Abstract
The present study reports the effect of high molecular weight bacterial fructan (levan) and glucan (reuteran) on growth and carbohydrate partitioning in transgenic sugarcane plants. These biopolymers are products of bacterial glycosyltransferases, enzymes that catalyze the polymerization of glucose or fructose residues from sucrose. Constructs, targeted to different subcellular compartments (cell wall and cytosol) and driven by the Cauliflower mosaic virus-35S: maize-ubiquitin promoter, were introduced into sugarcane by biolistic transformation. Polysaccharide accumulation severely affected growth of callus suspension cultures. Regeneration of embryonic callus tissue into plants proved problematic for cell wall-targeted lines. When targeted to the cytosol, only plants with relative low levels of biopolymer accumulation survived. In internodal stalk tissue that accumulate reuteran (max 0.03 mg/g FW), sucrose content (ca 60 mg/g FW) was not affected, while starch content (<0.4 mg/g FW) was increased up to four times. Total carbohydrate content was not significantly altered. On the other hand, starch and sucrose levels were significantly reduced in plants accumulating levan (max 0.01 mg/g FW). Heterologous expression resulted in a reduction in total carbohydrate assimilation rather than a simple diversion by competition for substrate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The market for liquid biofuel through fermentation of starch and sugars has expanded many-fold in the last few years. This demand has renewed research and speculation into factors that determine yield in plants (Smith 2008). Sugarcane is a C4 grass crop (Moore and Maretzki 1996) that accumulates sucrose in the internodal tissue of the stalk to levels of up to 20 % of fresh weight, or exceeding 50 % of dry weight (Komor et al. 1981; Rae et al. 1996). The plant is unusual in its ability to store sucrose in relatively mature sink tissue, compared to growth apices and developing organs such as tubers. Mechanisms involved in accumulation of sucrose in crops like sugarcane are not well understood and little is known with regard to factors that control the extent of sucrose storage in stalks. Advances made with routine transformation methodology and increasing availability of genomic tools allow for new experimentation and assessment of hypotheses (Rossouw et al. 2007; Wu and Birch 2007). Recent studies have been aimed at the development of sugarcane as a vehicle for production of sugar-derived biomaterials (Basnayake et al. 2012; Hamerli and Birch 2011). Several examples of the creation of transgenic plants expressing heterologous enzymes converting sucrose into non-metabolizable glucans (Zhang et al. 2007) or fructans (Caimi et al. 1996; Sévenier et al. 1998; Hellwege et al. 2000; Weyens et al. 2004) have been reported. No convincing evidence was thus far presented showing that total carbohydrate content was significantly increased in any of these plants.
Fructan accumulates naturally as the primary reserve carbohydrate in 12–15 % of the higher plants (Hendry and Wallace 1993; Cairns 2003). Synthesis from sucrose is extrachloroplastic, without the involvement of phosphorylated sugars or nucleotide co-factors, and it accumulates in the vacuole of both photosynthetic and storage cells. Plant derived fructans have a low degree of polymerization (DP) that seldom exceeds 100 fructose units, due to the presence of fructan-degrading enzymes. Short chain products harvested from plants limit technical application of this polymer (Pilon-Smits et al. 1996). This hurdle may be overcome by the production of microbial polysaccharides in plants lacking degrading enzymes. The molecular weight of microbial polysaccharides exceeds those of plant polymers by at least two orders of magnitude, while the DP can exceed 100,000 monomer units.
Bacterial homopolysaccharides (HoPSs) are synthesized from sucrose through the action of only one enzyme belonging to the group glycosyltransferases, also termed glycansucrases (Bauer et al. 2009). Group members involved in the biosythesis of fructans and glucans are termed fructosyltransferases (FTFs; EC 2.4.1.10) and glucosyltransferases (GTFs; EC 2.4.1.5), respectively. Levan and reuteran are examples of bacterial HoPS that may be exploited commercially, in particularly by the food industry (Roberfroid 1999; Bauer et al. 2009). Levan is composed of β-2,6 linkages and several related FTF-encoding genes have been identified from lactic acid bacteria (Korakli and Vogel 2006). Reuteran is a highly branched glucan with predominately α-(1 → 4) glucosidic bonds. Two strains of L. reuteri (121 and ATCC 55730) have been implicated in reuteran production and the corresponding genes identified (Kralj et al. 2002, 2005).
We incorporated bacterial glycansucrases genes, encoding enzymes for levan and reuteran production, into sugarcane. No published paper has yet described the exploitation of sugarcane as vehicle for transgenic biopolymer production. Sugarcane lacks the enzymes for fructan production as well as enzymes for hydrolysis of bacterial HoPSs, while high levels of sucrose, the sole substrate for bacterial glycansucrases, accumulate. Objectives for this study include (i) co-production of sucrose and potentially valuable biopolymers such as fructan and glucan and (ii) establishment of plants with altered source–sink balance for the study of carbon partitioning.
