Abstract
Carnivory in plants evolved as an adaptation strategy to nutrient-poor environments. Thanks to specialized traps, carnivorous plants can gain nutrients from various heterotrophic sources such as small insects. Digestion in traps requires a coordinated action of several hydrolytic enzymes that break down complex substances into simple absorbable nutrients. Among these, several pathogenesis-related proteins including β-1,3-glucanases have previously been identified in digestive fluid of some carnivorous species. Here we show that a single acidic endo-β-1,3-glucanase of ~50 kDa is present in the digestive fluid of the flypaper-trapped sundew (Drosera rotundifolia L.). The enzyme is inducible with a complex plant β-glucan laminarin from which it releases simple saccharides when supplied to leaves as a substrate. Moreover, thin-layer chromatography of digestive exudates showed that the simplest degradation products (especially glucose) are taken up by the leaves. These results for the first time point on involvement of β-1,3-glucanases in digestion of carnivorous plants and demonstrate the uptake of saccharide-based compounds by traps. Such a strategy could enable the plant to utilize other types of nutritional sources e.g., pollen grains, fungal spores or detritus from environment. Possible multiple roles of β-1,3-glucanases in the digestive fluid of carnivorous sundew are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carnivory in plants represents an interesting example of adaptation to nutrient-poor habitats and has arisen independently in several lineages of angiosperms (Albert et al. 1992). The carnivorous syndrome involved evolution of different morphological features associated with attraction, trapping, and digestion of prey by several types of specialized traps (Juniper et al. 1989; Givnish et al. 2011; Bauer et al. 2012; Krol et al. 2012). An insect prey represents the main additional source of nutrients for carnivorous plants. Mainly nitrogen, phosphorus and sulfur are obtained from complex substances in a digestion fluid that is secreted into the traps by specialized glands of different structural complexity. Biochemically, the digestion process comprises a coordinated action of different hydrolytic enzymes. The best characterized are the proteases, esterases and acidic phosphatases, the activities of which have directly been linked to the digestion process (Juniper et al. 1989). Other enzymes detected in traps include chitinases, glucanases, thaumatin-like proteins and ribonucleases (Matušíková et al. 2005; Eilenberg et al. 2006; Hatano and Hamada 2008; Rottloff et al. 2011; Ishisaki et al. 2012a, b; Schulze et al. 2012). Several of the latter enzymes are known as plant defense components, and belong to a group of so called pathogenesis-related proteins (Boller 1987; Mithöfer 2011). For example chitinases (PR-3) inhibit fungal growth in vitro as well as in planta (Boller 1993), while in carnivorous plants they act in digestion of the insect cutin and facilitate the other digestive enzymes to access the inner parts of the prey (Juniper et al. 1989; Matušíková et al. 2005). This dual role has also been demonstrated in silico through comparison of chitinase gene sequences in carnivorous and non-carnivorous plants (Renner and Specht 2012). More recently, β-1,3-glucanases (PR-2) have also been identified in the pitcher fluid of Nepenthes alata (Hatano and Hamada 2008, 2012) and Venus flytrap (Dionaea muscipula) (Schulze et al. 2012).
Plant β-1,3-glucanases (glucan endo-1,3-glucosidases, GH-17, E.C. 3.2.1.39) are an abundant class of hydrolytic enzymes that catalyze the cleavage of 1,3-β-d-glucosidic bonds in β-1,3-glucans. These enzymes can break up cell walls of invading pathogens and restrict their growth (Schlumbaum et al. 1986; Sela-Buurlage et al. 1993; Jach et al. 1995; Moravčíková et al. 2007; Pauchet et al. 2009). Beta-1,3-glucanases (for simplicity henceforth only glucanases) also act during abiotic stresses e.g., after wounding, cold or heavy metal exposure (Ernst et al. 1996; Wu and Bradford 2003; Piršelová et al. 2011). Besides stress-related functions, they are also important for normal plant growth and development playing a role in the turnover of glucans during microsporogenesis (Worrall et al. 1992), pollen germination and pollen tube growth (Roggen and Stanley 1969; Ori et al. 1990), fertilization (Lotan et al. 1989; Ori et al. 1990), embryogenesis (Dong and Dunstan 1997; Helleboid et al. 1998), seed germination (Vögeli-Lange et al. 1994), regulation of transport through vascular tissues (Levy et al. 2007) and cellulose biosynthesis (Meier et al. 1981). Glucanases often accompany and act synergically with other PR proteins (mostly chitinases). Since insects do not contain glucans, the role of such glucan-cleaving enzymes in the digestive processes of carnivorous plants appears questionable.
The aim of this study was to explore the occurrence and activity of glucanases in the digestion process of a flypaper-trapped carnivorous plant. In vitro-grown sundew (Drosera rotundifolia L.) was chosen as a subject because of its small size, easily accessible adhesive traps and possibility to exclude a bias from microbial contamination. In sundews (Droseraceae), the digestive processes take place on leaves covered on the upper side by specialized stalked glands called tentacles. They secrete a droplet of sticky, shiny mucilage with a mix of different hydrolytic enzymes such as protease, esterase, acidic phosphatase and chitinase (Clancy and Coffey 1977; Juniper et al. 1989; Matušíková et al. 2005). Though so far there has been no report on enzymatic activity of glucanases in sundew, expression of a glucanase gene (Matušíková et al. 2004) in mesophyll and secretory cells of sundew tentacles has previously been reported (Libantova et al. 2009). Using a plant-derived β-1,3-glucan laminarin we show that glucanases are active and decompose the substrate on the traps, moreover, the released degradation products are absorbed from the leaf surface. To the best of our knowledge this is the first report on cleavage and subsequent uptake of sugar-based substrate by carnivorous traps. We discuss the possible roles of glucanases as producers of potential saccharides with various utility in digestion process of carnivorous plants.
