedure previously described by Rosas et al. (1998) to dissociate non-specifically bound host proteins. Briefly, vesicular fluid was removed, the cyst was thoroughly washed with ice-cold PBS and incubated with 50 mM glycine–HCl, pH 2.5; 0.1 % Triton X-100; 0.15 mM NaCl for 30 s; pH was restored by adding Tris–HCl, pH 9, and cyst was fixed for 72 h in Zamboni solution. Afterwards, the specimens were included in paraffin and 6 µm sections were cut. Slides were placed on poly-l-lysine-treated microslides (Sigma). Peroxidase activity was blocked by treatment with 3 % H2O2 in PBS for 10 min at room temperature. After washing with PBS, the slides were blocked with 5 % BSA in PBS plus 0.1 % Triton X-100 (pH 7.4) for 1 h at 37 °C. Solutions were removed and the slides were incubated overnight at 4 °C either with rabbit pre-immune sera or with rabbit hyper-immune anti-HP6/TSOL18 sera. Those sera were employed as primary antibody source at a dilution of 1:1000 in 1 % BSA in PBS plus 0.1 % Triton X-100 (PBS/A-T). After washing three times in PBS/A-T, 5 min each, slides were covered with a biotinylated goat anti-rabbit IgG (ImmunO Universal Kit, MP Biomedicals, Eschwege, Germany) for 30 min at 37 °C, rinsed with PBS/A-T, and treated with streptavidin–peroxidase conjugate (HRP conjugated, Universal Kit, MP Biomedicals) for 30 min at 37 °C. Peroxidase activity was visualized by incubating the samples with 3′3-diaminobenzidine (DAB-Plus Kit, Zymed, South San Francisco, CA, USA). The slides were counterstained with Mayer’s hematoxylin, dehydrated, cleared, mounted, and observed through an optical microscope (Nikon, Tokyo, Japan) using the MetaMorph Imaging System v.4.5 software.
Mouse immunization for cysticercosis protection studies
The immunogenicity of the carrot-derived HP6/TSOL18 (line 5) was evaluated in female BALB/cAnN mice (6–8 weeks-old), cared for in accordance with federal regulations for animal experiments (NOM-062-ZOO-1999, Ministry of Agriculture, Mexico). The immunization protocol was approved by the Institutional Animal Care Committee.
Mice were divided into three different groups (n = 7), each receiving one of the following treatments: total soluble protein extracted from 10 mg of freeze-dried HP6/TSOL18 transgenic line 5 administered orally by gavage, or as a recombinant GST-HP6/TSOL18 expressed in E. coli by s.c. immunization. Control mice were orally immunized with soluble extract derived from 10 mg of a non-transformed carrot line (WT group). Subsequently, mice were boosted at day 10 and challenged 2 weeks after the last immunization.
Parasite and infections
Parasites for challenge infection were obtained from 2 to 3-month-old intraperitoneally infected mice as previously described (Toledo et al. 2001). Two weeks after the last immunization, mice were intraperitoneally infected with 20 small (2 mm in diameter), non-budding T. crassiceps cysticerci in 0.9 ml of PBS. Mice were sacrificed 30 days after infection, and cysts were harvested from peritoneal cavity and counted as previously reported (Fragoso et al. 2011). The effect of vaccination was evaluated by the mean number of cysticerci recovered from each mouse.
Statistical analysis
Data were analyzed by one-way ANOVA analysis using the STATISTICA 2.7 software, and Student’s t tests for independent samples. Differences were regarded as significant for P < 0.05.
Results
Expression of HP6/TSOL18 antigen in transgenic carrot cells
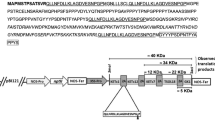
In this study, the pBI121 vector was selected to drive the transfer and expression of a synthetic hp6/tsol18 gene. The strategy, based on the use of restriction sites with SmaI and SacI, allowed the successful cloning of the gene into the pBI121 vector, yielding the pBin-HP6/TSOL18 construct, as confirmed by sequencing. A positive clone was propagated and used for Agrobacterium-mediated transformation. This vector is schematically represented in Fig. 1.