Materials and methods
Bacterial strains, DNA methodology and construction of glycosyltransferase coding sequences
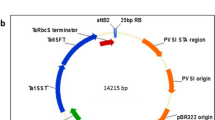
Routine procedures were used for DNA isolation, subcloning and restriction analysis (Ausubel et al. 1994). For cytosol targeting, two glycosyltransferase open reading frames (ORFs), truncated to exclude native secretion peptides, were constructed (Fig. 1) based on the fructansucrase gene (ftfA) from Lactobacillus sanfranciscensis TMW 1.392 (Tieking et al. 2005) and the glucansucrase gene (ftfO) from L. reuteri ATCC 55730 (Kralj et al. 2005). A third construct included a bacterial secretion signal, assembled with an ORF encompassing the entire fructansucrase gene (ftfA) of L. reuteri LTH5448 (Schwab and Gänzle 2006). Appropriate primer pairs used to construct ORFs of the native genes are given in Table 1. To determine whether mutations had been introduced by PCR, the ORFs were cloned into the E. coli pGEM®-T Easy Vector System (Promega) and verified by nucleotide sequencing as described elsewhere (Bauer et al. 2005). ORFs were subsequently excised and placed under control of a strong, constitutive, tandem Cauliflower mosaic virus (CaMV)-35S: maize polyubiquitin (UBI-1) promoter, which drives stable, high-level gene expression in sugarcane (Wei et al. 2003). Table 1 also lists gene- and vector sequence-specific PCR primer pairs used to verify the correct orientation of cloned products, and sequence specific primer pairs for confirmation of transcription with RT-PCR.
Plant tissue and transformation
Growth of sugarcane embryogenic callus for biolistic transformation was initiated from immature leaf roll tissue harvested from the commercial variety NCo310 (Taylor et al. 1992; Snyman et al. 1996). Constructs were co-transformed with the pEmuKN expression vector containing the npt-II selectable marker (Bower and Birch 1992). Selection was done on geneticin-containing medium and putative transgenic plants from independent transformation events were regenerated and hardened off based on standard protocols (Snyman et al. 1996). Plants were grown in a greenhouse for at least 12 months before characterization. Callus and cell suspension cultures were grown on modified Murashige–Skoog (MS) medium (Murashige and Skoog 1962) containing 58 mM sucrose, 0.5 g/L casein and 13.6 μM 2,4 dichlorophenoxyacetic acid.
Cell suspension cultures
Suspension cultures were initiated from embryonic callus (1–2 g) inoculated into 50 mL MS medium. Cell viability was dependent on a gradually increase in culture volume (up to 150 mL). Cultures were replenished with 75 mL fresh MS medium on a weekly basis after removing exactly half of the previous week’s growth medium from settled cultures. Clumping callus material was removed when cells in suspension reached an OD600 of 0.3. Due to pronounce differences in growth speed of individual transgenic lines, growth could not be synchronized. All transgenic lines representative of an individual construct were subjected to the same period of growth and analyzed as a group when the slowest member reached a minimum OD600 of 0.6. Cells were washed three times with 25 mM sodium acetate buffer (pH 5.5) containing 1 mM CaCl2, and collected by centrifugation. Both fresh and dry weights were noted.
DNA extraction and PCR analysis
Genomic DNA was extracted from plant material using a Nucleon PhytoPure DNA extraction kit (Tepnel Life Sciences, Manchester, UK). Lines containing ORFs of interest were selected with vector- and gene-specific PCR primer pairs (Table 1).
RNA extraction and RT-PCR analysis
Plant material was ground in liquid nitrogen and total RNA extracted from ca. 0.25 g tissue according to the method of Chang et al. (2003). Ten micrograms of total RNA were treated with Turbo DNA-free DNase I (Ambion Inc., Austin, TX, USA) and purified by RNeasy MinElute columns (Qiagen Inc., Valencia, CA, USA). The presence of transcripts was confirmed with RT-PCR. RT reactions were performed using SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA, USA), and PCR experiments contained 200 nmoles each of gene-specific primer (Table 1). Products were sequenced to verify correct amplification.
Protein extraction
Callus and suspension-cultured cells were ground in liquid nitrogen (ca. 1 g fresh weight) in the presence of 5 mL Tris–HCl buffer (50 mM, pH 8) containing 1 mM EDTA, 1 mM CaCl2, 5 mM DTT and 2 % (m/v) PVPP. Insoluble residues were removed by centrifugation at 10,000g for 5 min. Protein was precipitated from supernatant by drop-wise adding 80 % ice-cold acetone, while stirring (Klibanov 2003; Abdel-Fattah et al. 2005; Esawy et al. 2008), and collected by centrifugation (13,000g for 5 min). Protein concentration was determined by the method of Bradford (1976) using the BioRad microassay (Hercules, CA, USA) with BSA as a standard.
Extraction of metabolites and water-soluble polysaccharides
Buffer for extracting sugars from callus and suspension cultures contained 30 mM Hepes (pH 7.8), 6 mM MgCl2 and 80 % ethanol (v/v). Sugar extraction from suspension-cultured cells were performed in 10 volumes (m:v) buffer. Ground callus tissue or plant material (0.5–1.0 g) was homogenized in 20 volumes extraction buffer (m:v). Sugars were extracted for 2 h at 80 °C and the liquid fraction removed by centrifugation. Sugar extraction was repeated and clarified samples pooled. Raw material remaining after extraction was washed twice with 70 % ethanol, dried and polysaccharides extracted twice with 5 volumes water (15 h at 37 °C, followed by 1 h at 50 °C). Aqueous extracts were clarified by centrifugation and polysaccharides precipitated overnight at −20 °C with 5 volumes ethanol, washed three times with 70 % ethanol and vacuum dried.