Materials and methods
Plant material
Plants of Drosera rotundifolia L. (Finnish cultivar „Vikulan Järvi“) were a kind gift from Terttu Kämäräinen (Department of Biology/Botany, University of Oulu, Finland). Plants were cultivated aseptically on agar media as described previously (Bobák et al. 1995). To imitate the presence of a glucan-rich substance, 10 μL of 1.15 or 20 % (w/v) laminarin (from Laminaria digitata, Sigma) in 0.05 M sodium acetate buffer (pH 5.2) was pipetted to the center of each leaf of the plants. Sodium acetate buffer was used as a control treatment. Digestive processes were allowed to proceed for 0, 24, 48, 72, 96, 144 and 192 h, respectively. Digestive mucilage was eluted and collected; nine cut leaf blades (total fresh weight of ca. 0.15 g) from individual plants were submerged one-by-one in 250 μL of 0.05 M sodium acetate buffer (pH 5.2) for 20 s, and the buffer was pipetted up and down seven times. After removal of leaves, the remnants of the liquid were captured into collection tubes by brief spinning of leaves in perforated 0.75 mL Eppendorf tube. Samples were frozen in liquid nitrogen and stored at −80 °C until analyzed.
To explore specificity of induction, sundew plants were induced with 10 μL of 1.15 % (w/v) gelatine (Merck) and leaf eluates were analyzed at a single time point of 48 hpi. Mucilage was collected and stored as described.
Detection of glucanase activity of sundew leaves in situ
Sundew leaves were detached from the plant and slightly squeezed on agar plates consisting of 0.1 M sodium acetate buffer (pH 5.0), 1 % (w/v) laminarin and 0.8 % (w/v) agarose. Plates were incubated for 48 h at 37 °C. Afterward the leaves were carefully removed and plates were stained with 0.5 mg L−1 Congo Red (Sigma) (Inbar and Chet 1991). Cleared zones in a red background indicated glucanase activity. Congo Red-stained plates were digitalized using a GS-800 calibrated densitometer (BioRad).
Analyses of glucanase activity in sundew leaf eluates
Aliquots (30 μL) of leaf eluates were separated on 12.5 % (w/v) SDS-containing polyacrylamide gel slabs (1.5 mm) with laminarin (0.01 %) (Sigma) incorporated as an enzyme substrate. For separation under native conditions the gels lacked SDS. Samples were mixed with one-third volume of 3 × SDS loading buffer and subsequently loaded on gels without any heat treatment before the run to preserve the enzyme activity. Standard buffer conditions for separation and detection of total-, acidic/neutral and basic/neutral protein fractions (respectively) were according to Laemmli (1970). The gel electrophoresis ran at 8 °C at a constant current of 18 mA. When the loading dye entered the separating gel, the current was increased to 24 mA and gels were run for another cca. 2 h until the loading dye reached the bottom of the gel. After electrophoresis of total proteins, enzymes were re-naturated in 1 % Triton X-100 (in 0.05 M sodium acetate buffer, pH 5.2) for 1 h with constant shaking. All the gels were incubated at 37 °C in sodium acetate buffer for 1 h to enable digestion of the substrate and then fixed in a destaining solution (20 % (v/v) methanol, 7 % (v/v) acetic acid in water) for 5 min. Glucanase activity was visualized by boiling the gels for 2.5 min in 0.1 % (w/v) 2,3,5-triphenyl tetrazolium chloride (Sigma) dissolved in 1 M NaOH (Pan et al. 1991). Areas with glucanase activity appeared as dark-red bands on non-stained (transparent) background. Intensity of the bands was taken as a measure of the glucanase activity. For quantification, the gels were scanned using a GS-800 calibrated densitometer (BioRad). Densitometry was performed from the image files using Image J analysis software (NIH; http://rsbweb.nih.gov/ij/). Glucanase activity was expressed as a raw integrated density corresponding to a sum of pixel values in areas of constant size after a background correction. Total protein profile of the samples was visualized by staining with Coomassie Brilliant Blue R250.
Analyses of protease activity in sundew leaf eluates
Overall proteolytic activity of leaf eluates was measured using Protease Fluorescent Detection Kit (Sigma) according to manufacturer instructions. The activity of individual proteases was detected in 16 μL sample aliquots (mixed with 3× concentrated SDS loading buffer) upon a separation in SDS–polyacrylamide gels with 0.025 % gelatin (w/v) as a copolymerized substrate in the separating gel (1 mm thick). After separation and renaturation of proteins as described above, the gels were incubated in 0.05 M sodium acetate buffer (pH 5.2) for 20 h and then stained with Coomasie Briliant Blue for 2 h. Gels were destained for additional cca. 5 h, scanned and processed as described above. Areas of proteolytic activity were visible as bright bands on dark-blue (stained) background.
Analyses of laminarin degradation
Laminarin content in leaf eluates was detected fluorometrically following the protocol of Kauss (1989). Briefly, samples were 10 times diluted in 0.05 sodium acetate (pH 5.2). Aliquots (20 μL) were mixed with 600 μL dye mix (4 volumes of 0.1 % (w/v) aniline blue in water and two volumes of 1 M glycine/NaOH buffer, pH 9.5) and incubated overnight in the dark at 8 °C. After a brief centrifugation, the fluorescence intensity of 200 μL mixture aliquots was measured with a Titertek Fluoroscan II plate reader (excitation at 355 nm, emission at 460 nm). The dilution factor for tested samples was determined empirically to obtain fluorescence intensity within the calibration range.