Map of the binary vector pBin-HP6/TSOL18 constructed to express the hp6/tsol18 gene in carrot calli. The gene of interest hp6/tsol18 is under control of the CaMV 35S promoter, along with the 5′UTR from tobacco etch virus (TEV). In addition, an nptII expression cassette is included to allow for kanamycin-based selection of transformed lines
After transformation procedure by Agrobacterium infection, multiple independent kanamycin-resistant (KanR) carrot calli were generated 1 month after co-cultivation. The nptII and TEV-UTR regions present in the T-DNA were successfully amplified in all lines, as reflected by the presence of 600-bp amplicons for the nptII transgene (Fig. 2a), and 150-bp amplicon for the TEV-UTR region (Fig. 2b), thus confirming the presence of the heterologous DNA in the carrot lines. No PCR product was amplified in reactions with DNA from wild-type calli.
Detection of hp6/tsol18 expression cassette in transformed carrot lines by PCR. a Detection of the nptII gene. PCR analysis was conducted using genomic DNA extracted from transgenic or wild-type calli and nptII-specific primers. Lanes M 100-bp ladder (New England Biolabs); 1 WT wild-type callus; 2 positive control (plasmid pBin-HP6/TSOL18); 3–9 transgenic lines 1–7, respectively. b Detection of TEV UTR. PCR analysis was conducted using genomic DNA extracted from transgenic or wild-type calli and TEV-UTR-specific primers. Lanes: M 100-bp ladder; 1–7 transgenic lines 1–7 respectively; 8 WT wild-type callus; 9 positive control (plasmid pBin-HP6/TSOL18)
Lines 3, 4, and 5 were selected to determine the number of transgene copies by q-PCR. To assess the number of transgene copies in the carrot line genome, standardized curves were obtained from the q-PCR analysis for the nptII and DcPRP1 genes in serial dilutions of genomic DNA samples. Slopes (S) and intercepts (I) derived from standard curves for both transgene and endogenous genes were calculated to determine the copy number. S and I values for the nptII primers curve were −3.578 and 30.2, respectively, with a 90.92 % primer efficiency. For the DcPRP1 primers curve, S and I values were −3.58 and 31.9, respectively, with a 91.53 % primer efficiency. Correlation coefficients of the standard curves for both genes showed acceptable values (0.9998 and 0.9982, respectively). Table 1 shows Ct mean values for the transgenic and WT carrot lines. Based on these data, it can be concluded that a single insertion of the transgene occurred in the selected transgenic lines.
To assess the ability of transgenic carrot cell lines to synthesize the recombinant HP6/TSOL18 protein, an initial ELISA screening was performed. Carrot lines 2–5 showed higher HP6/TSOL18 expression (Fig. 3a), and therefore, these four lines were selected to detect the recombinant protein by Western blot. Rabbit anti-GST-HP6/TSOL18 sera detected the presence of the expected 18-kDa protein in samples from transgenic carrot lines (Fig. 3b, lines 2–5). In addition, extracts from transgenic lines showed a band of higher molecular weight, approximately 40-kDa. The positive control showed a 40-kDa protein, in agreement with the theoretical molecular weight of the GST-HP6/TSOL18 fusion protein, while an unexpected 20-kDa band, which might correspond to a degradation product, was also observed.
a Accumulation levels of the HP6/TSOL18 protein in carrot calli lines detected by ELISA. Carrot lines 2–5 showed HP6/TSOL18 expression. b Immunodetection of the HP6/TSOL18 antigen in carrot total protein extracts. Western blot was conducted using a rabbit polyclonal anti-GST-HP6/TSOL18 antibody to assess the presence of the HP6/TSOL18 protein in total protein calli extracts. Lanes 1 purified recombinant GST-HP6/TSOL18 (positive control, 500 ng); lanes 2–5 protein extracts from transgenic carrots lines 2, 3, 4, 5; lane 6 protein extract from wild-type carrot callus
Line 5 was selected for further analysis. HP6/TSOL18 expression levels in this line were confirmed by ELISA. OD values for the line 5, significantly higher than those of the WT carrot line, were used to estimate accumulation levels according to the standard curve obtained with recombinant GST-HP6/TSOL18. Expression levels for line 5 were estimated in 14 µg g−1 dry-weight of carrot calli.
