Abstract
Sphingolipids play an important role in signal transduction pathways that regulate physiological functions and stress responses in eukaryotes. In plants, recent evidence suggests that their metabolic precursors, the long-chain bases (LCBs) act as bioactive molecules in the immune response. Interestingly, the virulence of two unrelated necrotrophic fungi, Fusarium verticillioides and Alternaria alternata, which are pathogens of maize and tomato plants, respectively, depends on the production of sphinganine-analog mycotoxins (SAMs). These metabolites inhibit de novo synthesis of sphingolipids in their hosts causing accumulation of LCBs, which are key regulators of programmed cell death. Therefore, to gain more insight into the role of sphingolipids in plant immunity against SAM-producing necrotrophic fungi, we disrupted sphingolipid metabolism in Nicotiana benthamiana through virus-induced gene silencing (VIGS) of the serine palmitoyltransfersase (SPT). This enzyme catalyzes the first reaction in LCB synthesis. VIGS of SPT profoundly affected N. benthamiana development as well as LCB composition of sphingolipids. While total levels of phytosphingosine decreased, sphinganine and sphingosine levels increased in SPT-silenced plants, compared with control plants. Plant immunity was also affected as silenced plants accumulated salicylic acid (SA), constitutively expressed the SA-inducible NbPR-1 gene and showed increased susceptibility to the necrotroph A. alternata f. sp. lycopersici. In contrast, expression of NbPR-2 and NbPR-3 genes was delayed in silenced plants upon fungal infection. Our results strongly suggest that LCBs modulate the SA-dependent responses and provide a working model of the potential role of SAMs from necrotrophic fungi to disrupt the plant host response to foster colonization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Long-chain bases (LCBs) are metabolic precursors of complex sphingolipids, whose predominant forms in plants are C18 aminoalcohols differing by modifications at C4 (E-desaturation, hydroxylation) and at C8 (E/Z-desaturation). Biosynthesis of LCBs involves the condensation between l-serine and palmitoyl-CoA in a reaction catalyzed by the serine palmitoyltransferase (SPT), a heterodimeric enzyme composed of two subunits named LCB1 and LCB2. The first LCB produced in plants is sphinganine, which is fully saturated and contains two hydroxyl groups. Sphinganine can be further modified by the addition of a hydroxyl group at C4 to yield phytosphingosine, and/or by introduction of double bonds at C4 and C8 to produce other LCBs (Lynch and Dunn 2004; Chen et al. 2009).
LCBs play prominent roles in signal transduction pathways regulating many aspects of plant development including cell growth, proliferation, differentiation, and cell death (Worrall et al. 2003; Sperling and Heinz 2003; Chen et al. 2006, 2008; Shi et al. 2007; Dietrich et al. 2008; Teng et al. 2008; Saucedo-García et al. 2011), as well as responses to abiotic (Ng et al. 2001; Coursol et al. 2003; Chen et al. 2012; Dutilleul et al. 2012) and biotic stress (Koga et al. 1998; Suharsono et al. 2002; Wang et al. 2008; Takahashi et al. 2009; Peer et al. 2010).
The relevance of sphingolipids in plant immunity has been known for some time but in recent years there have been major breakthroughs. Sphingolipids from the fungal pathogen Magnaporthe grisea induced phytoalexin accumulation, cell death, and increased resistance to infection in rice cells (Koga et al. 1998). In this sphingolipid-mediated response, a heterotrimeric G protein and the small GTPase OsRac1 activate the OsMAPK6 transduction pathway (Suharsono et al. 2002; Lieberherr et al. 2005). In Nicotiana benthamiana, sphingolipid biosynthesis is crucial at early stages of the resistance defense response as evidenced by the fact that the gene for the SPT LCB2 subunit is strongly induced upon infection with the non-host pathogen Pseudomonas cichorii, but only a minor and transient induction is observed in response to the host pathogen P. syringae pv. tabaci (Takahashi et al. 2009). In Arabidopsis leaves inoculated with P. syringae a higher and sustained increase of phytosphingosine levels occurs in an incompatible interaction that leads to resistance, as compared with the compatible one (Peer et al. 2010). This body of evidence suggests that LCBs are early transducers required for plants’ immune response. Under this new role for sphingolipids, it is relevant to analyze the cases of two unrelated genera of necrotrophic fungi, Fusarium verticillioides and Alternaria alternata f. sp. lycopersici, which have evolved to produce sphinganine-analog mycotoxins (SAMs) that disrupt sphingolipid biosynthesis (Gilchrist and Grogan 1976; Desjardins et al. 1995; Akamatsu et al. 1997).
Alternaria alternata f. sp. lycopersici is the causal agent of tomato stem canker, and it can also infect leaves, stems, and fruits (Gilchrist and Grogan 1976). F. verticillioides is a non-obligate plant pathogen commonly associated with maize, causing root, stalk, and ear rot (Kommedahl and Windels 1981). The role of SAM production in pathogenicity has been demonstrated for both pathogens [reviewed by Sánchez-Rangel and Plasencia (2010)]. When a Fusarium musae strain that neither colonizes maize nor produces SAMs is transformed with the entire FUM cluster responsible for FB1 production, it synthesizes the toxin and infects maize seedlings (Glenn et al. 2008). A. alternata f. sp. lycopersici pathogenicity depends on the production of another SAM, the AAL-toxin, to infect susceptible tomato plants (Gilchrist and Grogan 1976; Akamatsu et al. 1997).
SAMs competitively inhibit ceramide synthase activity in distinct plant species including Arabidopsis, causing significant accumulation of LCBs which in turn induces programmed cell death (PCD) (Abbas et al. 1994; Spassieva et al. 2002; Shi et al. 2007; Saucedo-García et al. 2011). SAM-induced PCD in Arabidopsis resembles the hypersensitive response (HR) triggered in plant cells at the site of pathogen infection, which includes ROS accumulation, callose deposition, and a dose-dependent induction of defense-related gene expression (Stone et al. 2000; Gechev et al. 2004). Hypersensitive cell death is an effective defense response against biotrophic pathogens that derive nutrients from living host tissues, but not against necrotrophic pathogens as they benefit from host cell death (Glazebrook 2005).
The activation of effective defense responses against biotrophs and necrotrophs is finely regulated by phytohormones (Glazebrook 2005; Spoel and Dong 2008; Pieterse et al. 2009, 2012). Upon pathogen attack, the phytohormone concentration and/or sensitivity change depending mainly on the lifestyle and infection strategy of the invading pathogen. Salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) are considered the primary signals in the regulation of the plant defense response. The SA-dependent pathway is typically effective against biotrophic pathogens, whereas the JA-/ET-dependent pathways counteract necrotrophic pathogens (Glazebrook 2005). Mostly, these signaling pathways cross-communicate in an antagonistic or synergistic manner, activating a specific set of defense-related genes that eventually determine the outcome of the immune response (Pieterse et al. 2012). Interestingly, some evidence suggests an association between sphingolipid-mediated signaling and phytohormones in plant immunity. The FB1-induced PCD in Arabidopsis is accompanied by the induction of JA-, ET-, and SA-dependent genes (Asai et al. 2000; Stone et al. 2000). Likewise, AAL-toxin-induced PCD in tomato also requires the JA and ET signaling pathways (Egusa et al. 2009; Zhang et al. 2011; Mase et al. 2012).
In order to gain more insight into the role of sphingolipids in the defense response against necrotrophs, we used virus-induced gene silencing (VIGS) of SPT LCB2 subunit to disrupt sphingolipid biosynthesis in N. benthamiana. Silencing of SPT altered the cellular content of LCBs and provoked a notable increase in the total levels of sphinganine and sphingosine, compared to control plants infiltrated with the viral vector. LCB elevation caused SA accumulation and constitutive expression of NbPR-1, the canonical marker of SA signaling. Moreover, SPT-silenced N. benthamiana plants became susceptible to Alternaria alternata f. sp. lycopersici infection, which was associated with an ameliorated expression of defense genes and possibly with the activation of SA-dependent responses. Our results provide genetic and biochemical evidence that the elevation of endogenous LCBs activates SA-dependent responses, and suggest that SAMs role in fungal virulence is to induce PCD, and possibly to subvert the signaling pathways required for disease resistance.
Materials and methods
Plant materials and growth conditions
Nicotiana benthamiana plants were grown in plastic pots containing a mixture of Sunshine Mix 4 and vermiculite (2:1 ratio), in a glasshouse set at 25 °C day temperature under natural photoperiod. For seedlings treatment with FB1, sphinganine, or SA, N. benthamiana wild-type seeds were surface-sterilized in 2.5 % sodium hypochlorite containing 0.05 % Tween20, rinsed three times in sterile water, and imbibed on GB-5 medium supplemented with 0.75 % sucrose and 0.8 % agar. Seeds were germinated and grown for 10 days in a Lab-Line growth chamber at 22 °C under 8 h light/16 h dark cycle (GE lamps, 80 μmol m−2 s−1). Seedlings were transferred to fresh medium supplemented with 5 μM FB1, 25 μM sphinganine, or 0.5 mM SA. Control seedlings were transferred to GB5 medium with 0.1 % methanol or 0.05 % ethanol, as they were used to prepare stock solutions of SA and sphinganine, respectively. Leaves were collected after 24 and 48 h, and immediately frozen in liquid nitrogen for RNA extraction.