Sugar analysis with NADP+-coupled assay
Sugar (glucose, fructose and sucrose) concentrations were measured by means of a NADP+-coupled assay (Jones et al. 1977) with modifications (Basson et al. 2010). The reaction buffer (200 μL) contained 158 mM Tris–HCl (pH 7), 3.3 mM ATP, 0.8 mM NADP+ and 5.27 mM MgCl2. Phosphorylation of glucose was monitored by following the reduction of NADP+ to NADPH after the simultaneous addition of hexokinase (1.5 U) and glucose-6-phosphate dehydrogenase (1.0 U). Fructose concentrations were measured in the same reaction mixture after the addition of phosphoglucoisomerase (4.0 U). Finally, hydrolysis of sucrose was measured after the addition of invertase (60 U). Assays were monitored at 340 nm on a Powerwave X Microplate Spectrophotometer with KC4 Kineticalc for Windows Version 2.7 (Bio-Tek Instruments, Winooski, VT, USA).
Monosaccharide analysis
Polysaccharides were hydrolyzed in 5 M trifluoro acetic acid at 100 °C for 2 h, vacuum dried and washed twice with 100 % methanol. Glucose and fructose concentrations were determined with the NADP+-coupled assay as described above.
Sucrase activity assays
Sucrase activity was determined by measuring enzymatic release of glucose and fructose from sucrose. Protein extracts were incubated at 35 °C in 25 mM sodium acetate buffer (pH 4.7) containing 1 mM CaCl2 and 50 mM sucrose. Aliquots were withdrawn at 30-min intervals over a period of 3 h and reactions stopped with 2 M NaOH (5:1). Glucose and fructose releases were quantified enzymatically with the NADP+-coupled system (described above).
Radiolabeling experiments
Washed suspension-cultured cells (1.0–1.5 g fresh weight) were resuspended in 2 mL sodium acetate labeling buffer (25 mM, pH 5.5) containing 1 mM CaCl2 and 50 mM sucrose. After the addition of 0.06 MBq of 14C-labeled sucrose, samples were incubated for 20 h at 30 °C and unincorporated label removed by five consecutive washes in ice-cold buffer (6000×g for 1 min). Metabolites and water-soluble polysaccharides were extracted as described above, and incorporated label counted.
Aliquots of protein (20 μL), extracted from washed suspension-cultured cells (1 g fresh weight), were incubated for 20 h at 30 °C in 120 uL labeling buffer containing 0.04 MBq of 14C-labeled sucrose. Products were precipitated with 5 volumes ethanol, washed eight times with 70 % ethanol, and incorporated label counted. Radioactivity was determined with a Beckman LS 1801 scintillation counter in 1 mL Ultima Flo M scintillation cocktail containing 50 μL extracts.
Histological study
Callus cells were fixed for 12 h with 2 % paraformaldehyde and 4 % glutaraldehyde in 0.1 M phosphate buffer (pH 7.2). Glutaraldehyde was washed out from the fixing material by placing specimens in phosphate buffer. Fixed tissue was dehydrated in a graded ethanol series and imbedded in Kulzer 7100 resin (Labonord, Templemars, France) or paraffin wax. Histological sections (3–5 μm) were attached to glass slides using Haupt’s solution and rinsed with xylene to remove wax. Specimens were treated with periodic acid-Schiff (PAS) reagent that stains selectively for polysaccharide compounds, and post-stained with hematoxylin. After removing excess stain, the sections were dehydrated in an ethanol series for a few seconds, embedded in euparel, covered with glass and dried at room temperature. Specimens were viewed and photographed under light microscope. Cell sections from the following lines were studied: SL 1.3, 4.3, 8.1 and 8.2; RL 1.3, 2.2 and 3.2; RR 1.2, 1.3, 1.4 and 3.1.
Determination of starch and soluble sugars content
Plant material (100–300 mg of leaf or internodal tissue) was extracted three times with 80 % ethanol at 80 °C, extracts filtered through a 0.22-μm membrane filter, and sucrose, glucose and fructose contents determined. Sugars were quantified with NADP-coupled assays as described above. Starch was extracted by incubating the pellet with 20 mM NaOH for 1 h at 80 °C. After neutralization with 1 M acetic acid, the extracts were incubated in 20 mM sodium acetate (pH 4.6) with 5 U of amyloglucosidase at 37 °C overnight. The amount of glucose released was determined with NAD-coupled assays.
Sampling of internodal tissue from mature plants
Four stalks of each clone (3 clones per line) were randomly selected totaling 12 stalks per line or transformation event. Internodes were numbered from the top of the culm, and stalks separated into internodal sections. The ‘first internode’ is defined as the node immediately below the node to which the last fully expanded leaf subtends (Moore 1987). The leaves and leaf sheath were removed from the stalks. Internodal sections (e.g. 9 and 10) were pooled, cut into small pieces, finely homogenized in liquid nitrogen and the fresh weight measured. Subsamples were dried to a constant weight at 80 °C and dry weight noted.
Statistical analyses
Quantitative analyses are presented as mean values and significant differences were analyzed by Student’s t-test. Differences reaching a minimal confidence level of 95 % were considered as being statistically significant.
Results
Transgenic sugarcane callus accumulating fructans and glucans
Three bacterial glycansucrase ORFs were introduced into sugarcane to analyze whether fructan and glucan accumulation can be induced. Lines containing the fructansucrase coding sequences of L. sanfranciscensis (strain TMW 1.392) and L. reuteri (strain LTH5448) were designated SL and RL, respectively (Table 1). Those containing the glucansucrase coding region of L. reuteri ATCC 55730 were designated RR.