Carrot-derived-HP6/TSOL18 is highly immunogenic in mice
To analyze the immunogenic potential of the carrot-based material expressing the HP6/TSOL18 protein, either calli from the transgenic line 5 or WT calli were used to immunize groups of BALB/cAnN mice. The levels of local and systemic antibodies induced by immunization with the transgenic callus line 5 were estimated by ELISA and compared to those induced by immunization with wild-type callus. This is an acceptable and widely used method for this purpose, since the statistical analysis validates the difference between treatments. According to the statistical analysis of the OD values, significantly higher levels of anti-HP6/TSOL18 IgG serum antibodies were detected in mice immunized with freeze-dried tissue from the line 5 or pure recombinant GST-HP6/TSOL18 (P < 0.05; Fig. 4a). Moreover, an increase in anti-HP6/TSOL18 mucosal IgA antibody levels was detected in the intestines of mice orally boosted with the transformed carrot line 5 (P < 0.05; Fig. 4b).
Levels of anti-HP6/TSOL18 IgG antibodies detected by ELISA in sera (a), IgA antibodies detected in intestinal washes (b) from mice immunized either with recombinant GST-HP6/TSOL18, line 5 carrot callus, or wild-type carrot callus (WT). Asterisk denotes statistical difference with respect to the WT-immunized group (P < 0.05)
Anti-TSOL18 antibodies recognized several structures of T. crassiceps cysticerci
As shown in Fig. 5, anti-HP6/TSOL18 antibodies specifically recognized several structures of T. crassiceps cysticerci: Tegument (T), parenchyma (P), distal cytoplasm region (DC), and the perinuclear cytoplasm region (PC). No reaction was detected using pre-immune sera.
Immunohistochemical staining of Taenia crassiceps (ORF) metacestodes. Sections were incubated with rabbit sera before and after immunization with the recombinant HP6/TSOL18 protein. Bound antibodies were detected using biotinylated goat anti-rabbit IgG plus streptavidin-peroxidase conjugate, and counterstained with hematoxylin. a and c Controls incubated with rabbit pre-immune sera. b and d Cysticerci structures recognized by the rabbit anti-recombinant GST-HP6/TSOL18 sera. T tegument; P parenchyma; DC distal cytoplasm region; PC perinuclear cytoplasm region
Protective effect of oral immunization with the HP6/TSOL18-carrot line 5 against murine cysticercosis
The effect in mice of oral immunization with total extract of freeze-dried calli of HP6/TSOL18-expressing carrot line 5 or wild-type carrot alone is shown in Table 2. Orally administered HP6/TSOL18-carrot significantly reduced the expected parasite load at a similar level to that of GST-HP6/TSOL18 recombinantly expressed in a bacterial system and s.c. administered. When orally administered, non-transgenic carrot calli alone failed to reduce the expected parasite load.
Discussion
In this study, carrot was selected as a convenient platform to explore the development of an affordable oral vaccine based on the T. solium HP6/TSOL18 antigen. It is remarkable that after transformation (as confirmed by conventional PCR) with a single T-DNA insertion (as confirmed by qPCR), carrot cells synthesized and accumulated the HP6/TSOL18 antigen, which was detected by both ELISA and Western blot analysis. Even though all assessed lines carry a single T-DNA copy, they showed different antigen yields, which may be due to differential transgene insertion sites into the carrot genome (Kim et al. 2007). The unexpected 40-kDa band observed in Western blot may correspond to HP6/TSOL18 associated to endogenous carrot proteins or a dimeric form of the antigen, since it was not observed in the wild-type cell line. Expression levels of up to 14 µg TSOL18 g−1 dry-weight carrot biomass were achieved (line 5), which are in the expected range according to previous experiences with carrot-produced antigens. For instance, the B subunit of E. coli heat-labile enterotoxin (LTB) accumulated at 23 µg g−1 dry-weight levels (Marquet-Blouin et al. 2003; Rosales-Mendoza et al. 2008).