Chemicals
FB1, LCBs (phytosphingosine, sphinganine, and sphingosine), and SA were purchased from Sigma Chemical Company. A FB1 stock solution (1 mM) was prepared in water, a 50 mM solution of each LCB was prepared in absolute ethanol, and a SA stock solution (125 mM) was prepared in 50 % methanol. For HPLC analysis, HPLC-grade solvents (J.T. Baker), and naphthalene-2,3-dicarboxyaldehyde (NDA; Fluka) were employed. NDA stock solution (20 mM) was prepared in acetonitrile. Stock solutions were protected from light and stored at −20 °C, except the FB1 stock solution that was stored at 4 °C.
VIGS of NbLCB2
The TRV vector was kindly provided by Dr. David Baulcombe from the Sainsbury Laboratory (Norwich, UK, http://www.tsl.ac.uk). A 602-bp cDNA fragment of the N-terminal region of NbLCB2 was amplified from total RNA of N. benthamiana leaves with the LCB2_602 primers listed in Supplementary Table S1. This fragment was cloned into pGEM-T Easy vector (Promega), sequenced and reamplified with the LCB2-BamHI and LCB2-HindIII primers. The resulting product was digested with BamHI and HindIII and cloned into the pTRV vector. The new plasmid was named pTRVSPT. Agrobacterium tumefaciens strain C58C1 was transformed with the pTRVSPT construct by electroporation. Cultures of A. tumefaciens C58C1 containing pBINTRA6, pTRV, or pTRVSPT were used to infiltrate leaves of N. benthamiana plants at 5-leaf stage using a needleless syringe (Ratcliff et al. 2001). Plants were grown for 4 weeks and evidence of gene silencing of NbLCB2 was obtained by semi-quantitative RT-PCR using two different primer sets: LCB2_1069 and LCB2_256. Viral replication and systemic infection were confirmed with TRV_Cp and TRV_Mp primers. All primer sets used in this study are listed in Supplementary Table S1.
Sphingolipids extraction and total LCBs quantification
Total LCBs from leaves of TRV and TRVSPT plants were extracted following the method described by Markham et al. (2006) and 1 nmol of d20:1 was used as internal standard per sample. Approximately 15- to 20-leaf disk per plant were employed so a representative sample was collected. Sphingolipids were hydrolyzed for 16 h at 110 °C in 10 % barium hydroxide. LCBs were extracted with 2 mL of diethylether and the lipid extract was redissolved in 200 μL of ethanol. NDA derivatization was accomplished by adding the following stock solutions: 140 μL of 25 mM sodium tetraborate buffer pH 9.5, 30 μL of 2.5 mM KCN, and 10 μL of 2.5 mM NDA. The tube was sealed with PTFE film and heated at 60 °C for 20 min. LCBs were quantified based on the recovery of the d20:1 standard. The HPLC system consisted of a Shimadzu LC-10ADVP pump, a RF-10AXL fluorescence detector, and a CR5A integrator. Derivatized LCBs were separated in a SUPELCO C18 column (15 cm × 4.6 mm, 5 μm) using 94 % acetonitrile as mobile phase at a flow rate of 1.2 mL min−1. Fluorescent derivatives were monitored at λexc of 419 nm and λem of 493 nm.
Alternaria sp. isolation and identification
Alternaria sp. was isolated from leaves of TRVSPT plants showing disease symptoms. Briefly, the leaves were placed on potato dextrose agar medium (PDA) and incubated for 5 days at room temperature under fluorescent light. Fungal colonies were transferred to PDA agar and incubated under the same conditions for 5–7 days. A single-spore isolate was obtained by preparing a spore suspension from the PDA culture in 10 mL of sterile water and streaking a loophole on water agar. The isolate was named A2SS and the identification was based on its macroscopic and microscopic characteristics. This was further confirmed by comparative sequence-based identification using the nuclear ribosomal internal transcribed spacer (ITS) region (ITS1, 5.8S rRNA, and ITS2) located between the nuclear small (18S) and large subunit (28S) rRNA genes.
DNA was obtained from the A2SS strain with DNAzol reagent (Invitrogen) according to manufacturer’s instructions. The ITS was amplified with the universal primers ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) and ITS5 (5′-GGAAGTAAAAGTCGTAACAAGG-3′; White et al. 1990), and the 600-bp product was resolved and purified from 2 % agarose gel for sequencing.
Pathogen inoculation
Alternaria sp. strains were grown on PDA for 2–3 weeks at room temperature under fluorescent light. Spores were collected in sterile water and the suspension was adjusted to 1 × 105 conidia/mL by counting in a hemacytometer. Detached leaves or stem pieces (2 cm length) of N. benthamiana TRV and TRVSPT plants were placed in a petri dish with a sterile moist filter paper and inoculated with 10 μL of the conidial suspension, sealed with parafilm, and incubated at 22 °C under 8 h light/16 h dark photoperiod (GE lamps, 80 μmol m−2 s−1). Control tissues were inoculated with the same volume of sterile water. Disease symptoms were recorded photographically. For spore counting, stems were transferred to eppendorf tubes and spores were released from the tissue by vigorous shaking in 1 mL water and counted in a hemacytometer.
Semi-quantitative RT-PCR analysis
Total RNA was extracted from 200 to 300 mg of leaf tissue using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Total RNA was calibrated spectrophotmetrically and in an agarose gels using the 18S rRNA band, and then 1 μg was used to synthesize the first-strand cDNA with oligo(dT) primer and Improm-II™ reverse transcriptase (Promega). PCR was performed with a denaturing temperature of 94 °C for 3 min, and annealing and extension temperatures of 58 and 72 °C, respectively, for 40 s. To compare levels of each transcript, a systematic analysis of primer concentration, total RNA amounts, and number of amplification cycles were conducted. Calibration curves with varying cycle number (22–32 cycles) were constructed to determine the proper number of cycles to quantify each transcript (Supplementary Fig. S1). Transcript levels of the eukaryotic elongation factor 1-alpha (NbEF1α) were used for normalization thus allowing a reproducible analysis of the various transcripts within the three or four replicates performed. The gene-specific primers used are indicated in Supplementary Table S1. Densitometric analysis of the PCR-generated fragments were analyzed and quantified with the Quantity software (Bio-Rad).
SA extraction and quantification by HPLC
SA was extracted from leaves as described by Meuwly and Métraux (1993) using o-anisic acid (o-ANI, 300 ng) as internal standard. HPLC analysis was performed in the equipment described above, setting fluorescence detection wavelengths at 305 nm excitation/405 nm emission for SA, and 305/365 for o-ANI. Dilutions of the extracts of free and conjugated SA were prepared in mobile phase (40 % methanol and 60 % water acidified with 4 % acetic acid), and were resolved by isocratic elution with a flow rate of 1.2 mL min−1. Under these HPLC conditions, SA eluted at ~10 min and o-ANI at 5 min. o-ANI recovery ranged from 46 to 74 %, and SA values were adjusted for yield losses.
Statistical analysis
Data was analyzed with the general linear model analysis of variance and Tukey’s test to determine the statistical significance at 0.05 and 0.01 probability using the Statistix® software package.
Accession number
NbLCB2 cDNA sequence was deposited in GenBank/EMBL databases (accession number AM902524).
Results
VIGS of N. benthamiana SPT LCB2 subunit dramatically affected its vegetative and reproductive development
A 1069-bp cDNA fragment of NbLCB2 (GenBank accession no. AM902524) was used to subclone a 602-bp cDNA into the TRV vector and the construct was named pTRVSPT (Fig. 1a). N. benthamiana plants at 5-leaf stage were agroinfiltrated with the empty TRV vector (as control) or pTRVSPT construct. Four weeks later, non-silenced TRV-infected control plants did not showed morphological differences compared to not agroinfiltrated plants (not shown). In contrast, TRVSPT infected plants developed dramatic changes in their phenotype, qualitatively similar to those reported by Takahashi et al. (2009), such as significant growth reduction (Fig. 1b, right) and abnormal leaf morphology (Fig. 1c). TRVSPT plant leaves lost their typical heart-shape and developed irregular morphology. Moreover, a strong effect on flower development and morphology was also observed as flowers of TRVSPT plants showed several defects such as bending and shortening of the corolla tube, alterations in the number, and shape of petals (Fig. 1d). Also spontaneous cell death was observed by the presence of necrotic areas on some leaves (Fig. 1e).