Microbial EPS are water soluble, unlike the majority of plant polysaccharides. This presented us with a convenient way to selectively screen for polymer accumulation in transformants. As starch loses crystallinity when heated above 60 °C, care was taken not to exceed this temperature when extracting biopolymers from plant tissue. Transformants screening positive for the accumulation of water-soluble polysaccharides in callus tissue are presented in Supplementary Figs. S1–S3. Callus tissue was not of the same age (1–3 months old) when harvested for analysis, since tissue of individual lines from the same construct differed in rates of biomass production (growth). As a consequence, interpretations of data presented in Supplementary Figures were only applied as a means of screening for positive transformants. Even so, reuteran accumulated to levels 1–2 order of a magnitude higher than levan (Supplementary Figs. S1–S3). Where levan was targeted to the cell wall, on average, 5× higher levels were accumulated compared to lines putatively expressing levan in the cytoplasm.
Subcellular targeting of transgene products
Cellular location of transgenic polymer was investigated by conducting histological studies with callus cells. During the fixation and embedding procedures, care was taken to retain the water-soluble polysaccharides. Thin sections of embedded callus material were stained specifically for polysaccharide-based compounds. Compared to the WT (Fig. 2a), the cytoplasm of SL and RR cells stained darker in all cases (Fig. 2b). In sections from RR lines 1.2 and 3.1, granule formation was more marked (Fig. 2c). Cell wall-targeted levan (RL) lines showed striking coloration along the cell rims (Fig. 2d).
Periodic acid-Schiff (PAS) histochemistry (carbohydrate) of the developing callus tissue in sugar cane. Intense violet/magneta staining confirms carbohydrate presence. a Wild type showing moderate staining in cytoplasm and cell membrane. b Putative cytosolic β-2,6 fructan (SL) line showing intense staining of the cytosol (400×). c Putative cytosolic α-1,4-α-1,6-glucan (RR) line. Developing callus cells show the augmented presence of cytoplasmic granules. d Cell wall-targeted β-2,6 fructan (RL) line showing the intense PAS staining of the outer perimeter. The inset is a magnification of mature callus cells showing intense PAS staining of the cell wall
Glycansucrase activity in callus paralleled with growth rate in suspension cultures
Northern blots could not detect mRNA for the glycosyltransferases. To confirm expression of the bacterial genes, total RNA was extracted from callus tissue and purified preparations subjected to the more sensitive technique, RT-PCR. Single amplification products of the expected size (depicted in Table 1) were reproducibly obtained. As sucrase activity in plant tissue could not be detected by conventional enzyme assays, activity was quantified with sensitive radiochemical detection using 14C-labeled sucrose (Figs. 3a, 4a, 5a). Specific activity estimates were expressed on a cellular dry weight rather than a total protein basis to avoid distortion by the accumulation of storage proteins. As only trace amounts were synthesized, the structure of the radiolabeled enzyme products was not determined. Among highest levan accumulators, specific activity in cell wall-targeted RL 3.3 tissue (Fig. 3a) was approximately seven times higher than in putative cytoplasmic SL 4.3 (Fig. 4a). Specific activity displayed by the most productive reuteran line RR 1.2 (Fig. 5a) was two times higher than the highest levan producer (RL 3.3). While pronounced differences in polysaccharide accumulation between individual lines originating from the same construct were evident, activity ranges were clearly construct dependent. Incorporation of radioactivity in glucan lines was higher than in levan lines, among which cell wall-targeted lines displayed the highest specific activity. The same trend was observed when screening callus tissue for polysaccharide accumulation (Supplementary Figs. S1–S3). Intracellularly produced radiolabeled product, measured over a period of 20 h, was quantifiable only for SL 8.2 and SL 8.1, both putative cytosolic expressed levan lines (Fig. 4c). Not surprisingly these lines were the highest accumulators in callus tissue (Supplementary Fig. S2).
Sugarcane lines accumulating cell wall-targeted fructan (RL) compared with wild type (WT). a Formation of radiolabeled water-soluble polysaccharide from [14C] sucrose in the presence of total proteins extracted from suspension cultures. Values shown are means with standard errors indicated by bars (n = 5). b Growth of suspension cultures was maintained for 30 days
Sugarcane lines accumulating putatively cytosolic fructan (SL) compared with wild type (WT). a Formation of radiolabeled water-soluble polysaccharide from [14C] sucrose in the presence of total proteins extracted from suspension cultures. b Growth of suspension cultures was maintained for 9 days. c Formation of radiolabeled water-soluble polysaccharide from [14C] sucrose by cultures in cell suspension. Values shown in a and c are mean ± SE (n = 5)
Sugarcane lines accumulating putatively cytosolic polyglucan (RR) compared with wild type (WT). a Formation of radiolabeled water-soluble polysaccharide from [14C] sucrose in the presence of total proteins extracted from suspension cultures. Values shown are means with standard errors indicated by bars (n = 5). b Growth of suspension cultures was maintained for 60 days
An inverse correlation between specific activity and growth rate among the cell lines from all three constructs was observed (Figs. 3b, 4b, 5b). Growth of suspension cultures representative of lines where levan was targeted to the cell wall (RL) was more inhibited compared to putative cytosolic targeting (SL). Levels of bacterial polysaccharide accumulation in lines RL 1.1 and RL 3.2 (Fig. 3a) were similar to levels in lines SL 6.1 and SL 4.3 (Fig. 4a). However, suspension cultures took 30 days (Fig. 3b) to reach optical densities (OD600 ~ 0.1), similar to those of 9-day-old SL lines (Fig. 4b). Growth of suspension cultures putatively accumulating reuteran in the cytoplasm (RR lines) was most inhibited, reaching cellular densities not higher than 0.2 in 60 days.