The total reducing sugar content of leaf exudates was measured spectrophotometrically according to Miller (1959). Sample aliquots (50 μL) were mixed with 150 μL of DNS reagent and boiled for 5 min in a water bath. Samples were then let to cool down at a room temperature and water-diluted 20 times to obtain values within the linear range. Absorbance at 540 nm was measured using UV-1800 UV/VIS Spectrophotometer (Shimadzu). Serial dilutions of glucose in 0.05 M sodium acetate buffer (pH 5.2) were used for preparation of the standard calibration curve.
Products of laminarin hydrolysis were assayed using thin-layer chromatography by separating 1 μL aliquots of leaf eluates on pre-coated silica-gel plates (Merck). The plates were developed twice with the solvent system of chloroform, acetic acid, and water (3:10:1, by vol.) at a room temperature. Saccharide spots were visualized by dipping the plates shortly (1 s) in aniline-diphenylamine-phosphoric acid reagent (2 % (w/v) diphenylamine, 2 % (v/v) aniline, 12.75 % (v/v) phosphoric acid in acetone) and then heating the plate at 100 °C for 15 min. Glucose, maltose and non-treated laminarin were used as reference samples. After scanning the plates, semi-quantification of individual sugar spots was performed using Image J software as described above.
Statistical analysis
Experiments were performed in three replicates. Data were analyzed by Student’s t test and 2-way ANOVA.
Results
Digestion processes in sundew leaves were induced with 20 % (excessive amount) or 1.15 % (limited amount) of laminarin substrate as described. The leaf eluates were assayed for proteolytic and glucanolytic activity. Furthermore, amounts of laminarin and its degradation products were determined in eluates.
In-gel analyses of tentacle secretions
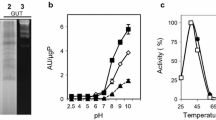
Sundew tentacles secrete a mucilage that degrades a glucan-rich substrate in agar plates (Fig. 1a, b). Therefore, eluted secretions were further studied for presence and activity of glucanases. For this, a droplet of laminarin solution was positioned on sundew leaves. Within the first 24 h the marginal tentacles responded mechanically and bent inwards indicating the presence of a (digestion) stimulating substance (Fig. 1c). This response did not occur on control plants treated with buffer that lacked any digestible substrate. Leaf exudates were collected at different time points after induction and separated in polyacrylamide gels with laminarin. Separations under native conditions identified a single acidic ~50 kDa glucanase isoform in all samples, including those from non-induced plants (Fig. 2a). No enzyme fraction was detected upon separation of basic protein fractions (Fig. 2b).
Plate clearing assay on Petri dishes with laminarin-containing substrate reveals strong glucanase activity at places of sundew tentacles, and is visible as clear (white) zones on dark background a. Such an activity is very weak on leaves induced with buffer only b. Tentacles on the leaves of sundew c bend inwards within 24 h after application of a droplet of digestible substrate laminarin (L) but not after loading of buffer (B)
Polyacrylamide gel separations of sundew eluates from control (C) and laminarin-induced (L) plants. A strong single glucanase band (pink-colored) was detected upon SDS-PAGE separation and subsequent staining for glucanases (left panel), while in general there was a low amount of proteins detectable in the lines after subsequent staining with Coomassie Brilliant Blue (right panel) a. Similarly, a single glucanase was identified after separation of acidic and neutral proteins under native-PAGE conditions (left panel), but no glucanase isoform was observed among basic and neutral proteins (right panel) b. Shown are samples from plants induced with 1.15 % laminarin for 48 h. M molecular size marker (Page Ruler™ Plus Prestained Protein Ladder, Thermo Scientific)
Upon induction of plants the enzymes activity significantly changed with time (P < 10−6) (Fig. 3). When laminarin was applied in limited amount, an increase with time was observed that peaked at 96 h. Afterward the glucanase activity decreased till the end of experiment (192 hpi) (Fig. 3a). When the plants were induced by excessive amount of laminarin the enzyme activity increased with time and remained high till the end of the experiment (192 hpi) (Fig. 3b). Compared to laminarin, gelatine as a proteinaceous substrate appeared as a weaker inducer of glucanase activity; the increase in plants induced by it for 48 h was not significant (Fig. 4).
The activity of glucanases in sundew eluates at different time points post induction with laminarin (0–192 hpi) was detected after separation in polyacrylamide gels, and expressed as raw integrated density (RID) for each band a, b. The amounts of laminarin and reducing sugars (as glucose equivalents) were also determined in each sample c, d. As a substrate, 1.15 % a, c or 20 % laminarin b, d were applied to leaves. Data from plants induced with buffer (gray squares) or laminarin (black triangles) indicate mean ± SE values of three replicates (R1–R3). The asterisk on the panel c indicates to the single time point at which the glucose release upon induction with a lower dose of substrate was significant (P < 0.05). On the other panels a, b, d, all the values of plants induced for >48 h are significantly different from controls
Activity of glucanases (expressed by raw intensity data, RID) in eluates excreted by sundew. The leaves were induced by acetate buffer (C), 1.15 % laminarin (L) and 1.15 % gelatin (G) as digestible substrate, respectively, for 48 h. Asterisk indicates a significant increase with respect to buffer-treated control at P < 0.05. Data represent mean ± SE values of three replicates
Hydrolysis of laminarin on sundew leaves
To explore whether laminarin acts only as an inducer of glucanase activity or it is readily hydrolyzed in digestive fluid, amounts of the (residual) laminarin and of the released reducing saccharides were measured in leaf eluates (Fig. 3). The results showed that with progressing time of the experiment the amount of laminarin in secretions continuously decreased indicating to its decomposition (Fig. 3c, d). Contemporarily, the amount of total reducing sugars gradually increased. At the limited substrate the elevation was low and significant at a single time point (48 hpi; P < 0.05), while it was much more obvious after induction with excessive amounts of laminarin (Fig. 3d). In control plants only small fluctuating amounts of reducing sugars were detected.