VIGS of SPT LCB2 subunit in N. benthamiana. a Scheme of NbLCB2 cDNA and the region used to generate the VIGS construct pTRVSPT. The positions of the primer sets LCB2-256 and LCB2-1069 used for RT-PCR analyzes are marked by arrows. b VIGS phenotype characterized by growth restriction and alterations in vegetative and reproductive development. The TRV (left) and TRVSPT plants (right) were photographed 4 weeks after agroinfiltration. c Morphological changes in leaves of TRVSPT plants. d Alterations in flower development of TRVSPT plants. e Spontaneous cell death associated to SPT-silencing. f Endogenous NbLCB2 mRNA levels in the leaves of TRV and TRVSPT plants. NbEF1α mRNA levels were used as RNA loading control
Efficiency of virus-induced SPT-silencing was evaluated by semi-quantitative RT-PCR using the LCB2_256 and LCB2_1069 primer sets (Fig. 1a). NbLCB2 transcript was detected in TRV control plants and 20–50 % reduction was typically observed in TRVSPT plants (Fig. 1f). Similar levels of viral transcripts (coat and movement proteins) were detected in control and SPT-silenced plants (Supplementary Fig. S2), confirming that viral infection did not interfere with the phenotype and that morphological changes were caused by the silencing of the SPT.
Effects of SPT-silencing on sphingolipid LCB composition
Because SPT is the first enzyme in sphingolipid biosynthesis (Gable et al. 2000; Breslow et al. 2010), we evaluated the effect of LCB2 gene silencing on LCB composition in N. benthamiana leaves 4 weeks after agroinfiltration. Phytosphingosine (t18:0), sphingosine (d18:1), and sphinganine (d18:0) were identified by the retention time of the corresponding standards (Fig. 2). Some other LCBs were detected as NDA derivatives of the LCBs released by sphingolipid hydrolysis from TRV- and SPT-silenced leaves (Supplementary Fig. 3, peaks 1–9). LCB composition of TRV control leaves was similar to those reported previously for other Solanacea, such as tobacco and tomato, and also Arabidopsis (Sperling et al. 2005; Markham et al. 2006; Buré et al. 2011), in which trihydroxylated LCBs such as phytosphingosine are more abundant than the dihydroxylated LCBs sphinganine and sphingosine (Fig. 2; Supplementary Fig. 3a, b). It is likely that some LCBs detected but not identified (Supplementary Fig. 3a, b) correspond to the isomers of 8-phytosphingenine (t18:1-E and t18:1-Z) that have been detected in N. tabacum leaves under similar analytical conditions (Sperling et al. 2005), or to the isomers of 4,8-sphingadienine (d18:2-EE and d18:2-EZ) which were also detected in tobacco leaves, cultured cells, and in tomato leaves (Sperling et al. 2005; Markham et al. 2006; Buré et al. 2011).
Total content (nmol g−1 FW) of phytosphingosine (t18:0), sphingosine (d18:1), and sphinganine (d18:0) in leaves of N. benthamiana TRV and TRVSPT plants 4 weeks after agroinfiltration. A total of 15–20 leaf disks were collected from each plant and pooled so values are expressed as the mean ± SD of four independent plants. Different letters above each set of bars indicate a significant difference (p < 0.01, Tukey’s test) in levels for the indicated LCB
Regarding TRVSPT leaves, the LCB profile was notably altered as the levels of phytosphingosine and other LCBs decreased (Fig. 2; Supplementary Fig. 3a), whereas the abundance of sphinganine and sphingosine were consistently found to be higher than in the TRV leaves (Fig. 2; Supplementary Fig. 3b). In TRVSPT leaves phytosphingosine levels were reduced by 50 %, whereas sphingosine and sphinganine levels increased threefold and fourfold, respectively, compared with TRV control leaves (Fig. 2). A general decrease of LCBs was not observed as expected, and importantly, the results revealed that SPT-silencing in N. benthamiana had multiple effects on sphingolipid metabolism, and that the abundance of bioactive LCBs can be selectively affected by the action of SPT.
VIGS of SPT activated SA-dependent responses
The increased levels of sphinganine and sphingosine found in TRVSPT plants correlated with the spontaneous cell death phenotype (Fig. 1e). This response was comparable to the PCD developed by Arabidopsis mutants affected in different reactions of sphingolipid metabolism (acd5, acd11, and erh1), which was associated to increased levels of SA, (Greenberg et al. 2000; Brodersen et al. 2005; Wang et al. 2008). Therefore, we determined SA levels in TRVSPT-silenced plants. As shown in Fig. 3a, N. benthamiana TRV leaves had low basal levels of total SA (~500 ng g−1 FW) in accordance with values reported by Catinot et al. (2008), while in TRVSPT leaves a 50 % increase in the content of total SA was found as compared to TRV leaves (p < 0.01). Furthermore, SA accumulation provoked by VIGS of SPT caused a strong constitutive expression of NbPR-1 gene that was not observed in TRV plants (Fig. 3b). The abundance of NbPR-1 transcripts was determined at saturation phase of PCR, demonstrating that the viral infection did not contribute to NbPR-1 induction in TRVSPT plants. These results strongly suggest a link between sphingolipid metabolism and SA signaling, possibly through the LCBs which increased by the SPT-silencing in N. benthamiana.
VIGS of SPT activated the salicylic acid (SA) signaling pathway. a Content of total SA in TRV and TRVSPT plant leaves. Samples were taken 5 weeks after agroinfiltration. Values are expressed as the mean ± SD of five independent plants. The asterisk above bars denotes a significant difference (p < 0.01, Tukey’s test). b VIGS of SPT caused constitutive expression of the SA-inducible NbPR-1 gene. Lane numbers correspond to independent plants
Sphinganine and FB1 restricted the growth of N. benthamiana wild-type seedlings and up-regulated the SA-inducible NbPR-1 gene
In order to confirm whether the activation of the SA-dependent signaling pathway in TRVSPT plants was mediated by elevation of LCBs levels, we exposed 10-day-old N. benthamiana wild-type seedlings to FB1 or exogenous sphinganine and evaluated NbPR-1 transcript levels. First, we assessed the susceptibility of N. benthamiana seedlings to FB1 because it was reported that the most of the Solanaceae, and in particular the Nicotiana species, are insensitive to SAMs (Mesbah et al. 2000; Brandwagt et al. 2001). As shown in Fig. 4a, FB1 induced a dose-dependent growth arrest and restricted root hair development. At the lowest dose (1 µM) a discrete reduction (24 %) was observed, whereas 5 µM FB1 caused a significant decrease of 64 % (p < 0.01, Fig. 4b), but did not induce chlorosis or cell death. These results indicated that N. benthamiana exhibited an intermediate sensitivity to FB1 according to the classification made by Brandwagt et al. (2001). Growth reduction caused by FB1 may be related to LCBs accumulation, as seedlings developed in presence of sphinganine showed a similar inhibition (Supplementary Fig. S4).
Sphinganine and FB1 up-regulated the SA-responsive gene PR-1 in N. benthamiana wild-type seedlings. a N. benthamiana had intermediate sensitivity to FB1. N. benthamiana 10-day-old seedlings were transferred to GB5 medium with or without FB1, and photographs were taken 10 days later. b Comparison of seedlings root length after 10 days of FB1 exposure (n = 10). Values are expressed as the mean ± SD. Different letters indicate a significant difference (p < 0.01, Tukey’s test). Inhibition of root growth and hypocotyl elongation were observed, but the cotyledons emerged and became green. The cotyledons and roots never developed necrosis symptoms. c FB1 and sphinganine induced the expression of the SA marker gene PR-1 in N. benthamiana. Ten-day-old seedlings were transferred to GB5 media supplemented with 5 µM FB1, 25 µM sphinganine, or 0.5 mM SA. Leaves were collected after 24 and 48 h of treatment and immediately frozen in liquid nitrogen for RNA extraction. Control seedlings correspond to 10-day-old seedlings transferred to fresh GB5 medium and the sample was taken after 48 h. Mock treatments with 0.05 % ethanol or 0.1 % methanol were included as they were used to prepare stock solutions of sphinganine and SA, respectively. Results are representative of two independent experiments
To examine whether LCBs were involved in the activation of SA-dependent signaling, 10-day-old seedlings were transferred to agar plates supplemented with 5 µM FB1, 25 µM sphinganine or 500 µM SA, whereas control seedlings were transferred to agar plates without chemicals and mock treatments containing 0.05 % ethanol or 0.1 % methanol. Noticeably, FB1 and sphinganine-induced NbPR-1 expression at 24 h of exposure, and the response was heightened at 48 h, especially with sphinganine, while in the control and mock-treated seedlings there was no induction of gene expression (Fig. 4c). These results support the hypothesis that LCBs are regulators of the SA-dependent pathway. Furthermore, it clearly opens the possibility that necrotrophic fungi that produce SAMs take advantage of the LCB-induced activation of the SA-dependent signaling pathway that leads to PCD, as part of their virulent strategy that contributes to a succesful colonization.