Early efforts to conduct anti-cysticercosis vaccination programs were hampered by difficulties associated with antigen production and the limited effectiveness of crude parasite extracts as vaccines, as well as prohibitive production costs. For this reason, innovations to override such limitations are much-needed (Molinari et al. 1993, 1997; Wang et al. 2003; Wu et al. 2005; Lightowlers 2006; Cai et al. 2008; Sciutto et al. 2008). The introduction of recombinant vaccines and robust production systems yielded promising vaccine candidates that have been evaluated in field trials (Huerta et al. 2001; Sciutto et al. 2007; Morales et al. 2008; Assana et al. 2010). Carrot cells can be propagated and cultured at industrial scale in bioreactors under well-established GMP-compliant procedures, as exemplified by the case of Protalix, a carrot-made glucocerebrosidase used to treat Gaucher´s disease (Aviezer et al. 2009); this successful case points out the potential of this platform for the production and delivery of biopharmaceuticals, preserving their biological activity. In our case, the immunogenic activity of plant-made HP6/TSOL18 was evaluated in BALB/cAnN mice. A successful induction of specific humoral responses at the systemic and intestinal mucosa levels was observed when a prime-boost immunization scheme by s.c. and oral route was followed, as previously reported by other groups (Molina et al. 2005; Webster et al. 2006). A four-dose immunization schedule using the carrot-made HP6/TSOL18 vaccine was first employed to test its immunogenic properties. Thereafter, the protection induced by the vaccine orally applied was tested.
Orally administered carrot-made HP6/TSOL18 vaccine induced a significant reduction in parasite load against the experimental murine T. crassiceps cysticercosis. Considering the high recognition of anti-HP6/TSOL18 antibodies in several structures of T. crassiceps cysticerci (Fig. 5), the observed protection could be due in part to effector mechanisms of antibodies attached to the parasite, mediated either by antibody-dependent cytotoxicity or by cellular immunity. Remarkably, the observed protection was similar to that attained by s.c. administration of the E. coli-made antigen, reflecting a high immunogenicity of the plant-made HP6/TSOL18 antigen.
The availability of two oral vaccine candidates that promote either a humoral (HP6/TSOL18) or a cellular immunity (S3Pvac) can be taken into account to design new, more potent vaccination schedules. The combined use of both vaccine candidates could offer a broader immune protection. Therefore, those parasites that could escape HP6/TSOL18-induced antibodies would be susceptible to the cellular immune response enhanced by S3Pvac. In addition, the expression system used in this study seems more effective than the papaya system previously used to express S3Pvac. For instance, a protein extract obtained from 40 mg of freeze-dried calli (from three lines processed in parallel) was used to immunize mice with S3Pvac, while the HP6/TSOL18 carrot-made vaccine consisted of a protein extract derived from 10 mg of a single carrot line. These differences justified the use of the carrot system to express the S3Pvac vaccine. It is also noteworthy that vaccine components from both papaya and carrot obviate the use of adjuvants to improve vaccine efficacy, required when E. coli-based recombinant GST-HP6/TSOL18 or synthetic S3Pvac was employed. Adjuvant compounds from plant cells may explain the high immunogenic activity of carrot-made vaccines, as suggested by several research groups (Mojica-Henshaw et al. 2003; Rosales-Mendoza and Salazar-González 2014).