Hydrolysis of laminarin by sundew glucanases was analyzed in more detail by thin layer chromatography (Fig. 5). Little amounts of glucose and other simple saccharides were detectable in the samples from control plants and the induced plants at time 0 hpi (Suppl. Fig. S1), probably as lysis products of the single, naturally occurring d-glucurono-d-mannan polysaccharide (>2.103 kDa) in the mucilage (Rost and Schauer 1977). With prolonged induction time, laminarin on leaves (in both excessive and limited amount) was progressively degraded into typical oligosaccharides with degree of polymerization 2–6 and glucose (Fig. 5a). This pattern indicates to cleavage of β -1,3-d-glucosidic bonds inside the laminarin chain that is characteristic for endo-1,3-β-glucanases (Hrmová and Fincher 1993).
Thin-layer chromatography plates of sundew eluates collected at different time points post induction (0–192 hpi) with a substrate. There was applied 1.15 % a or 20 % laminarin b. Below the plates the graphs show the raw integrated densities (RID) of spots corresponding to individual laminarin degradation products (s1–s6) for the period of the experiment. Data indicate mean ± SE values of three replicates (on the plate loaded in order 1–3 for each time point). As reference saccharides (RefS), glucose and maltose were co-separated
The pattern of hydrolytic products revealed that their amount does not proportionally increase over the whole experiment. When limited amount of laminarin was applied, little amounts of simple saccharides were detected at the end of the experiment (192 hpi) while laminarin became depleted from leaves (Fig. 5a). This suggests that laminarin is not only decomposed in tentacle secretions but products are also taken up by leaves. The observed pattern corroborates well with the activity of glucanases as well as with the data on amounts of laminarin and reducing saccharides (Fig. 3). At excessive laminarin on leaves the amount of released sugars remained high till the end of the experiment (Fig. 5b). The phenomenon of oligosaccharide uptake by leaves under these conditions was not obvious, nevertheless, the accumulation rate of simple saccharides was non-linear (Fig. 3d).
Proteolytic processes on the sundew leaves
Very low proteolytic activities were measured in sundew plants induced with laminarin as well as in controls (Fig. 6). Exception was a small but significant (P < 0.01) increase at excessive amount of substrate on leaves 72 hpi (Fig. 6b). Hence, proteases are not (or weakly) induced by glucan-rich substrate in sundew secretions. Nevertheless, the measured activity can be attributed to a minimum of three protein fractions of different sizes (90, 75 and 60 kDa, respectively) as detected in gelatin-polyacrylamide gels (Fig. 6c). Unfortunately, intensities of these bands could not be reliably quantified using the given system.
Proteolytic activity (expressed as trypsin equivalents) of sundew eluates collected at different time points post induction (0–192 hpi). Plants were induced with a buffer (gray squares), or laminarin solution (black triangles) in limited 1.15 % a or excessive 20 % b amount (respectively). The activities correspond to minimum of three protease fractions as detected after separation on SDS-PAGE (c; for clarity, the grayscale of gel was inverted). Data represent mean ± SE values of three replicates. Asterisk indicates a significant increase with respect to buffer-treated control at P < 0.05. The last line of the gel (GI) indicates a sample from the plant induced by piece of 1.5 % gelatine. M, protein size marker (Page Ruler™ Plus Prestained Protein Ladder, Thermo Scientific)
Discussion
To survive in nutrient-poor habitats, carnivorous plants produce specialized features such as traps and digestive glands enabling them to gather additional nutrients from various heterotrophic sources. This ability is reinforced by releasing the cocktail of endogenous hydrolytic enzymes that cleave complex substances into simpler absorbable nutrients. Recently, a glucanase has been identified in the digestive fluid from the Venus fly trap (Schulze et al. 2012), and another two glucanases have been detected in digestive fluids of unopened pitchers of Nepenthes alata (Hatano and Hamada 2012). Here we report on the presence of a single ~50 kDa acidic endoglucanase in secretions of sundew tentacles. For comparison, the enzyme identified in the Venus flytrap showed homology with a 37.4 kDa acidic enzyme from Arabidopsis (Schulze et al. 2012). Albeit secretion of digestive enzymes has long been proposed as inducible by the presence of prey (Rost and Schauer 1977; Adlassnig et al. 2012), the sundew glucanase is present in the secretions of non-stimulated plants, too. Following the stimulation of sundew glands with a glucan-rich substrate the activity of the glucanase increases, reaches maxima after several days and subsequently declines till the end of the experiment. This pattern coincides with release rate of reducing sugars, at concurrent decrement of source substrate on the leaves. It must be noted, however, that the observed process does not appear as fixed scenario. At high excess of substrate the enzyme activity (and release rate of reducing sugars) is kept high even at the end of the experiment, thus availability of the substrate appears to condition the enzyme induction.