VIGS of SPT compromised N. benthamiana resistance against the necrotrophic fungal pathogen Alternaria alternata
As described above, SPT-silencing had strong effects on plant development, and a very interesting additional phenotype observed was that our TRVSPT plants grown in the greenhouse often developed necrotic lesions in stems and leaves (Fig. 5a, b) that were not observed in TRV or not agroinfiltrated plants. From these lesions, we isolated and obtained a monosporic fungal culture (strain A2SS). In PDA, the fungus developed black colonies (Fig. 5c) and brown conidia with transverse and longitudinal septa that are characteristic of Alternaria sp. morphology (Fig. 5d). Molecular identification by the sequence of the ITS narrowed the isolate to two possible species: A. alternata and A. tenuissima. This result was relevant because N. benthamiana, like most Nicotiana species, is resistant to A. alternata (Brandwagt et al. 2001), thus SPT-silencing and disruption of sphingolipid metabolism was directly associated to susceptibility to this necrotroph.
VIGS of SPT conferred susceptibility to the necrotrophic pathogen Alternaria alternata. Stem (a) and foliar lesions (b) on a TRVSPT plant. The macroscopic (c) and microscopic (d) morphology of the fungi isolated from the lesion on the TRVSPT leaf correspond to A. alternata. The strain isolated was cultivated on 0.5 × PDA and photographed 15 days later. For pathogenicity assays, excised N. benthamiana TRV and TRVSPT leaves were inoculated with 1 × 103 conidia of Alternaria brassiciola (e) or Alternaria sp. A2SS strain (f) and photographed 10 dpi. The inoculated leaves were kept in separate humid chambers at 22 °C. Only the TRVSPT leaves inoculated with A2SS strain (Alternaria sp.) developed symptoms of infection and spread of the fungus (indicated by white arrows)
To rule out that TRVSPT plants susceptibility was generalized to other Alternaria species, detached leaves of TRV and TRVSPT plants were inoculated with 103 conidia of either A. brassicicola or Alternaria sp. A2SS. A. brassicicola could not infect the TRV leaves nor the TRVSPT (Fig. 5e) while, as expected, the A2SS strain infected only TRVSPT leaves causing necrotic expanded lesions, and on the inoculated TRV leaves only a slight chlorosis was observed (Fig. 5f). These results indicate that SPT-silencing confered a selective susceptibility to A. alternata, highlighting the potential role of LCB in the defense response, as the virulence of the tomato pathotype (A. alternata f. sp. lycopersici) depends on the disruption of sphingolipid biosynthesis and LCBs accumulation caused by the AAL-toxin (Gilchrist and Grogan 1976; Spassieva et al. 2002). This prompted us to analyze the defense response of N. benthamiana TRVSPT plants challenged with A. alternata f. sp. lycopersici.
N. benthamiana SPT-silenced plants became susceptible to the non-host pathogen A. alternata f. sp. lycopersici and exhibited altered defense gene expression
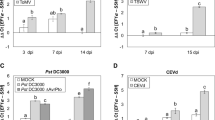
To test the relevance of the altered LCB homeostasis in N. benthamiana TRVSPT plants in the defense response against a SAM-producing fungus, we performed pathogenicity assays using an A. alternata f. sp. lycopersici aa strain (provided by Dr. Liancheng Du, University of Nebraska). A conidial suspension was used to inoculate detached leaves and stems from TRV and TRVSPT plants. Sterile water was used to spot control leaves. As expected, A. alternata f. sp. lycopersici was unable to infect the TRV leaves and the inoculated regions developed only mild symptoms of chlorosis (Fig. 6a). In contrast, TRVSPT leaves developed intense chlorosis and necrotic lesions on the inoculated sites, and an increase in fungal growth was observed at 10 days after inoculation (dai, Fig. 6a). Because A. alternata f. sp. lycopersici is the causal agent of tomato stem canker disease, pathogenicity tests were performed also on N. benthamiana stems. In the TRVSPT stems, fungal proliferation was evident at 6 dai, whereas very discrete or no signs of fungal growth were observed in TRV stems (Fig. 6b). In the control stems inoculated with sterile water (TRV and TRVSPT) no signs of infection or fungal development were observed (data not shown). Colonization levels were determined by counting conidia collected from the stems at 6 dai. Fungal colonization of TRVSPT plant stems correlated with a tenfold increase in conidia number compared with TRV plant stems (Fig. 6c). These results are consistent with the hypothesis that LCBs are modulators of the defense response against pathogens, including necrotrophs, so the altered LCB homeostasis in N. benthamiana TRVSPT plants suppressed the resistance against the non-host and necrotrophic pathogen A. alternata f. sp. lycopersici.
VIGS of SPT compromised Nicotiana benthamiana resistance against Alternaria alternata f. sp. lycopersici. Excised leaves (a) and cut stems (b) from TRV and TRVSPT plants were inoculated with 1 × 103 conidia. The inoculated tissues were kept in separate humid chambers at 22 °C, and photographed 10 days after inoculation (dai) in the case of leaves, and stems at 6 dai. Orange boxes close-up shown in leaves photographs at the right. Infection symptoms were observed only on tissues of TRVSPT plants. Controls inoculated with sterile water did not develop disease symptoms (not shown). c Quantitative analysis of spore production. Conidia were released from each stem, suspended in water 6 dai and counted in a hemacytometer. Values are mean of 40 stem pieces and are representative from two independent experiments. The asterisk denotes significant difference and was determined by Tukey’s mean comparison test (p < 0.01). d Time course analysis of pathogenesis-related (PR) gene expression by semi-quantitative RT-PCR in leaves of TRV and TRVSPT plants inoculated with A. alternata f. sp. lycopersici (1 × 103 conidia). NI corresponds to non-inoculated control leaves. EF1α transcript was used as control for normalization. Results are representative of three independent experiments
Because LCBs are signal transducers in plants, we investigated whether the susceptibility of N. benthamiana TRVSPT plants to A. alternata f. sp. lycopersici might be associated with a delayed or reduced expression of defense-related genes. Necrotrophic infection often results in the activation of PR genes encoding proteins with potent antifungal activities (Glazebrook 2005). To examine this, we analyzed by semi-quantitative RT-PCR the expression of NbPR-1, NbPR-2, and NbPR-3 genes in inoculated TRV and TRVSPT leaves. At 8 h post-inoculation (hpi), an induction of NbPR-2 and NbPR-3 expression was observed in TRV leaves which was increased at later times (24 and 48 hpi, Fig. 6d), suggesting that glucanases and chitinases are induced during N. benthamiana defense response against this necrotroph. NbPR-1 expression was induced at 24 hpi and increased at 48 hpi (Fig. 6d). As anticipated, the TRVSPT leaves showed a lower expression of NbPR-2 and NbPR-3 genes early (8 and 24 hpi) during infection, compared to the inoculated TRV leaves (Fig. 6d) which correlated with its compromised resistance to A. alternata f. sp. lycopersici infection. In contrast, high transcript levels of NbPR-1 gene were observed at 8 hpi in TRVSPT leaves, whereas accumulation of this transcript occurred at 24 hpi in TRV leaves (Fig. 6d). This result could be explained by the fact that NbPR-1 was constitutively expressed in TRVSPT plants due to SA accumulation. In order to test whether activation of the SA pathway observed in TRVSPT plants had an adverse effect on the JA-mediated response, we analyzed transcript levels of the JA-inducible LOX gene in N. benthamiana TRV and TRVSPT in response to methyl jasmonate (MeJA). TRV leaves showed a strong and dose-dependent induction of LOX expression, whereas in TRVSPT leaves a dose-dependent response was also observed but lower levels were detected (Fig. S5). Overall, these results indicate that the misregulation of LCB homeostasis caused by VIGS of SPT LCB2 subunit and the activation of SA-dependent responses affected the expression of defense genes compromising N. benthamiana resistance to the non-host pathogen Alternaria alternata f. sp. lycopersici.
Discussion
N. benthamiana phenotypic features associated to SPT-silencing
Because NbLCB2 is a single-copy gene in the N. benthamiana genome (Gan et al. 2009), the VIGS strategy was highly effective as reflected in the dramatic phenotype observed using our pTRVSPT construct. The significant growth restriction and abnormal leaf development reproduces the phenotype observed by Takahashi et al. (2009), highlighting the importance of sphingolipids as structural and signaling molecules during plant growth and development (Pata et al. 2010; Zäuner et al. 2010). Furthermore, the phenotype caused by pTRVSPT is more severe than those previously reported for the same gene (Gan et al. 2009; Takahashi et al. 2009), and such differences might be due to the cDNA sequence cloned in the VIGS vector. While in the report by Takahashi et al. (2009) and this study, similar cDNA sequences corresponding to the 5′-cDNA end were used, Gan et al. (2009) employed a 303-bp cDNA from the 3′-end. The growth phenotype of N. benthamiana TRVSPT plants was also comparable to those observed in Arabidopsis LCB1 RNAi-suppressed lines and in LCB2 knock-out mutants that show altered leaf morphology and severe growth restriction, the latter associated to an impaired cell expansion. Therefore, as suggested, plants might adjust their growth mechanisms to compensate the reduction of sphingolipid biosynthesis (Chen et al. 2006; Dietrich et al. 2008). The aberrant shape of leaves from N. benthamiana TRVSPT plants also denotes an impaired regulation of cell growth and polarity establishment. Proteomic studies have revealed an enrichment of some polarity-related proteins in lipid rafts such as PIN1, SKU5, and COBRA, suggesting a role for these domains in the polar expansion of plant cells (Willemsen et al. 2003; Mongrand et al. 2004; Borner et al. 2005). Moreover, recent evidence has emerged on sphingolipids involvement in a secretory pathway for polar auxin transport. Disruption of sphingolipids biosynthesis in tobacco BY2 cultured cells and Arabidopsis seedlings by FB1 alters the polar localization of the auxin transporter PIN1 to the plasma membrane (Aubert et al. 2011; Markham et al. 2011).