Further experiments to assess the effect of additional booster doses to the immunization schedule, as well as the antigen stability in plant biomass, will be required. Our results are in agreement with several trials that have demonstrated the potential of carrot cells to deliver functional antigens by the oral route (Rosales-Mendoza and Tello-Olea 2015). These properties as delivery vehicle could be attributed to the plant cell-bioencapsulation that diminishes antigen degradation, while allowing a proper antigen release with a subsequent uptake by M cells.
In conclusion, carrot-made HP6/TSOL18 vaccine possesses immunogenic activity able to reduce the parasite load in mice, and thus, it is proposed as a promising candidate along with the S3Pvac antigens as an oral anti-cysticercosis vaccine (Hernández et al. 2007). Oral formulations composed by minimally processed plant biomass offer clear advantages, since starting materials for plant biomass production are inexpensive, and no cold-chain is required once plant cells are freeze-dried. Moreover, oral administration is safer and simplifies treatment.
Author contribution statement
SRM and ES designed the research; EME, DGA, MH, JC, JSG, ARM, and GR generated and characterized the carrot lines; TG, EME, ES, GF and SRM analyzed data; ES, GF and SRM wrote the manuscript. All authors contributed to editing and approving the final version of the manuscript.
Abbreviations
- ERT:
-
Enzyme replacement therapy
- GST-HP6/TSOL18:
-
Glutathione S-transferase-HP6/TSOL18 fusion protein
- NC:
-
Neurocysticercosis
- CNS:
-
Central nervous system
References
Assana E, Kyngdon CT, Gauci CG, Geerts S, Dorny P, De Deken R (2010) Elimination of Taenia solium transmission to pigs in a field trial of the HP6/TSOL18 vaccine in Cameroon. Int J Parasitol 40:515–519
Aviezer D, Brill-Almon E, Shaaltiel Y, Hashmueli S, Bartfeld D, Mizrachi S, Liberman Y, Freeman A, Zimran A, Galun E (2009) A plant-derived recombinant human glucocerebrosidase enzyme—a preclinical and phase I investigation. PLoS One 4:e4792
Batson A (1998) Win–win interactions between the public and private sectors. Nat Med 4(5 Suppl):487–491
Benitez L, Garate T, Harrison LJ, Kirkham P, Brookes SM, Parkhouse RM (1996) Cloning and sequencing of the gene encoding the principal 18-kDa a secreted antigen of activated oncospheres of Taenia saginata. Mol Biochem Parasitol 78:265–268
Berman S, Giffin RB (2004) Global perspectives on vaccine financing. Expert Rev Vaccines 3:557–562
Betancourt MA, de Aluja AS, Sciutto E, Hernández M, Bobes RJ, Rosas G, Hernández B, Fragoso G, Hallal-Calleros C, Aguilar L, Flores-Peréz I (2012) Effective protection induced by three different versions of the porcine S3Pvac anticysticercosis vaccine against rabbit experimental Taenia pisiformis cysticercosis. Vaccine 30:2760–2767
Cai X, Yuan G, Zheng Y, Luo X, Zhang S, Ding J, Jing Z, Lu C (2008) Effective production and purification of the glycosylated TSOL18 antigen, which is protective against pig cysticercosis. Infect Immun 76:767–770
Dabral N, Moreno-Lafont M, Sriranganathan N, Vemulapalli R (2014) Oral immunization of mice with gamma-irradiated Brucella neotomae induces protection against intraperitoneal and intranasal challenge with virulent B. abortus 2308. PLoS One 9(9):e107180
Daniell H (2006) Production of biopharmaceuticals and vaccines in plants via the chloroplast genome. Biotechnol J 1:1071–1079