The activity of glucanase in time does not appear to corroborate the dynamics of the proteases in sundew secretions. Previously, Matušíková et al. (2005) have observed a similar discrepancy between chitinase and proteolytic activities of sundew secretions. Albeit mechanical irritation of highly sensitive tentacles clearly does activate (at least to some extent) the enzymes in mucilage (Matušíková et al. 2005), their activity appears to depend on the chemical nature of the substrate on leaves. For example, induction with gelatine caused four-fold increase of the proteolytic activity at 48 hpi, whereas it had a low impact on the chitinase- (Matušíková et al. 2005) or glucanase activity (this study). Thus, different stimuli can activate different components of the plant digestive mechanism. The type of substrate might also determine the efficacy of the prey decay machinery, while it is poorly (or not at all) activated when stimulus is only mechanical or lacks nutrients (e.g., water, acetate buffer). A specific induction after the perception of appropriate signals seems to be reasonable in nutrient-poor habitats as it may limit the cost of carnivory to the plant and optimizes the use of available resources (Gallie and Chang 1997).
The biological role of glucanases (along with other PR proteins) in carnivorous traps has primarily been considered in the context of facing microbial pathogens (Juniper et al. 1989; Matušíková et al. 2005; Hatano and Hamada 2008; Schulze et al. 2012). A standby antimicrobial defense seems to be reasonable considering a high microbial community diversity resulting from a broad variety of prey and high concentrations of nutrients and organic material on adhesive traps. Indeed, some carnivorous plants can avoid and control (at least to some extent) microbial colonization of their traps (Pranjic 2004; Buch et al. 2013). However, considering the extraordinary cost of carnivory nature (Givnish et al. 1984; Thorén et al. 1996), the purely defense capability might not satisfactorily justify the resource investment into the production of a rich cocktail of PR enzymes in mucilage (Schulze et al. 2012). In this sense, additional benefit of such proteins has recently been suggested in certain plant species as a result of evolutional shift toward carnivory (Renner and Specht 2012; Schulze et al. 2012). This concept appears to be valid for e.g., chitinases mostly with respect to decomposition of insect carcass (Juniper et al. 1989; Matušíková et al. 2005). Moreover, the jasmonate cascade, that is known to regulate the PR enzymes in plants (Turner et al. 2002), has recently been demonstrated to facilitate carnivory in sundew since it triggers leaf bending upon capture of prey (Nakamura et al. 2013).
Here we present the first (though still indirect) evidence that a glucanase contributes to digestive processes of CPs. Since a common insect prey of sundews lacks glucans, such a role of this enzyme in sundew secretions might appear as doubtful. From early years, however, experimental examples are known of a ‘vegetarian’ nutrition of some flypaper-trapped carnivorous plants that augment the conventional insect diet (Harder and Zemlin 1968; Juniper et al. 1989). This consists of glucan-rich airborne organic particles, such as pollen grains, fungal spores, seeds, detritus or other random fragments. It is likely, that in natural conditions certain CPs, both episodically and continuously, could benefit from the aerial rain of such particles (Harder and Zemlin 1968; Juniper et al. 1989). However, before the present study the digestion of glucan-rich diet has never been studied in any depth with respect to hydrolytic enzymes present in the carnivorous traps. The cell wall glucan-polysaccharides are not a direct source of N, S or P for CPs (in contrast to e.g., chitin of insect cuticle that is an N-acetyl-derivative of glucose). Nevertheless, their decomposition by glucanases (and perhaps some other enzymes with possible glucanohydrolytic activities such as thaumatin-like proteins and beta-glucosidases, that could be present in sundew mucilage, too) could enable access and utilization of other, nutritionally more rich parts. This has been shown indirectly by feeding Pinguicula plants grown on nutrient-deficient media solely with Pinus pollen leading to stimulated growth and flowering (Harder and Zemlin 1968).
Our results further show that the simple sugars released from the laminarin are taken up from the leaves. Except for a few works on some Nephentales (reviewed by Juniper et al. 1989), Aldrovanda vesiculosa (Fabian-Galan and Salageanu 1968) and Drosera capensis (Adlassnig et al. 2012) there is little knowledge on decomposition or assimilation of sugars in digestive secretions of CPs. The main reason for this might be that the assimilation of organic carbon in itself is of a low ecological importance (Adamec 1997). On the other hand, once made accessible, assimilation of energy rich saccharide molecules could (partially) compensate for high energetic costs to carnivory. In aquatic Utricularia species, the autotrophically fixed carbon is preferentially allocated to growing shoot apices and traps to cover the costs of carnivory (Sirová et al. 2010). The ability to digest and absorb sugars from heterotrophic sources may become advantageous especially in situations when access to sunlight is limited (e.g., due to shadowing by surrounding plants).
Still, the putative role of glucanases does not necessarily has to be restricted to defense or delivering nutrition to plant. Hypothetically, several other roles might also be considered since glucanases degrade polysaccharides into simpler forms with diverse utility in the processes of carnivory. For example, it has been shown that sweetness of extra-floral nectar is the primary means by which pitcher plants attract prey (Bennet and Ellison 2009). The saccharide composition also alters viscoelasticity of the digestive fluid and thereby affects retention efficiency of the trap (Gaume and Forterre 2007; Bauer et al. 2009). Furthermore, some saccharides (especially sucrose) promote endocytosis that supplements the nutrient uptake by carriers in many carnivorous plants including those from the genus Drosera (Etxeberria et al. 2005). The latter strategy enables CPs to relocate a part of prey digestion into the interior of the gland, while smaller amounts of digestive enzymes have to be secreted (Adlassnig et al. 2012). Finally, the carbon substances in traps of some carnivorous plants have been suggested to benefit the associated microbes (Sirová et al. 2009, 2010; Koopman et al. 2010; Takeuchi et al. 2011). In protocarnivorous species that do not produce digestive enzymes at all (e.g., Sarracenia or Heliamphora) a functioning phytoelm community is crucial for prey utilization (Koopman et al. 2010). For other carnivorous species there is still considerable uncertainty about the functional and ecological benefits of microbes to carnivory (Takeuchi et al. 2011).