Silencing efficiency of our construct is also highlighted by the dramatic effect observed on flower development that was not described before in previous reports of N. benthamiana SPT-silenced plants. These results reinforce previous evidence found in Arabidopsis on the essential role of sphingolipid biosynthesis during reproductive development. The expression of Arabidopsis LCB2a gene is higher in the flower buds and mature flowers compared with other organs such as leaves and stem, whereas null mutants of the two functional LCB2 genes display gametophytic lethality and loss of pollen viability (Dietrich et al. 2008). Similarly, the sbh1 sbh2 double mutant (affected on LCB C-4 hydroxylase genes) does not progress from vegetative to reproductive growth (Chen et al. 2008).
Another observed feature of the N. benthamiana TRVSPT plants phenotype was the presence of spontaneous cell death lesions, which coincides with previous reports linking sphingolipid biosynthesis and metabolism to the regulation of PCD. Inhibition of sphingolipid biosynthesis by SAMs causes cell death in several plant species (Abbas et al. 1994; Lamprecht et al. 1994; Stone et al. 2000; Saucedo-García et al. 2011). These observations are consistent with the fact that in Arabidopsis, several mutations affecting sphingolipid metabolism such as acd5, acd11, and erh1 that affect a ceramide kinase, a sphingosine transporter and an inositol-phosphoryl-ceramide synthase, respectively, cause cell death as well (Brodersen et al. 2002; Liang et al. 2003; Wang et al. 2008). Importantly, several lines of evidence support that PCD activation is mediated by increased LCB levels. Spraying or infiltration of Arabidopsis and N. benthamiana leaves with sphinganine, sphingosine, or phytosphinganine, or the addition of sphinganine to tobacco BY2 cultured cells promote cell death (Shi et al. 2007; Takahashi et al. 2009; Lachaud et al. 2010). In accordance with these findings, overexpression of SPT LCB2 subunit in N. benthamiana causes cell death, probably due to accumulation of LCBs (Takahashi et al. 2009). Conversely, mutations in Arabidopsis LCB1 or LCB2a genes, or the inhibition of SPT activity with myriocin, attenuate FB1-induced LCBs accumulation, and prevent cell death (Shi et al. 2007; Saucedo-García et al. 2011).
Silencing of the SPT LCB2 subunit in N. benthamiana significantly affects LCB homeostasis
As described above, two previous studies have reported VIGS of SPT LCB2 subunit in N. benthamiana (Gan et al. 2009; Takahashi et al. 2009). However, both of them lacked or were unsuccessful in analyzing LCBs levels. This is understandable given the diversity and low levels of these metabolites found in plant tissues. Indeed, as a first attempt, we tried to determine free LCBs in TRV and TRVSPT plants, but they were below detection limits. For this reason, we performed sphingolipid hydrolysis and analyze the NDA derivatives of the released LCBs. Because SPT is the enzyme that catalyzes the first reaction in sphingolipid biosynthesis, we hypothesized that VIGS of the LCB2 subunit caused a general reduction in LCBs levels, especially of sphinganine, which is the first LCB synthesized and is a substrate for desaturases and hydroxylases to generate the other LCBs (Chen et al. 2009). However, we found that while the trihydroxylated LCB phytosphingosine, which is quite abundant, decreased 50 %, levels of the dihydroxylated LCBs sphinganine and sphingosine increased considerably. This differential effect of SPT-silencing on LCBs profile revealed the complexity of the LCBs metabolism and regulation, and is in agreement with the results from Chen et al. (2006). In Arabidopsis, reduction of SPT activity in LCB1-RNAi plants caused large increases (approximately fourfold) in the total content of sphinganine (and also a 2.5-fold increase of phytosphingosine), while some trihydroxylated LCBs such as the phytosphingenine isomers (t18:1-E and t18:1-Z) decreased. These results could be attributed to differences in LCB levels among taxonomic groups, as reported by Markham et al. (2006). Interestingly, the elevation of sphinganine and sphingosine levels in N. benthamiana TRVSPT plants partially mimics the effect of SAMs in de novo biosynthesis of sphingolipids, but this interesting similarity must be confirmed by further sphingolipidomic analyzes.
LCBs act as signal transducers in SA pathway
Because both, LCBs and SA have a key role in regulating cell death and plant immune response [reviewed by Berkey et al. (2012), Vlot et al. (2009), An and Mou (2011)], we hypothesized that they may share signaling pathways. Moreover, all the phenotypic characteristics associated with VIGS of SPT in N. benthamiana plants such as growth reduction, cell death, and susceptibility to a necrotroph could be associated with SA accumulation. A primary function of phytohormones is the regulation of plant growth and development, and interestingly, many Arabidopsis mutants with SA accumulation develop a dwarf phenotype like N. benthamiana TRVSPT plants (Rivas-San Vicente and Plasencia 2011). Furthermore, we recently found that FB1 and sphinganine induce a dose-dependent accumulation of free SA in germinating maize embryos (de la Torre-Hernández et al. 2010). The comparison of SA levels between TRV and TRVSPT plants indicated that indeed the latter had a discrete but significant elevation of total SA which correlated with a strong constitutive expression of NbPR-1 gene. Likewise, the Arabidopsis mutants acd5, acd11, and erh1, which have an altered sphingolipid metabolism, contain elevated (3- to 80-fold increase) SA levels and strongly express AtPR-1 (Greenberg et al. 2000; Brodersen et al. 2005; Wang et al. 2008). Taken together, these evidences strongly suggest that an increase of sphingoid intermediaries, especially LCBs, activate the SA-dependent pathway. Then, in order to define if the LCB elevation observed in TRVSPT plants was responsible of the activation of the SA pathway, we treated N. benthamiana wild-type seedlings with FB1 or sphinganine and determined NbPR-1 transcript levels. This gene, the canonical marker of SA signaling (Mur et al. 2006; Spoel et al. 2007; Koornneef et al. 2008; Denoux et al. 2008), showed an induced expression upon exposure to both compounds. Moreover, the same effect has been observed in Arabidopsis, indicating that LCBs positively regulate the SA-dependent pathway. Arabidopsis wild-type seedlings treated with SAMs (FB1 and AAL-toxin) show a dose-dependent induction of PR-1 expression and other defense-related genes (Stone et al. 2000; Gechev et al. 2004). In tobacco BY2 cells, sphinganine (25 μM) rapidly induces the expression of the defense genes NtPR-3 and NtMYB2, but not of NtPR-1 (Lachaud et al. 2011). In the latter case, NtPR-1 expression could have not been detected because the cells were harvested 15 min after sphinganine treatment, which could be a very short time to activate SA signaling. Our results indicate that NbPR-1 expression is induced after 24 h, and this time coincides with the induction caused by SAMs in Arabidopsis (Stone et al. 2000; Gechev et al. 2004).
It is worth mentioning that even though most Nicotiana species are resistant to Alternaria alternata f. sp. lycopersici and insensitive to SAMs (determined by a root elongation assay in presence of 0.2 μM AAL-toxin; Brandwagt et al. 2001), when higher FB1 doses were used, inhibition of root elongation and hypocotyl expansion on N. benthamiana seedlings was recorded, a very similar effect to the species with intermediate SAM sensitivity (Brandwagt et al. 2001). These observations are also comparable with the FB1-induced growth restriction reported on maize, tomato (Lamprecht et al. 1994), and Arabidopsis (Stone et al. 2000; Shi et al. 2007; Markham et al. 2011; Saucedo-García et al. 2011; Watanabe and Lam 2011). However, unlike the aforementioned species, N. benthamiana seedlings exposed to FB1 did not develop symptoms of chlorosis or cell death. In this sense, it is important to mention that the tomato gene Asc-1 that mediates insensitivity to SAMs and resistance to Alternaria alternata f. sp. lycopersici (Gilchrist and Grogan 1976) is conserved in most Nicotiana species (Brandwagt et al. 2001). Asc-1 is a single codominant gene that codes an enzyme with ceramide synthase activity that is not effectively inhibited by SAMs, therefore LCB accumulation induced by AAL-toxin in resistant (Asc/Asc) tomato leaves is discrete compared to that caused in susceptible (asc/asc) leaves (Spassieva et al. 2002). The same could happen in Nicotiana sp. because even though tobacco is SAMs insensitive to low doses of AAL-toxin (Brandwagt et al. 2001), 1 µM FB1 causes an approximately tenfold increase in sphinganine levels in BY-2 cultured cells (Aubert et al. 2011).