Regeneration of callus tissue into transgenic plants and plant development
The presence of transcripts in mature plant tissue was confirmed with RT-PCR (results not presented). Mature SL and RR plant lines positive for HoPSs production were indistinguishable from wild-type plants based on morphology, however, growth rate was visibly retarded. Regeneration of embryonic callus tissue into plants proved problematic for cell wall-targeted levan lines (13 RL lines in total, Supplementary Fig. S1). Only two lines, RL1.3 and RL2.2, could be regenerated into plants. These lines accumulated the lowest amount of levan as measured in callus tissue (Fig. 3a), no bacterial polymer could be detected in internodal tissue of mature plants (data not shown), and plants were indistinguishable from wild type in morphology and growth rate.
Carbohydrate content in transgenic sugarcane producing levan and reuteran
The starch and low molecular mass neutral sugar (sucrose, glucose, and fructose) contents in internodal tissue of control plants and transformants were compared and related to the water-soluble fructan/glucan content. Metabolite content was expressed in terms of fresh weight to facilitate comparisons with previously published data. Trends remained the same when data were expressed in dry weight (data not shown).
Levan lines SL1.1, SL1.3 and SL4.3 accumulated significant (P < 0.05) levels of fructan (Fig. 6a). The sucrose content in internodal tissue of stalks harvested from these lines was also significantly reduced, with amounts being only about two-third of the level observed in control plants (Fig. 6b). No significant effect on glucose and fructose contents was observed (data not shown), while starch content was significantly reduced, being up to six times higher in the WT (Fig. 6c). Ambiguously, reduction in starch was least pronounced in the highest fructan accumulator (SL4.3).
Only two RR lines were successfully regenerated from callus tissue and significantly increased amounts of water-soluble polysaccharides were detected when compared to wild-type controls (Fig. 7a). Sucrose, glucose and fructose contents remained unchanged (data not shown). Starch content was significantly increased, up to four times in internodes 9 and 10 (Fig. 7b). These results spurred analyses of the starch content over the length of the stalks. In all samples, starch content was increased and remained stable, even in older internodes.
Polysaccharide content of mature sugarcane lines accumulating polyglucan (RR) compared with wild type (WT). a Glucose levels in hydrolyzed water-soluble polysaccharides extracted from internodes 9 and 10. b Starch content in sugarcane internodal tissue. Sucrose content in transgenic plants was not significantly different from WT. Values shown are mean ± SE (n = 3)
Discussion
Sugarcane is an economically important plant that lacks glycansucrases, but accumulates high levels of the enzyme’s substrate, sucrose, in internodal tissue of the stalk. We introduced a pair of bacterial FTFs and a GTF into sugarcane. This study investigated the effects of heterologous accumulation of the gene products, levan (fructan) and reuteran (glucan), on plant development and carbon partitioning.
Sugarcane and sucrose accumulation
The biology of sucrose accumulation in sugarcane provides an interesting physiological system for the study of carbohydrate partitioning. Figure 8 depicts the range of sucrose concentrations as measured on internodal tissue of various sugarcane sources. Data assembly from literature and statistical analysis are shown in Supplement (Uys 2006, 2009). Internodal tissue matures progressively toward the base of the stalk with a corresponding increase in sucrose concentration up to an astonishingly high concentration of 0.5 M. The plant is unusual in its ability to store sucrose in relatively mature sink tissue, compared to growth apices and developing organs such as tubers. Accumulation of bacterial polysaccharides and the effect on carbohydrate assimilation were therefore studied by measuring target metabolites in internodal tissue.
Sucrose concentrations (converted to mM) as measured for internodes 3–10 (216 points in total) from 27 different sugarcane sources. Lines connecting a series of points represent a single set of measurements. The thick line depicts the sample median (50 percentile). Data were collected from the literature and collated as shown in Supplement. Min minimum, max maximum
Growth inhibition in levan and reuteran transformants
Several publications noted that plant transformants expressing fructansucrase exhibit aberrant phenotypes and/or growth appear to be inhibited (Pilon-Smits et al. 1996; Cairns 2003). Tissue damage by transgenic levan accumulation has been suggested and possible mechanisms have been reviewed, however, the query remains unresolved (Cairns 2003). With the aid of suspension cultures, this study convincingly demonstrated that heterologous accumulation of bacterial HoPSs, levan (fructan) and glucan (glucan), in transgenic sugarcane leads to growth inhibition. Suspension cultures are ready for the analysis within months, while it may take up to 2 years to generate mature sugarcane plants from embryonic callus tissue. Control and transgenic lines can be grown in a uniform environment and at a fraction of the costs compared to greenhouse- and field-grown plants. Suspension cultures furthermore allow for the creation of homogenous replicates for rapid and reproducible sampling.
Low rates of product accumulation in levan and reuteran transformants
Callus tissue as well as internodal tissue harvested from mature plants accumulated HoPSs at low levels, in accordance with most other studies where plants express transgenic fructansucrase (Cairns 2003). Low rates were accompanied with low message expression and low enzyme quantities. Not only growth rate but also survival of plants regenerated from callus tissue was severely impaired. This study supports the notion that biopolymer accumulation is a function of lethal negative selection of transformants displaying high levels of expression. The variation of expression levels between transgenic tissue containing the same construct is not correlated with the number of inserted transgenes, but appears to be caused by plant sequences in the proximity of the insertion site (Weising et al. 1988; van der Meer et al. 1994). The mechanism of HoPS-mediated developmental interferences is unclear. Considering that the 35S promoter is active in phloem tissue, developmental aberrations have been blamed on high MW HoPSs disturbing the source to sink transport (Pilon-Smits et al. 1996). This problem was not overcome by targeting levan to the cell perimeter (RL lines, this study).