Conclusion
We conclude that a glucanase acts in sundew digestive secretions and degrades a glucan-rich substrate into simple saccharides. Furthermore, our indirect approach showed these are readily taken up by the digestive glands indicating that the glucanases contribute to the plant digestion processes. Further research is needed to reveal the substrate specificity, regulation of activity and possible other functions, which the glucanases can fulfil in the secretion of tentacles. Use of radioactively-labeled substrate would provide more direct evidence on uptake of glucanase digestive products and clarify how the gained sugar-rich resources allocate in the plants under natural conditions. It also remains to elucidate how such saccharide-base resources influence the uptake of others (e.g., nitrogen) nutrients and whether they provide any ecophysiological benefit to the CPs, for instance under growth conditions encumbering photosynthesis. In support to the assumption that carnivory represents an evolutionary shift from defense (Mithöfer 2011; Hatano and Hamada 2012; Schulze et al. 2012) this work broadens the list of defense components with a possibly direct role in feeding of the CPs. Phylogenetic analyses of glucanase gene sequences within Caryophyllales might reveal whether functional specialization of these enzymes occurred during evolution as it was shown for chitinases (Renner and Specht 2012).
Abbreviations
- CP:
-
Carnivorous plant
- Hpi:
-
Hours post induction
- PR:
-
Pathogenesis-related
References
Adamec L (1997) Mineral nutrition of carnivorous plants: a review. Bot Rev 63:273–299
Adlassnig W, Koller-Peroutka M, Bauer S, Koshkin E, Lendl T, Lichtscheidl IK (2012) Endocytotic uptake of nutrients in carnivorous plants. Plant J 71:303–313
Albert VA, Williams SE, Chase MW (1992) Carnivorous plants: phylogeny and structural evolution. Science 257:1491–1495
Bauer U, Willmes C, Federle W (2009) Effect of pitcher age on trapping efficiency and natural prey capture in carnivorous Nepenthes rafflesiana plants. Ann Bot 103:1219–1226
Bauer U, Clemente CJ, Renner T, Federle W (2012) Form follows function: morphological diversification and alternative trapping strategies in carnivorous Nepenthes pitcher plants. J Evol Biol 25:90–102
Bennet KF, Ellison AM (2009) Nectar, not colour, may lure insects to their death. Biol Lett 5:469–472
Bobák M, Blehová A, Krištín J, Ovečka M, Šamaj J (1995) Direct plant regeneration from leaf explants of Drosera rotundifolia cultured in vitro. Plant Cell Tiss Org Cult 43:43–49
Boller T (1987) Hydrolytic enzymes in plant disease resistance. In: Kosuge T, Nester EW (eds) Plant microbe interactions: molecular and genetic properties. Macmillan, New York, pp 385–413
Boller T (1993) Antimicrobial functions of the plant hydrolases, chitinase and ß-1,3-glucanase. In: Fritig B, Legrand M (eds) Mechanisms of plant defense responses. Kluwer Academic Publishers, Dordrecht, pp 391–400
Buch F, Rott M, Rottloff S, Paetz C, Hilke I, Raessler M, Mithöfer A (2013) Secreted pitfall-trap fluid of carnivorous Nepenthes plants is unsuitable for microbial growth. Ann Bot 111:375–383
Clancy FGA, Coffey MD (1977) Acid phosphatase and protease release by the insectivorous plant Drosera rotundifolia. Can J Bot 56:480–488
Dong JZ, Dunstan DI (1997) Endo chitinase and beta-1,3-glucanase genes are developmentally regulated during somatic embryogenesis in Picea glauca. Planta 201:189–194
Eilenberg H, Pnini-Cohen S, Schuster S, Movtchan A, Zilberstein A (2006) Isolation and characterization of chitinase genes from pitchers of the carnivorous plant Nepenthes khasiana. J Exp Bot 57:2775–2784
Ernst D, Bodemann A, Schmelzer E, Langebartels C, Sandermann H (1996) Beta-1,3-Glucanase mRNA is locally, but not systemically induced in Nicotiana tabacum L. cv Bel W3 after ozone fumigation. J Plant Physiol 148:215–221
Etxeberria E, Baroja-Fernandez E, Munoz FJ, Pozueta-Romero J (2005) Sucrose-inducible endocytosis as a mechanism for nutrient uptake in heterotrophic plant cells. Plant Cell Physiol 46:474–481
Fabian-Galan G, Salageanu N (1968) Considerations on the nutrition of certain carnivorous plants (Drosera capensis and Aldrovanda vesiculosa). Rev Roum Biol Bot 13:275–280
Gallie DR, Chang SC (1997) Signal transduction in the carnivorous plant Sarracenia purpurea. Regulation of secretory hydrolase expression during development and in response to resources. Plant Physiol 115:1461–1471
Gaume L, Forterre Y (2007) A viscoelastic deadly fluid in carnivorous pitcher plants. PLoS ONE 2:e1185
Givnish TJ, Burkhardt EL, Happel RE, Weintraub JD (1984) Carnivory in the bromeliad Brocchinia reducta with a cost/benefit model for the general restriction of carnivorous plants to sunny, moist, nutrient poor habitats. Am Nat 124:479–497