De Aluja AS (2008) Cysticercosis in the pig. Curr Trop Med Chem 8:368–374
De Aluja AS, Suárez-Marín R, Sciutto-Conde E, Morales-Soto J, Martínez-Maya JJ, Villalobos N (2014) Evaluation of the impact of a control program against taeniasis-cysticercosis (Taenia solium). Salud Publica Mex 56:259–265
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: version II. Plant Mol Biol Rep 1:19–21
Flisser A, Gauci CG, Zoli A, Martinez-Ocana J, Garza-Rodriguez A, Dominguez-Alpizar JL, Maravilla P, Rodriguez-Canul R, Ávila G, Aguilar-Vega L, Kyngdon C, Geerts S, Lightowlers MW (2004) Induction of protection against porcine cysticercosis by vaccination with recombinant oncosphere antigens. Infect Immun 72:5292–5297
Fragoso G, Esquivel-Guadarrama F, Santana MA, Bobes RJ, Hernández B, Cervantes J, Segura R, Goldbaum FA, Sciutto E, Rosas G (2011) Heterologous prime-boost oral immunization with GK1 peptide from Taenia crassiceps cysticerci induces protective immunity. Clin Vaccine Immunol 18:1067–1076
Gandhi G, Lydonb P, Cornejoc S, Brenzel L, Wrobel S, Chang H (2013) Projections of costs, financing, and additional resource requirements for low- and lower middle-income country immunization programs over the decade, 2011–2020. Vaccine 31:137–148
Garcia HH, Del Brutto OH, Cysticercosis Working Group in Peru (2005) Neurocysticercosis: updated concepts about an old disease. Lancet Neurol 4:653–661
Gauci CG, Flisser A, Lightowlers MW (1998) Taenia solium oncosphere protein homologous to host-protective Taenia ovis and Taenia saginata 18 kDa antigens. Int J Parasitol 28:757–760
Gecchele E, Merlin M, Brozzetti A, Falorni A, Pezzotti M, Avesani L (2015) A comparative analysis of recombinant protein expression in different biofactories: bacteria, insect cells and plant systems. J Vis Exp 97:e52459. doi:10.3791/52459
Gonzalez AE, Gauci CG, Barber D, Gilman RH, Tsang VC, Garcia HH, Verastegui M, Lightowlers MW (2005) Vaccination of pigs to control human neurocysticercosis. Am J Trop Med Hyg 72:837–839
Harrison GB, Heath DD, Dempster RP, Gauci C, Newton SE, Cameron WG, Robinson CM, Lawrence SB, Lightowlers MW, Rickard MD (1996) Identification and cDNA cloning of two novel low molecular weight host-protective antigens from Taenia ovis oncospheres. Int J Parasitol 26:195–204
Hernández M, Cabrera-Ponce JL, Fragoso G, López-Casillas F, Guevara-García A, Rosas G, León-Ramírez C, Juárez P, Sánchez-García G, Cervantes J, Acero G, Toledo A, Cruz C, Bojalil R, Herrera-Estrella L, Sciutto E (2007) A new highly effective anticysticercosis vaccine expressed in transgenic papaya. Vaccine 25:4252–4260
Hernández M, Rosas G, Cervantes J, Fragoso G, Rosales-Mendoza S, Sciutto E (2014) Transgenic plants: a 5-year update on oral antipathogen vaccine development. Expert Rev Vaccines 13:1523–1536
Huerta M, De Aluja AS, Fragoso G, Toledo A, Villalobos N, Hernández M, Gevorkian G, Acero G, Diaz A, Alvarez I, Avila R, Beltran C, Garcia G, Martinez JJ, Larralde C, Sciutto E (2001) Synthetic peptide vaccine against Taenia solium pig cysticercosis: successful vaccination in a controlled field trial in rural Mexico. Vaccine 20:262–266
Jayashi CM, Gonzalez AE, Castillo Neyra R, Kyngdon CT, Gauci CG, Lightowlers MW (2012) Characterisation of antibody responses in pigs induced by recombinant oncosphere antigens from Taenia solium. Vaccine 30:7475–7480
Kim SI, Veena JH, Gelvin SB (2007) Genome-wide analysis of Agrobacterium T-DNA integration sites in the Arabidopsis genome generated under non-selective conditions. Plant J 51:779–791