Subcellular targeting of transgene products
Differential localization of the gene product may modulate the detrimental effects reported for FTF (fructan) expression. Fluorescence experiments have however shown the miss-localization of levan regardless of the putative specificity of the targeting sequences (for review see Cairns 2003). For example, the sporamin vacuolar targeting sequence is known to localize levansucrase into the endomembrane system rather than the vacuole (Turk et al. 1997). To circumvent ambiguous targeting, this study avoided the addition of foreign targeting sequences to the plant expression constructs. In the absence of targeting sequences, transgene products have been established to accumulate in the cytosol.
Thin cross-sections of resin embedded callus cells were selectively stained for polysaccharide-based compounds. Transgenic cells, expressing reuteransucrase from L. reuteri (RR) and fructansucrases from L. sanfranciscensis (SL), stained positive for increased polysaccharide accumulation in the cytosol. The expression of levan in the cytoplasm may disrupt cellular spatial organization so severely that the cell dies (Cairns 2003). Expression of reuteran in sugarcane (this study) resulted in plants with increased starch content. Glucan (starch and reuteran) accumulation in reuteran lines RR 1.2 and RR3.1 was furthermore correlated with augmented granule formation. Glucan appears to precipitate in the cytosol above a certain threshold level. The 35S promoter is active in phloem tissue and high DP HoPSs may perturb metabolite transport for instance by precipitation, as previously suggested (Pilon-Smits et al. 1996). Among RR lines, growth in suspension of lines 1.2 and 3.1 were most severely inhibited. Surprisingly, these were the only RR lines that could be generated into mature plants. Soluble glucans occupy much larger volumes than granules and have potentially serious impacts on the osmotic and volume regulation of cells (Raven 2005). Formation of granules, rather than soluble glucan, in the cytosol might have allowed these plants to survive. Cell rims of RL plants displayed marked coloration, a clear indication that the gene product (levan) is localized at the cell perimeter. The FTF gene of L. reuteri was not truncated and contains a native bacterial secretion sequence, explaining its defaults to the extracellular compartment in plants. While targeting to the cell perimeter may avoid direct effects on cytoplasmic organization, growth of RL suspension cultures was more inhibited than with putative cytosolic targeting (SL). Absence of granules in tissue culture cells of RL lines could not be explained. Plants with measurable levels of fructan could not be generated from RL callus tissue. This study highlights the complexities and far reaching consequences arising from introducing polymers with high DP in plants.
Carbon partitioning
Endogenous sugars are subject to hourly transport and turnover, while HoPSs accumulate over months. Instantaneous concentrations of endogenous carbohydrates such as sucrose, starch, and glucose, cannot assess the extent of HoPS accumulation as a proportion of primary metabolism. We therefore compared accumulation of transgenic polymer with the flux of sugar through photoassimilation as suggested by Cairns (2003). Table 2 summarizes parameters associated with transgenic levan/reuteran accumulation and compares values with results from other studies. Estimate of flux into HoPSs was, in all cases, less than 0.1 % of primary metabolism (also see Cairns 2003), suggesting that pilfering sucrose for transgenic biopolymer production is unlikely to impact on the steady-state sucrose pool or fluxes of photoassimilate.
Starch content in levan (fructan) accumulators (SL lines) was significantly reduced relative to controls. A number of studies reported on lowered starch concentrations when compared to WT or empty vector controls. To our knowledge, only two studies reported on an increase in starch (Turk et al. 1997; Jenkins et al. 2002), associated with a decreased translocation of photoassimilates from leaves (Turk et al. 1997). Reduced starch concentration has been considered due to a competition for carbon by fructansucrase and a resulting diversion of carbon flow from primary metabolism (van der Meer et al. 1994). Considering the low rate of levan synthesis in transgenic plants (see Table 2), metabolic competition is, however, an unlikely cause for reduced starch accumulation. Total reduction in carbohydrate accumulation is furthermore non-stoichiometric; the sum of fructan, starch and sucrose concentrations in transformants is significantly lower than in WT or empty vector controls. Heterologous expression clearly results in a reduction in total assimilation rather than a simple diversion by competition.
GTF enzymes of LAB generally share a common structure composed of four distinct domains: a signal peptide, a variable domain, a catalytic domain, and a glucan-binding domain (Fig. 1). A truncated version of the reuteransucrase gene, gtfA, encompassing the catalytic and glucan-binding domains was expressed in sugarcane (RR lines). These two domains have been shown to be required for functional expression in Escherichia coli (Kralj et al. 2002, 2004). Unlike with levan, putative cytoplasmic expression of reuteran in sugarcane was positively correlated with starch content, a minor storage carbohydrate in sugarcane. No significant changes in low molecular mass neutral sugar (sucrose, glucose, and fructose) contents were observed. Cytoplasmic accumulation of reuteran may interfere with synthesis/breakdown metabolic cycles controlling starch formation. It is also conceivable that reuteran binds to starch and decreases the susceptibility of starch to hydrolysis. Little is unfortunately known about the processes involved in starch degradation outside of cereal endosperm (Kossmann and Lloyd 2000).