Givnish TJ, Barfuss MHJ, Van Ee B, Riina R, Schulte K, Horres R, Gonsiska PA, Jabaily RS, Crayn DM, Smith JAC, Winter K, Brown GK, Evans TM, Holst BK, Luther H, Till W, Zizka G, Berry PE, Sytsma KJ (2011) Phylogeny, adaptive radiation, and historical biogeography in Bromeliaceae: insights from an eight-locus plastid phylogeny. Am J Bot 98:872–895
Harder R, Zemlin I (1968) Blütenbildung von Pinguicula lusitanica in vitro durch Fütterung mit Pollen. Planta 78:72–78
Hatano N, Hamada T (2008) Proteome analysis of pitcher fluid of the carnivorous plant Nepenthes alata. J Proteome Res 7:809–816
Hatano N, Hamada T (2012) Proteomic analysis of secreted protein induced by a component of prey in pitcher fluid of the carnivorous plant Nepenthes alata. J Proteomics 75:4844–4852
Helleboid S, Bauw G, Belingheri L, Vasseur J, Hilbert JL (1998) Extracellular beta-1,3-glucanases are induced during early somatic embryogenesis in Cichorium. Planta 205:56–63
Hrmová M, Fincher GB (1993) Purification, characterization and gene structure of (1 → 3)-β-glucanase isoenzyme GIII from barley (Hordeum vulgare). Biochem J 289:453–461
Inbar J, Chet I (1991) Detection of chitinolytic activity in the rhizosphere using image analysis. Soil Biol Biochem 23:239–242
Ishisaki K, Arai S, Hamada T, Honda Y (2012a) Biochemical characterization of a recombinant plant class III chitinase from the pitcher of the carnivorous plant Nepenthes alata. Carbohydr Res 361:170–174
Ishisaki K, Honda Y, Taniguchi H, Hatano N, Hamada T (2012b) Heterogonous expression and characterization of a plant class IV chitinase from the pitcher of the carnivorous plant Nepenthes alata. Glycobiology 22:345–351
Jach G, Gornhardt B, Mundy J, Logemann J, Pinsdorf P, Leah R, Schell J, Maas C (1995) Enhanced quantitative resistance against fungal disease by combinatorial expression of different barley antifungal proteins in transgenic tobacco. Plant J 8:97–109
Juniper BE, Robins RJ, Joel DM (1989) The carnivorous plants. Academic Press, London
Kauss H (1989) Fluorometric measurement of callose and other 1,3-β-glucans. In: Linskens HF, Jackson JF (eds) Modern methods of plant analysis: Plant fibers. Springer, Berlin, pp 127–137
Koopman MM, Fuselier DM, Hird S, Carstens BC (2010) The carnivorous pale pitcher plant harbors diverse, distinct, and time-dependent bacterial communities. Appl Environ Microbiol 76:1851–1860
Krol E, Plachno BJ, Adamec L, Stolarz M, Dziubinska H, Trebacz K (2012) Quite a few reasons for calling carnivores ‘the most wonderful plants in the world’. Ann Bot 109:47–64
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Levy A, Erlanger M, Rosenthal M, Epel BL (2007) A plasmodesmata-associated beta-1,3-glucanase in Arabidopsis. Plant J 49:669–682
Libantova J, Kamarainen T, Moravcikova J, Matusikova I, Salaj J (2009) Detection of chitinolytic enzymes with different substrate specificity in tissues of intact sundew (Drosera rotundifolia L.). Mol Biol Rep 36:851–856
Lotan T, Ori N, Fluhr R (1989) Pathogenesis-related proteins are developmentally regulated in tobacco flowers. Plant Cell 1:881–887
Matušíková I, Libantová J, Moravčíková J, Mlynárová L, Nap JP (2004) The insectivorous sundew (Drosera rotundifolia L.) might be a novel source of PR genes for biotechnology. Biologia 59:719–725
Matušíková I, Salaj J, Moravčíková J, Mlynárová L, Nap JP, Libantová J (2005) Tentacles of in vitro-grown round-leaf sundew (Drosera rotundifolia L.) show induction of chitinase activity upon mimicking the presence of prey. Planta 222:1020–1027
Meier H, Kesting U, Poppe S (1981) Effect of native crude fiber on the digestibility of nitrogen and amino acids in pigs. Arch Tierernahr 31:187–193
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Mithöfer A (2011) Carnivorous pitcher plants: insights in an old topic. Phytochemistry 72:1678–1682
Moravčíková J, Libantová J, Heldák J, Salaj J, Bauer M, Matušíková I, Gálová Z, Mlynárová L (2007) Stress-induced expression of cucumber chitinase and Nicotiana plumbaginifolia beta-1,3-glucanase genes in transgenic potato plants. Acta Biol Plant 29:133–141
Nakamura Y, Reichelt M, Mayer VE, Mithöfer A (2013) Jasmonates trigger prey-induced formation of ‘outer stomach’ in carnivorous sundew plants. Proc R Soc B 280:20130228
Ori N, Sessa G, Lotan T, Himmelhoch S, Fluhr R (1990) A major stylar matrix polypeptide (sp41) is a member of the pathogenesis-related proteins superclass. EMBO J 9:3429–3436
Pan SQ, Ye XS, Kuc J (1991) A technique for detection of chitinase, beta-1,3-glucanase, and protein- patterns after a single separation using polyacrylamide-gel electrophoresis or isolelectrofocusing. Phytopathology 81:970–974