Compromised resistance of N. benthamiana SPT-silenced plants (TRVSPT) to the necrotrophic fungi Alternaria alternata f. sp. lycopersici
The involvement of SPT in non-host resistance was illustrated with the compromised resistance in N. benthamiana LCB1- and LCB2-silenced plants against the bacterial pathogen P. cichorii (Takahashi et al. 2009). Consistent with this finding, our SPT-silenced plants were more susceptible to the non-host necrotrophic fungi A. alternata f. sp. lycopersici, despite the presence of a functional Asc gene that confers resistance against this pathogen (Brandwagt et al. 2001). Because TRVSPT plants had altered levels of LCBs, in particular elevation of sphinganine resembling the effect of SAMs, we reasoned that this fact would generate a favorable growth condition for the necrotrophic pathogen. In order to gain more insight into the mechanisms operating in this compatible interaction, we evaluated the expression of NbPR-1, NbPR-2, and NbPR-3 genes that are part of the early defense responses and have a key role in limiting pathogen entry, growth, and spread. The induction pattern of expression of these defense genes in TRVSPT leaves in response to the fungal infection showed important alterations compared to TRV leaves. Lower levels of NbPR-2 and NbPR-3 transcripts were observed in TRVSPT leaves that correlated with the compromised resistance against A. alternata f. sp. lycopersici. The production of enzymes capable of degrading the cell walls of invading fungi (β-1,3-glucanases and chitinases coded by NbPR-2 and NbPR-3 genes) and the timing of this defense mechanism is an important component of plant disease resistance. Their role in plant immune response has been examined by their overexpression in transgenic plants that exhibit enhanced resistance to fungal infection and delayed disease symptoms (Jach et al. 1995; Dana et al. 2006). Furthermore, in vitro and in vivo experiments have demonstrated that the antifungal effect of PR-2 and PR-3 proteins is synergistically enhanced when both enzymes are present (Lawrence et al. 1996).
Constitutive expression of NbPR-1 induced by the accumulation of SA in TRVSPT plants (scarcely observed under semi-quantitative RT-PCR conditions) possibly led to an earlier induction in response to fungal infection, compared to TRV plants. This constitutive activation of the SA-dependent pathway in TRVSPT plants could be crucial in the susceptibility to A. alternata f. sp. lycopersici as phytohormone signaling plays a major role in determining the outcome of plant–pathogen interactions (Spoel and Dong 2008; Pieterse et al. 2009, 2012; Robert-Seilaniantz et al. 2011). A very tempting possibility is that the SAM-producing fungal necrotrophs promote LCBs elevation not only to induce the death of their hosts, but also to exploit their association with the SA-dependent pathway to antagonize or suppress the effective defense mechanisms activated by other phytohormones such as JA. In the most simplified models of hormone crosstalk in plant immunity, JA and ET work synergistically in resistance against necrotrophs, whereas SA pathway often antagonizes the JA-dependent defense responses (Spoel et al. 2007; Koornneef et al. 2008; Leon-Reyes et al. 2009, 2010; El Oirdi et al. 2011). Activation of the SA pathway in Arabidopsis (either by biotic stress or exogenous treatment) renders leaves more susceptible to Alternaria brassicicola, which exhibit severe disease symptoms. This correlates with a strong expression of PR-1 and a decreased expression of the JA-inducible PDF1.2 gene (Spoel et al. 2007). It has also been shown that low doses of SA are enough to trigger a fast and long-lasting antagonistic effect on defense genes regulated by JA (Koornneef et al. 2008). Notably, this also occurs in Solanaceae as SA-pretreatment (50 µM) of tomato leaves increases Botrytis cinerea infection and significantly lowers the expression of the JA-regulated PI-I and PI-II defense genes. Moreover, an aggressive B. cinerea isolate (B191) secretes an exopolysaccharide that promotes a twofold accumulation of SA in the host to suppress the JA pathway required for resistance (El Oirdi et al. 2011). Our TRVSPT plants showed activation of the SA pathway which might potentially suppress the JA-mediated response as determined by transcript levels of the LOX gene. Supporting the interpretation behind the experimental evidence with these fungi, there are many other cases reported on pathogens that take advantage of the phytohormone signaling crosstalk to suppress host defenses (Pieterse et al. 2009; Robert-Seilaniantz et al. 2011).
It is very probable that complex interactions among the hormonal pathways operate in the susceptibility of N. benthamiana TRVSPT plants to A. alternata f. sp. lycopersici, and future work will unravel whether the increased SA levels antagonize JA- and ET-dependent defense responses, and SA interaction with other hormones. Significantly, there is evidence that JA and ET are involved in the cell death triggered by this necrotroph in tomato (Egusa et al. 2009; Zhang et al. 2011), and also in the Arabidopsis and tomato cell death induced by FB1 and AAL-toxin, respectively (Asai et al. 2000; Gechev et al. 2004; Mase et al. 2012).
In summary, the work presented here provides evidence that alterations in the sphingolipids de novo biosynthesis that results in elevated LCB levels activate the SA signaling pathway as a downstream element. In this context, the SAMs produced by F. verticillioides and A. alternata f. sp. lycopersici would be displaying a double role: disruption of the host sphingolipid metabolism and activation of the SA pathway in order to divert defense responses mediated by other plant hormones. Together, both strategies would assure a successful invasion into the host.
Abbreviations
- SPT:
-
Serine palmitoyltransferase
- LCB:
-
Long-chain base
- d18:0:
-
Sphinganine
- d18:1(Δ4):
-
Sphingosine
- t18:0:
-
Phytosphingosine
- SAMs:
-
Sphinganine-analog mycotoxins
- FB1:
-
Fumonisin B1
- JA:
-
Jasmonic acid
- PCD:
-
Programmed cell death
- VIGS:
-
Virus-induced gene silencing
- PR:
-
Pathogenesis-related
- SA:
-
Salicylic acid
References
Abbas HK, Tanaka T, Duke SO, Porter JK, Wray EM, Hodges L, Sessions A, Wang E, Merril AH, Riley RT (1994) Fumonisin- and AAL-toxin-induced disruption of sphingolipid metabolism with accumulation of free sphingoid bases. Plant Physiol 106:1085–1093
Akamatsu H, Itoh Y, Kodama M, Otani H, Kohmoto K (1997) AAL-toxin-deficient mutants of Alternaria alternata tomato pathotype by restriction enzyme-mediated integration. Phytopathology 87:967–972
An C, Mou Z (2011) Salicylic acid and its function in plant immunity. J Int Plant Biol 53:412–428
Asai T, Stone JM, Heard JE, Kovtun Y, Yorgey P, Sheen J, Ausubel FM (2000) Fumonisin B1-induced cell death in Arabidopsis protoplasts requires jasmonate-, ethylene-, and salicylate-dependent signaling pathways. Plant Cell 12:1823–1835
Aubert A, Marion J, Boulogne C, Bourge M, Abreu S, Bellec Y, Faure JD, Satiat-Jeunemaitre B (2011) Sphingolipids involvement in plant endomembrane differentiation: the BY2 case. Plant J 65:958–971
Berkey R, Bendigeri D, Xiao S (2012) Sphingolipids and plant defense/disease: the “death” connection and beyond. Front Plant Sci 3:68
Borner GH, Sherrier DJ, Weimar T, Michaelson LV, Hawkins ND, Macaskill A, Napier JA, Beale MH, Lilley KS, Dupree P (2005) Analysis of detergent-resistant membranes in Arabidopsis. Evidence for plasma membrane lipid rafts. Plant Physiol 137:104–116
Brandwagt BF, Kneppers TJA, Van der Weerden GM, Nijkamp HJJ, Hille J (2001) Most AAL toxin-sensitive Nicotiana species are resistant to the tomato fungal pathogen Alternaria alternata f. sp. lycopersici. Mol Plant Microbe Interact 14:460–470
Breslow DK, Collins SR, Bodenmiller B, Aebersold R, Simons K, Shevchenko A, Ejsing CS, Weissman JS (2010) Orm family proteins mediate sphingolipid homeostasis. Nature 463:1048–1055