Abbreviations
- FW:
-
Fresh weight
- DP:
-
Degree of polymerization
- HoPS:
-
Homopolysaccharide
- FTF:
-
Fructosyltransferase
- GTF:
-
Glucosyltransferase
- PAS:
-
Periodic acid-Schiff
- MS:
-
Murashige–Skoog
- WT:
-
Wild type
References
Abdel-Fattah AF, Mahmoud DAR, Esawy MAT (2005) Production of levansucrase from Bacillus subtilis NRC 33a and enzymic synthesis of levan and fructo-oligosaccharides. Curr Microbiol 51:402–407
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1994) Current protocols in molecular biology. Wiley, New York
Basnayake SW, Morgan TC, Wu L, Birch RG (2012) Field performance of transgenic sugarcane expressing isomaltulose synthase. Plant Biotechnol J 10:217–225
Basson CE, Groenewald J-H, Kossmann J, Cronje C, Bauer R (2010) Sugar and acid-related quality attributes and enzyme activities in strawberry fruits: invertase is the main sucrose hydolysing enzyme. Food Chem 121:1156–1162
Bauer R, Volschenk H, Dicks LMT (2005) Cloning and expression of the malolactic gene of Pediococcus damnosus NCFB 1832 in Saccharomyces cerevisiae. J Biotechnol 118:353–362
Bauer R, van Bekker J, du Wyk N, Toit C, Dicks LMT, Kossmann J (2009) Exopolysaccharide production by lactose-hydrolyzing bacteria isolated from traditionally fermented milk. Int J Food Microbiol 131:260–264
Bower R, Birch RG (1992) Transgenic sugarcane plants via microprojectile bombardment. Plant J 2:409–416
Bradford MM (1976) Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein–dye binding. Anal Biochem 72:248–254
Caimi PG, McCole LM, Klein TM, Kerr PS (1996) Fructan accumulation and sucrose metabolism in transgenic maize endosperm expressing a Bacillus amyloliquefaciens SacB gene. Plant Physiol 110:355–363
Caimi PG, McCole LM, Klein TM, Hershey HP (1997) Cytosolic expression of the Bacillus amyloliquefaciens SacB protein inhibits tissue development in transgenic tobacco and potato. New Phytol 136:19–28
Cairns AJ (2003) Fructan biosynthesis in transgenic plants. J Exp Bot 54:549–567
Chang YC, Hsieh PW, Chang FR, Wu RR, Liaw CC, Lee KH, Wu YC (2003) Two new protopines argemexicaines A and B and the anti HIV alkaloid 6-acetonyldihydrochelerytrine from formosan Argemone mexicana. Planta Med 69:148–152
Esawy MA, Mahmoud DAR, Fattah AFA (2008) Immobilisation of Bacillus subtilis NRC33a levansucrase and some studies on its properties. Braz J Chem Eng 25:237–246
Gerrits N, Turk SCHJ, van Dun KPM, Hulleman SHD, Visser RGF, Weisbeek PJ, Smeekens SCM (2001) Sucrose metabolism in plastids. Plant Physiol 125:926–934
Hamerli D, Birch RG (2011) Transgenic expression of trehalulose synthase results in high concentrations of the sucrose isomer trehalulose in mature stems of field-grown sugarcane. Plant Biotech J 9:32–37
Hellwege EM, Czapla S, Jahnke A, Willmitzer L, Heyer AG (2000) Transgenic potato (Solanum tuberosum) tubers synthesize the full spectrum of inulin molecules naturally occurring in globe artichoke (Cynara scolymus) roots. Proc Nat Acad Sci USA 97:8699–8704
Hendry GAF, Wallace RK (1993) The origin, distribution and evolutionary significance of fructans. In: Suzuki M, Chatterton NJ (eds) Science and technology of fructans. CRC Press, Boca Raton, pp 119–139
Jenkins LD, Snow AJ, Simpson RJ, Higgins TJ, Jacques NA, Pritchard J, Gibson J, Larkin PJ (2002) Fructan formation in transgenic white clover expressing a fructosyltransferase from Streptococcus salivarius. Funct Plant Biol 29:1287–1298
Jones MGK, Outlaw WH, Lowry OH (1977) Enzymic assay of 10−7 to 10−14 moles of sucrose in plant tissues. Plant Physiol 60:379–383
Klibanov AM (2003) Asymmetric enzymatic oxidoreductions in organic solvents. Curr Opin Biotechnol 14:427–431
Komor E, Thom M, Maretzki A (1981) The mechanism of sugar uptake by sugarcane suspension cells. Planta 153:181–192
Korakli M, Vogel RF (2006) Structure/function relationship of homopolysaccharide producing glycansucrases and therapeutic potential of their synthesised glycans. Appl Microbiol Biotechnol 71:790–803
Kossmann J, Lloyd J (2000) Understanding and influencing starch biochemistry. Crit Rev Plant Sci 19:171–226
Kralj S, van Geel-Schutten GH, Rahaoui H, Leer RJ, Faber EJ, van der Maarel MJEC, Dijkhuizen L (2002) Molecular characterization of a novel glucosyltransferase from Lactobacillus reuteri strain 121 synthesizing a unique, highly branched glucan with α-(1 → 4) and α-(1 → 6) glucosidic bonds. Appl Environ Microbiol 68:4283–4291
Kralj S, van Geel-Schutten GH, van der Maarel MJEC, Dijkhuizen L (2004) Biochemical and molecular characterization of Lactobacillus reuteri 121 reuteransucrase. Microbiol 150:2099–2112
Kralj S, Stripling E, Sanders P, van Gel-Schutten GH, Dijkhuizen L (2005) Highly hydrolytic reuteransucrase from probiotic Lactobacillus reuteri strain ATCC 55730. Appl Environ Microbiol 71:3942–3950
Moore PH (1987) Anatomy and morphology. In: Heinz DJ (ed) Sugarcane improvement through breeding. Elsevier Science Publisher, New York, pp 85–142