Pauchet Y, Freitak D, Heidel-Fischer HM, Heckel DG, Vogel H (2009) Immunity or digestion: glucanase activity in a glucan-binding protein family from Lepidoptera. J Biol Chem 284:2214–2224
Piršelová B, Kuna R, Libantová J, Moravčíková J, Matušíková I (2011) Biochemical and physiological comparison of heavy metal-triggered defense responses in the monocot maize and dicot soybean roots. Mol Biol Rep 38:3437–3446
Pranjic K (2004) Zur Ökologie karnivorer Pflanzen: Die Rolle von Mikroorganismen beim Abbau von Tieren durch fleischfressende Pflanzen. University of Vienna, Vienna, p 145
Renner T, Specht CD (2012) Molecular and functional evolution of class I chitinases for plant carnivory in the Caryophyllales. Mol Biol Evol 29:2971–2985
Roggen HS, Stanley RG (1969) Cell wall hydrolysing enzymes in wall formation as measured by pollen tube extension. Planta 84:295–303
Rost K, Schauer R (1977) Physical and chemical properties of the mucin secreted by Drosera capensis. Phytochemistry 16:1365–1368
Rottloff S, Stieber R, Maischak H, Turini FG, Heubl G, Mithöfer A (2011) Functional characterization of a class III acid endo chitinase from the traps of the carnivorous pitcher plant genus, Nepenthes. J Exp Bot 62:4639–4647
Schlumbaum A, Mauch F, Vögeli U, Boller T (1986) Plant chitinases are potent inhibitors of fungal growth. Nature 324:365–367
Schulze WX, Sanggaard KW, Kreuzer I, Knudsen AD, Bemm F, Thogersen IB, Braeutigam A, Thomsen LR, Schliesky S, Dyrlund TF, Escalante-Perez M, Becker D, Schultz J, Karring H, Weber A, Hojrup P, Hedrich R, Enghild JJ (2012) The protein composition of the digestive fluid from the Venus flytrap sheds light on prey digestion mechanisms. Mol Cell Proteomics 11:1306–1319
Sela-Buurlage MB, Ponstein AS, Bresvloemans SA, Melchers LS, Vandenelzen PJM, Cornelissen BJC (1993) Only specific tobacco (Nicotiana tabacum) chitinases and-1, 3-glucanase exhibit antifungal activity. Plant Physiol 101:857–863
Sirová D, Borovec J, Černá B, Rejmánková E, Adamec L, Vrba J (2009) Microbial community development in the traps of aquatic Utricularia species. Aquat Bot 90:129–136
Sirová D, Borovec J, Šantrůčková H, Šantrůček J, Vrba J, Adamec L (2010) Utricularia carnivory revisited: plants supply photosynthetic carbon to traps. J Exp Bot 61:99–103
Takeuchi Y, Salcher MM, Ushio M, Shimizu-Inatsugi R, Kobayashi MJ, Diway B, von Mering C, Pernthaler J, Shimizu KK (2011) In situ enzyme activity in the dissolved and particulate fraction of the fluid from four pitcher plant species of the genus Nepenthes. PLoS ONE 6:e25144
Thorén LM, Karlsson PS, Tuomi J (1996) Somatic cost of reproduction in three carnivorous Pinguicula species. Oikos 76:427–434
Turner JG, Ellis C, Devoto A (2002) The jasmonate signal pathway. Plant Cell 14:S153–S164
Vögeli-Lange R, Frundt C, Hart CM, Nagy F, Meins F (1994) Developmental, hormonal, and pathogenesis-related regulation of the tobacco class I beta-1,3-glucanase B promoter. Plant Mol Biol 25:299–311
Worrall D, Hird DL, Hodge R, Paul W, Draper J, Scott R (1992) Premature dissolution of the microsporocyte callose wall causes male sterility in transgenic tobacco. Plant Cell 4:759–771
Wu CT, Bradford KJ (2003) Class I chitinase and beta-1,3-glucanase are differentially regulated by wounding, methyl jasmonate, ethylene, and gibberellin in tomato seeds and leaves. Plant Physiol 133:263–273
Acknowledgments
This work was supported by the grant from the Slovak Grant Agency VEGA No. 2/0090/14 and MVTS COST FA1006. Financial support for P. Socha was provided by the Operational Programme Research and Development for the project: “Implementation of the research of plant genetic resources and its maintaining in the sustainable management of Slovak republic” (ITMS: 26220220097), co-financed from the resources of the European Union Fund for Regional Development. We are thankful to Dr. Ľubomír Adamec (Institute of Botany AS, Czech Republic) for helpful discussions and critical reviewing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
425_2013_1925_MOESM1_ESM.tif
Suppl. Fig. S1 Thin-layer chromatography plates of sundew eluates (repetition 1) collected at different time points post induction (0–192 hip) with acetate buffer instead of a digestible substrate. For comparison, a sample of plant induced with laminarin for 192 h is given (192L). As reference saccharides (RefS), glucose (G) and maltose (M) were co-separated. (TIFF 1736 kb)
Rights and permissions
About this article
Cite this article
Michalko, J., Socha, P., Mészáros, P. et al. Glucan-rich diet is digested and taken up by the carnivorous sundew (Drosera rotundifolia L.): implication for a novel role of plant β-1,3-glucanases. Planta 238, 715–725 (2013). https://doi.org/10.1007/s00425-013-1925-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-013-1925-x