Brodersen P, Petersen M, Pike HM, Olszak B, Skov S, Odum N, Jorgensen LB, Brown RE, Mundy J (2002) Knockout of Arabidopsis accelerated cell death11 encoding a sphingosine transfer protein causes activation of programmed cell death and defense. Genes Dev 16:490–502
Brodersen P, Malinovsky FG, Hématy K, Newman MA, Mundy J (2005) The role of salicylic acid in the induction of cell death in Arabidopsis acd11. Plant Physiol 138:1037–1045
Buré C, Cacas JL, Wang F, Gaudin K, Domergue F, Mongrand S, Schmitter JM (2011) Fast screening of highly glycosylated plant sphingolipids by tandem mass spectrometry. Rapid Commun Mass Spectrom 25:3131–3145
Catinot J, Buchala A, Abou-Monsour E, Métraux JP (2008) Salicylic acid production in response to biotic and abiotic stress depends on isochorismate in Nicotiana benthamiana. FEBS Lett 582:473–478
Chen M, Han G, Dietrich CR, Dunn TM, Cahoon EB (2006) The essential nature of sphingolipids in plants as revealed by the functional identification and characterization of the Arabidopsis LCB1 subunit of serine palmitoyltransferase. Plant Cell 18:3576–3593
Chen M, Markham JE, Dietrich CR, Jaworski JG, Cahoon EB (2008) Sphingolipid long-chain base hydroxylation is important for growth and regulation of sphingolipid content and composition in Arabidopsis. Plant Cell 20:1862–1878
Chen M, Cahoon EB, Saucedo-García M, Plasencia J, Gavilanes-Ruíz M (2009) Plant sphingolipids: structure, synthesis and function. In: Wada H, Murata N, Govindjee (eds) Lipids in photosynthesis: essential and regulatory functions. Springer, Dordrecht, pp 77–115
Chen M, Markham JE, Cahoon EB (2012) Sphingolipid Δ8 unsaturation is important for glucosylceramide biosynthesis and low-temperature performance in Arabidopsis. Plant J 69:769–781
Coursol S, Fan LM, Le Stunff H, Spiegel S, Gilroy S, Assmann SM (2003) Sphingolipid signalling in Arabidopsis guard cells involves heterotrimeric G proteins. Nature 423:651–654
Dana M, Pintor-Toro J, Cubero B (2006) Transgenic tobacco plants overexpressing chitinases of fungal origin show enhanced resistance to biotic and abiotic stress agents. Plant Physiol 142:722–730
de la Torre-Hernández ME, Rivas-San Vicente M, Greaves-Fernández N, Cruz-Ortega R, Plasencia J (2010) Fumonisin B1 induces nuclease activation and salicylic acid accumulation through long-chain sphingoid base build-up in germinating maize. Physiol Mol Plant Pathol 74:337–345
Denoux C, Galletti R, Mammarella N, Gopalan S, Werck D, De Lorenzo G, Ferrari S, Ausubel FM, Dewdney J (2008) Activation of defense response pathways by OGs and Flg22 elicitors in Arabidopsis seedlings. Mol Plant 1:423–445
Desjardins AE, Plattner RD, Nelsen TC, Leslie JF (1995) Genetic analysis of fumonisin production and virulence of Gibberella fujikuroi mating population A (Fusarium monilliforme) on maize (Zea mays) seedlings. Appl Environ Microbiol 61:79–86
Dietrich CR, Han G, Chen M, Howard R, Dunn TM, Cahoon EB (2008) Loss-of-function mutations and inducible RNAi suppression of Arabidopsis LCB2 genes reveal the critical role of sphingolipids in gametophytic and sporophytic cell viability. Plant J 54:284–298
Dutilleul C, Benhassaine-Kesri G, Demandre C, Rézé N, Launay A, Pelletier S, Renou JP, Zachowski A, Baudouin E, Guillas I (2012) Phytosphingosine-phosphate is a signal for AtMPK6 activation and Arabidopsis response to chilling. New Phytol 194:181–191
Egusa M, Ozawa R, Takabayashi J, Otani H, Kodama M (2009) The jasmonate signaling pathway in tomato regulates susceptibility to a toxin-dependent necrotrophic pathogen. Planta 229:965–976
El Oirdi M, El Rahman TA, Rigano L, El Hadrami A, Rodriguez MC, Daayf F, Vojnov A, Bouarab K (2011) Botrytis cinerea manipulates the antagonistic effects between immune pathways to promote disease development in tomato. Plant Cell 23:2405–2421
Gable K, Slife H, Bacikova D, Monaghan E, Dunn TM (2000) Tsc3p is an 80-amino acid protein associated with serine palmitoyltransferase and required for optimal enzyme activity. J Biol Chem 275:7597–7603
Gan Y, Zhang L, Zhang Z, Dong S, Li J, Wang Y, Zheng X (2009) The LCB2 subunit of the sphingolipid biosynthesis enzyme serine palmitoyltransferase can function as an attenuator of the hypersensitive response and Bax-induced cell death. New Phytol 181:127–146
Gechev TS, Gadjev IZ, Hille J (2004) An extensive microarray analysis of AAL-toxin-induced cell death in Arabidopsis thaliana brings new insights into the complexity of programmed cell death in plants. Cell Mol Life Sci 61:1185–1197
Gilchrist DG, Grogan RG (1976) Production and nature of a host-specific toxin from Alternaria alternata f. sp. lycopersici. Phytopathology 66:165–171
Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43:205–227
Glenn AE, Zitomer NC, Zimeri AM, Williams LD, Riley RT, Proctor RH (2008) Transformation-mediated complementation of a FUM gene cluster deletion in Fusarium verticillioides restores both fumonisin production and pathogenicity on maize seedlings. Mol Plant Microbe Interact 21:87–97
Greenberg JT, Silverman P, Liang H (2000) Uncoupling salicylic acid-dependent cell death and defense-related responses from disease resistance in the Arabidopsis mutant acd5. Genetics 156:341–350
Jach G, Gornhardt B, Mundy J, Logemann J, Pinsdorf E, Leah R, Schell J, Maas C (1995) Enhanced quantitative resistance against fungal disease by combinatorial expression of different barley antifungal proteins in transgenic tobacco. Plant J 8:97–109
Koga J, Yamauchi T, Shimura M, Ogawa N, Oshima K, Umemura K, Kikuchi M, Ogasawara N (1998) Cerebrosides A and C, sphingolipid elicitors of hypersensitive cell death and phytoalexin accumulation in rice plants. J Biol Chem 273:31985–31988
Kommedahl T, Windels CE (1981) Root-, stalk-, and ear-infecting Fusarium species on corn in the USA. In: Nelson PE, Toussoun TA, Cook RJ (eds) Fusarium: diseases, biology, and taxonomy. The Pennsylvania State University Press, University Park, pp 94–103
Koornneef A, León-Reyes A, Ritsema T, Verhage A, Den Otter FC, Van Loon LC, Pieterse CMJ (2008) Kinetics of salicylate-mediated suppression of jasmonate signaling reveal a role for redox modulation. Plant Physiol 147:1358–1368
Lachaud C, Da Silva D, Cotelle V, Thuleau P, Xiong TC, Jauneau A, Brière C, Graziana A, Bellec Y, Faure JD, Ranjeva R, Mazars C (2010) Nuclear calcium controls the apoptotic-like cell death induced by D-erythro-sphinganine in tobacco cells. Cell Calcium 47:92–100
Lachaud C, Da Silva D, Amelot N, Béziat C, Brière C, Cotelle V, Graziana A, Grat S, Mazars C, Thuleau P (2011) Dihydrosphingosine-induced programmed cell death in tobacco BY-2 cells is independent of H2O2 production. Mol Plant 4:310–318
Lamprecht SC, Marasas WFO, Alberts JF, Cawood ME, Gelderblom WCA, Shephard GS, Thiel PG, Calitz FJ (1994) Phytotoxicity of fumonisins and TA-toxin to corn and tomato. Phytopathology 84:383–391
Lawrence CB, Josten MHAJ, Tuzun S (1996) Differential induction of pathogenesis-related proteins in tomato by Alternaria solani and the association of basic chitinase isozyme with resistance. Physiol Mol Plant Pathol 48:361–377
Leon-Reyes A, Spoel SH, De Lange ES, Abe H, Kobayashi M, Tsuda S, Millenaar FF, Welschen RAM, Ritsema T, Pieterse CMJ (2009) Ethylene modulates the role of NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1 in cross talk between salicylate and jasmonate signaling. Plant Physiol 149:1797–1809
Leon-Reyes A, Du Y, Koornneef A, Proietti S, Körbes AP, Memelink J, Pieterse CMJ, Ritsema T (2010) Ethylene signaling renders the jasmonate response of Arabidopsis insensitive to future suppression by salicylic acid. Mol Plant Microbe Int 23:187–197
Liang H, Yao N, Song JT, Luo S, Lu H, Greenberg JT (2003) Ceramides modulate programmed cell death in plants. Genes Dev 17:2636–2641
Lieberherr D, Thao NP, Nakashima A, Umemura K, Kawasaki T, Shimamoto K (2005) A sphingolipid elicitor-inducible mitogen-activated protein kinase is regulated by the small GTPase OsRac1 and heterotrimeric G-protein in rice. Plant Physiol 138:1644–1652