Larralde C, Sciutto E (2006) El control de la Taenia solium en México quinientos años después de su llegada al Nuevo Mundo. Cisticercosis, guía para profesionales de la salud. Secretaría de Salud, Fundación Mexicana para la Salud, Instituto Nacional de Salud Pública, Fondo de Cultura Económica 7:182–237. ISBN 968-16- 8138-X
Lightowlers MW (2006) Cestode vaccines: origins, current status and future prospects. Parasitology 133:27–42
Marquet-Blouin E, Bouche FB, Steinmetz A, Muller CP (2003) Neutralizing immunogenicity of transgenic carrot (Daucus carota L.) derived measles virus hemagglutinin. Plant Mol Biol 51:459–469
Mojica-Henshaw MP, Francisco AD, De Guzman F, Tigno XT (2003) Possible immunomodulatory actions of Carica papaya seed extract. Clin Hemorheol Microcirc 29:219–229
Molina A, Veramendi J, Hervás-Stubbs S (2005) Induction of neutralizing antibodies by a tobacco chloroplast-derived vaccine based on a B cell epitope from canine parvovirus. Virology 342:266–275
Molinari JL, Soto R, Tato P, Rodriguez D, Retana A, Sepulveda J, Palet A (1993) Immunization against porcine cysticercosis in an endemic area in Mexico: a field and laboratory study. Am J Trop Med Hyg 49:502–512
Molinari JL, Rodriguez D, Tato P, Soto R, Arechavaleta F, Solano S (1997) Field trial for reducing porcine Taenia solium cysticercosis in Mexico by systematic vaccination of pigs. Vet Parasitol 69:55–63
Morales J, Martínez JJ, Manoutcharian K, Hernández M, Fleury A, Gevorkian G, Acero G, Blancas A, Toledo A, Cervantes J, Maza V, Quet F, Bonnabau H, De Aluja AS, Fragoso G, Larralde C, Sciutto E (2008) Inexpensive anti-cysticercosis vaccine: S3Pvac expressed in heat inactivated M13 filamentous phage proves effective against naturally acquired Taenia solium porcine cysticercosis. Vaccine 26:2899–2905
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–479
Parkhouse RM, Bonay P, González LM, Ferrer E, Gárate T, Aguilar CM, Cortez AMM, Harrison LJ (2008) TSOL18/HP6-Tsol, an immunogenic Taenia solium oncospheral adhesion protein and potential protective antigen. Parasitol Res 102:921–926
Rosales-Mendoza S, Salazar-González JA (2014) Immunological aspects of using plant cells as delivery vehicles for oral vaccines. Expert Rev Vaccines 13:737–749
Rosales-Mendoza S, Tello-Olea MA (2015) Carrot cells: a pioneering platform for biopharmaceuticals production. Mol Biotechnol 57:219–232
Rosales-Mendoza S, Soria-Guerra RE, de Jesús Olivera-Flores MT, López-Revilla R, Argüello-Astorga GR, Jiménez-Bremont JF, García-de la Cruz RF, Loyola-Rodríguez JP, Alpuche-Solís AG (2007) Expression of Escherichia coli heat-labile enterotoxin b subunit (LTB) in carrot (Daucus carota L.). Plant Cell Rep 26:969–976
Rosales-Mendoza S, Soria-Guerra RE, López-Revilla R, Moreno-Fierros L, Alpuche-Solís AG (2008) Ingestion of transgenic carrots expressing the Escherichia coli heat-labile enterotoxin B subunit protects mice against cholera toxin challenge. Plant Cell Rep 1:79–84
Rosales-Mendoza S, Soria-Guerra RE, Moreno-Fierros L, Han Y, Alpuche-Solís AG, Korban SS (2011) Transgenic carrot tap roots expressing an immunogenic F1-V fusion protein from Yersinia pestis are immunogenic in mice. J Plant Physiol 168:174–180
Rosas G, Cruz-Revilla C, Fragoso G, López-Casillas F, Pérez A, Bonilla MA, Rosales R, Sciutto E (1998) Taenia crassiceps cysticercosis: humoral immune response and protection elicited by DNA immunization. J Parasitol 84:516–523