Moore PH, Maretzki A (1996) Photoassimilate distribution in plants and crops. In: Zamski E, Schaffer AA (eds) Source–sink relationships in sugarcane. Marcel Dekker Inc, New York, pp 643–669
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco cultures. Plant Physiol 15:473–497
Pilon-Smits EAH, Ebskamp MJM, Paul MJ, Jeuken MJW, Weisbeek PJ, Smeekens SCM (1995) Improved performance of transgenic fructan-accumulating tobacco under drought stress. Plant Physiol 107:125–130
Pilon-Smits EAH, Ebskamp MJW, Jeuken IM, van der Meer RGF, Visser RGF, Weisbeek PJ, Smeekens SCM (1996) Microbial fructan production in transgenic potato plants and tubers. Ind Crop Prod 5:35–46
Rae AL, Perroux JM, Grof CLG (1996) Sucrose partitioning between vascular bundles and storage parenchyma in the sugarcane stem: a potential role for the ShSUT1 sucrose transporter. Planta 220:817–825
Raven JA (2005) Cellular location of starch synthesis and evolutionary origin of starch genes. J Phycol 41:1070–1072
Röber M, Geider K, Muller-Röber B, Willmitzer L (1996) Synthesis of fructans in tubers of transgenic starch-deficient potato plants does not result in an increased allocation of carbohydrates. Planta 199:528–536
Roberfroid MB (1999) What is beneficial for health? The concept of functional food. Food Chem Toxicol 37:1039–1041
Rossouw D, Bosch S, Kossmann J, Botha FC, Groenewald JH (2007) Downregulation of neutral invertase activity in sugarcane cell suspension cultures leads to a reduction in respiration and growth and an increase in sucrose accumulation. Funct Plant Biol 34:490–498
Schwab C, Gänzle MG (2006) Effect of membrane lateral pressure on the expression of fructosyltransferases in Lactobacillus reuteri. Syst Appl Microbiol 29:89–99
Sévenier R, Hall RD, van der Meer IM, Hakkert HJC, van Tunen AJ, Koops AJ (1998) High level fructan accumulation in a transgenic sugar beet. Nat Biotechnol 16:843–846
Smith AM (2008) Harnessing plant biomass for biofuels and biomaterials. Prospects for increasing starch and sucrose yields for bioethanol production. Plant J 54:546–558
Snyman SJ, Meyer GM, Carson DL, Botha FC (1996) Establishment of embryogenic callus and transient gene expression in selected sugarcane varieties. S Afr J Bot 62:151–154
Taylor P, Ko H, Adkins S, Rathus C, Birch R (1992) Establishment of embryogenic callus and high protoplast yielding suspension cultures of sugarcane (Saccharum spp. hybrids). Plant Cell Tissue Organ Cult 28:69–78
Tieking M, Ehrmann MA, Vogel RF, Gänzle MG (2005) Molecular and functional characterization of a levansucrase from the sourdough isolate Lactobacillus sanfranciscensis TMW 1.392. Appl Micorbiol Biotech 66:655–663
Turk SCHJ, de Roos K, Scott PA, van Kun K, Weisbeek P, Smeekens SCM (1997) The vacuolar sorting domain of sporamin transports GUS, but not levansucrase, to the plant vacuole. New Phytol 136:29–38
Uys L (2006) Computational systems biology of sucrose accumulation in sugarcane. M.Sc. (Biochemistry) thesis, University of Stellenbosch, South Africa
Uys L (2009) Coupling kinetic models and advection-diffusion equations to model vascular transport in plants, applied to sucrose accumulation in sugarcane. Ph.D. (Biochemistry) thesis, University of Stellenbosch, South Africa
van der Meer IM, Ebskamp MJM, Visser RGF, Weisbeek PJ, Smeekensa SCM (1994) Fructan as a new carbohydrate sink in transgenic potato plants. Plant Cell 6:561–570
Wei H, Wang M-L, Moore PH, Albert HH (2003) Comparative expression analysis of two sugarcane polyubiquitin promoters and flanking sequences in transgenic plants. J Plant Physiol 160:1241–1251
Weising K, Schell J, Kahl G (1988) Foreign genes in plants: transfer, structure, expression and applications. Annu Rev Genet 22:241–277
Weyens G, Ritsema T, Van Dun K, Meyer D, Lommel M, Lathouwers J, Rosquin I, Denys P, Tossens A, Marleen Nijs M, Turk T, Gerrits N, Bink S, Walraven B, Lefèbvre M, Smeekens S (2004) Production of tailor-made fructans in sugar beet by expression of onion fructosyltransferases. Plant Biotech J 2:321–327
Wu L, Birch RG (2007) Doubled sugar content in sugarcane plants modified to produce a sucrose isomer. Plant Biotech J 5:109–117
Zhang S, Dong JG, Wang T, Guo S, Glassman K, Ranch J, Nichols SE (2007) High level accumulation of a-glucan in maize kernels by expressing the gtfD gene from Streptococcus mutans. Transgenic Res 16:467–478
Acknowledgments
This work was supported by grants from the South African National Research Foundation and the South African Sugar Association.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bauer, R., Basson, C.E., Bekker, J. et al. Reuteran and levan as carbohydrate sinks in transgenic sugarcane. Planta 236, 1803–1815 (2012). https://doi.org/10.1007/s00425-012-1731-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-012-1731-x