Lynch DV, Dunn TM (2004) An introduction to plant sphingolipids and a review of recent advances in understanding their metabolism and function. New Phytol 161:677–702
Markham JE, Li J, Cahoon EB, Jaworski JG (2006) Separation and identification of major plant sphingolipid classes from leaves. J Biol Chem 281:22684–22694
Markham JE, Molino D, Gissot L, Bellec Y, Hématy K, Marion J, Belcram K, Palauqui JC, Satiat-JeuneMaître S, Faure JD (2011) Sphingolipids containing very-long-chain fatty acids define a secretory pathway for specific polar plasma membrane protein targeting in Arabidopsis. Plant Cell 23:2362–2378
Mase K, Mizuno T, Ishihama N, Fuji T, Mori H, Kodama M, Yoshioka H (2012) Ethylene signaling pathway and MAPK cascades are required for AAL-toxin-induced programmed cell death. Mol Plant Microbe Interact 25:1015–1025
Mesbah LA, van der Weerden GM, Nijkamp HJJ, Hille J (2000) Sensitivity among species of Solanaceae to AAL toxins produced by Alternaria alternata f. sp. lycopersici. Plant Pathol 49:734–741
Meuwly P, Métraux JP (1993) Ortho-anisic acid as internal standard for the simultaneous quantitation of salicylic acid and its putative biosynthetic precursors in cucumber leaves. Anal Biochem 214:500–505
Mongrand S, Morel J, Laroche J, Claverol S, Carde JP, Hartmann MA, Bonneu M, Simon-Plas F, Lessire R, Bessoule JJ (2004) Lipid rafts in higher plant cells: purification and characterization of Triton X-100-insoluble microdomains from tobacco plasma membrane. J Biol Chem 279:36277–36286
Mur LAJ, Kenton P, Atzorn R, Miersch O, Wasternack C (2006) The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol 140:249–262
Ng CK, Carr K, McAinsh MR, Powell B, Hetherington AM (2001) Drought-induced guard cells signal transduction involves sphingosine-1-phosphate. Nature 410:596–599
Pata MO, Hannun YA, Ng CKY (2010) Plant sphingolipids: decoding the enigma of the sphinx. New Phytol 185:611–630
Peer M, Stegmann M, Mueller MJ, Waller F (2010) Pseudomonas syringae infection triggers de novo synthesis of phytosphingosine from sphinganine in Arabidopsis thaliana. FEBS Lett 584:4053–4056
Pieterse CMJ, León-Reyes A, Van der Ent S, Van Wees SCM (2009) Networking by small-molecule hormones in plant immunity. Nat Chem Biol 5:308–316
Pieterse CMJ, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SCM (2012) Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol 28:28.1–28.33
Ratcliff F, Martin-Hernandez AM, Baulcombe DC (2001) Tobacco rattle virus as a vector for analysis of gene function by silencing. Plant J 25:237–245
Rivas-San Vicente M, Plasencia J (2011) Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot 62:3321–3338
Robert-Seilaniantz A, Grant M, Jones JDG (2011) Hormone crosstalk in plant disease and defense: more than just JASMONATE-SALICYLATE antagonism. Annu Rev Phytopathol 49:26.1–26.27
Sánchez-Rangel D, Plasencia J (2010) The role of sphinganine analog mycotoxins on the virulence of plant pathogenic fungi. Toxin Rev 29:73–86
Saucedo-García M, Guevara-García A, González-Solís A, Cruz-García F, Vázquez-Santana S, Markham JE, Lozano-Rosas MG, Dietrich CR, Ramos-Vega M, Cahoon EB, Gavilanes-Ruíz M (2011) MPK6, sphinganine and the LCB2a gene from serine palmitoyltransferase are required in the signaling pathway that mediates cell death induced by long chain bases in Arabidopsis. New Phytol 191:943–957
Shi L, Bielawski J, Mu J, Dong H, Teng C, Zhang J, Yang X, Tomishige N, Hanada K, Hannun YA, Zuo J (2007) Involvement of sphingoid bases in mediating reactive oxygen intermediate production and programmed cell death in Arabidopsis. Cell Res 17:1030–1040
Spassieva SD, Markham JE, Hille J (2002) The plant disease resistance gene Asc-1 prevents disruption of sphingolipid metabolism during AAL-toxin-induced programmed cell death. Plant J 32:561–572
Sperling P, Heinz E (2003) Plant sphingolipids: structural diversity, biosynthesis, first genes and functions. Biochim Biophys Acta 1632:1–15
Sperling P, Franke S, Lüthje S, Heinz E (2005) Are glucocerebrosides the predominant sphingolipids in plant plasma membranes? Plant Physiol Biochem 43:1031–1038
Spoel SH, Dong X (2008) Making sense of hormone crosstalk during plant immune responses. Cell Host Microbe 3:348–351
Spoel SH, Johnson JS, Dong X (2007) Regulation of tradeoffs between plant defenses against pathogens with different lifestyles. Proc Natl Acad Sci USA 104:18842–18847
Stone JM, Heard JE, Asai T, Ausubel FM (2000) Simulation of fungal-mediated cell death by fumonisin B1 and selection of fumonisin B1-resistant (fbr) Arabidopsis mutants. Plant Cell 12:1811–1822
Suharsono U, Fujisawa Y, Kawasaki T, Iwasaki Y, Satoh H, Shimamoto K (2002) The heterotrimeric G protein α subunit acts upstream of the small GTPase Rac in disease resistance of rice. Proc Natl Acad Sci USA 99:13307–13312
Takahashi Y, Berberich T, Kanzaki H, Matsumura H, Saitoh H, Tomonobu K, Terauchi R (2009) Serine palmitoyltransferase, the first step enzyme in sphingolipid biosynthesis, is involved in nonhost resistance. Mol Plant Microbe Int 22:31–38
Teng C, Dong H, Shi L, Deng Y, Mu J, Zhang J, Yang X, Zuo J (2008) Serine palmitoyltransferase, a key enzyme for de novo synthesis of sphingolipids, is essential for male gametophyte development in Arabidopsis. Plant Physiol 146:1322–1332
Vlot AC, Dempsey DMA, Klessig DF (2009) Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47:177–206
Wang W, Yang X, Tangchaiburana S, Ndeh R, Markham JE, Tsegaye Y, Dunn TM, Wang GL, Bellizi M, Parsons JF, Morrissey D, Bravo JE, Lynch DV, Xiao S (2008) An inositolphosphorylceramide synthase is involved in regulation of plant programmed cell death associated with defense in Arabidopsis. Plant Cell 20:3163–3179
Watanabe N, Lam E (2011) Arabidopsis metacaspase 2d is a positive mediator of cell death induced during biotic and abiotic stresses. Plant J 66:969–982
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Willemsen V, Frimi J, Grebe M, van der Toorn A, Palme K, Scheres B (2003) Cell polarity and PIN protein positioning in Arabidopsis require STEROL METHYLTRANSFERASE1 function. Plant Cell 15:612–625
Worrall D, Ng CKY, Hetherington AM (2003) Sphingolipids, new players in plant signaling. Trends Plant Sci 8:317–320
Zäuner S, Ternes P, Warnecke D (2010) Biosynthesis of sphingolipids in plants (and some of their functions). Adv Exp Med Biol 688:249–263
Zhang L, Jia C, Liu L, Zhang Z, Li C, Wang Q (2011) The involvement of jasmonates and ethylene in Alternaria alternata f. sp. lycopersici toxin-induced tomato cell death. J Exp Bot 62:5405–5418
Acknowledgments
We thank to Dr. Baulcombe and Sainsbury Laboratory (Norwich, UK) for the TRV vector and Agrobacterium strains used for VIGS, Dr. Liancheng Du (University of Nebraska) for the Alternaria alternata f. sp. lycopersici aa strain, Dra. Helena Porta-Ducoing. for the Alternaria brassiciola strain, and Dr. Arturo Guevara-García (IBT, UNAM) for providing N. benthamiana seeds. The authors gratefully acknowledge the critical comments and support received from Drs. Yvonne Rosenstein-Azoulay and Felipe Cruz-García. We appreciate the technical assistance of Manuela Nájera-Martínez and Diana Sánchez-Rangel, and Laurel Fabila-Ibarra for greenhouse work. This study was funded by Consejo Nacional de Ciencia y Tecnología (Grant No. CONACYT 50503,) and Facultad de Química, UNAM (PAIP 6290-08). Mariana Rivas-San Vicente received a doctoral fellowship (170394) from CONACYT.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rivas-San Vicente, M., Larios-Zarate, G. & Plasencia, J. Disruption of sphingolipid biosynthesis in Nicotiana benthamiana activates salicylic acid-dependent responses and compromises resistance to Alternaria alternata f. sp. lycopersici . Planta 237, 121–136 (2013). https://doi.org/10.1007/s00425-012-1758-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-012-1758-z