Sciutto E, Morales J, Martinez JJ, Toledo A, Villalobos MN, Cruz-Revilla C, Meneses G, Hernández M, Diaz A, Rodarte LF, Acero G, Gevorkian G, Manoutcharian K, Paniagua J, Fragoso G, Fleury A, Larralde R, De Aluja AS, Larralde C (2007) Further evaluation of the synthetic peptide vaccine S3Pvac against Taenia solium cysticercosis in pigs in an endemic town of Mexico. Parasitology 134:129–133
Sciutto E, Fragoso G, de Aluja AS, Hernández M, Rosas G, Larralde C (2008) Vaccines against cysticercosis. Curr Top Med Chem 8:415–423
Sciutto E, Fragoso G, Hernández M, Rosas G, Martinez JJ, Fleury A, Cervantes J, Aluja A, Larralde C (2013) Development of the S3pvac vaccine against murine Taenia crassiceps cysticercosis: a historical review. J Parasitol 99:693–702
Streatfield SJ, Howard JA (2003) Plant production systems for vaccines. Expert Rev Vaccines 2:763–775
Toledo A, Larralde C, Fragoso G, Gevorkian G, Manoutcharian K, Hernández M, Acero G, Rosas G, López-Casillas F, Garfias CK, Vázquez R, Terrazas I, Sciutto E (1999) Towards a Taenia solium cysticercosis vaccine: an epitope shared by Taenia crassiceps and Taenia solium protects mice against experimental cysticercosis. Infect Immun 67:2522–2530
Toledo A, Fragoso G, Rosas G, Hernández M, Gevorkian G, López-Casillas F, Hernández B, Acero G, Huerta M, Larralde C, Sciutto E (2001) Two epitopes shared by Taenia crassiceps and Taenia solium confer protection against murine T. crassiceps cysticercosis along with a prominent T1 response. Infect Immun 69:1766–1773
Wang QM, Sun SH, Hu ZL, Wu D, Wang ZC (2003) Immune response and protection elicited by DNA immunization against Taenia cysticercosis. Vaccine 21:1672–1680
Webster DE, Smith SD, Pickering RJ, Strugnell RA, Dry IB, Wesselingh SL (2006) Measles virus hemagglutinin protein expressed in transgenic lettuce induces neutralising antibodies in mice following mucosal vaccination. Vaccine 24:3538–3544
Weng H, Pan A, Yang L, Zhang C, Liu Z, Zhang D (2004) Estimating number of transgene copies in transgenic rapeseed by real-time PCR assay with HMG I/Y as an endogenous reference gene. Plant Mol Biol Rep 22:289–300
Wu L, Diao Z, Dengm X, Gao J, Zhou Z, Liu Y, Wang Y (2005) DNA vaccine against Taenia solium cysticercosis expressed as a modified hepatitis B virus core particle containing three epitopes shared by Taenia crassiceps and Taenia solium. J Nanosci Nanotechnol 5:1204–1210
Yusibov V, Streatfield SJ, Kushnir N (2011) Clinical development of plant-produced recombinant pharmaceuticals: vaccines, antibodies, and beyond. Hum Vaccin 7:313–321
Acknowledgments
The authors acknowledge Georgina Diaz and Daniel Garzón for their technical support, and Juan Francisco Rodriguez for the language edition of this manuscript. This research was partially supported by the CONACyT (201448, 152793), DGAPA (IG-200414), and The Programa de Investigación para el Desarrollo y la Optimización de Vacunas, Adyuvantes y Métodos Diagnósticos del Instituto de Investigaciones Biomédicas, UNAM.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Monreal-Escalante, E., Govea-Alonso, D.O., Hernández, M. et al. Towards the development of an oral vaccine against porcine cysticercosis: expression of the protective HP6/TSOL18 antigen in transgenic carrots cells. Planta 243, 675–685 (2016). https://doi.org/10.1007/s00425-015-2431-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-015-2431-0