Abstract
Grapevine bud fruitfulness is determined by the differentiation of uncommitted meristem (UCM) into either tendril or inflorescence. Since tendril and inflorescence differentiation have long been considered sequential steps in inflorescence development, factors that control the progression of floral meristem development may regulate the final outcome of UCM differentiation, and thus affect fruitfulness. A comparison of the expression profiles of the master regulators of floral meristem identity (FMI) during development of fruitful and non-fruitful buds along the same cane allowed associating the expression of a homolog of terminal flower 1 (TFL1, a negative regulator of FMI) to fruitful buds, and the expression of positive FMI regulators to non-fruitful buds. Combined with (a) cytokinin-induced upregulation of VvTFL1A expression in cultured tendrils, which accompanied cytokinin-derived tendril transformation into branched, inflorescence-like structures, (b) positive regulation of VvTFL1A expression by cytokinin, which was demonstrated in transgenic embryonic culture expressing GUS reporter under the control of VvTFL1A promoter, and (c) a significantly higher level of active cytokinins in fruitful positions, the data may support the assumption of cytokinin-regulated VvTFL1A activity’s involvement in the control of inflorescence development. Such activity may delay acquisition of FMI and allow an extended branching period for the UCM, resulting in the differentiation of inflorescence primordia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Grapevine is a perennial plant with a reproductive developmental cycle that spans two consecutive growing seasons. In the spring, after completion of bud dormancy cycle, young shoots emerge from mature buds that are carried on woody canes (shoots from the preceding season which were lignified at the end of the growing season; see Fig. S1 for growth cycle description). A young shoot may emerge from a mature bud with or without inflorescences, depending on differentiation of inflorescence primordia within that bud during its development on the young shoot in the previous spring.
Grape bud fruitfulness, expressed as the number of clusters per bud, is influenced by genetic background, environmental conditions and horticultural practices. Genotype has a major effect, as evidenced by the differences between cultivars in the number of clusters per shoot and in fruitful node position on the cane. High temperature and high light intensity during vegetative growth appear to have a positive influence, while shade and exogenous gibberellin have a marked negative influence on bud fruitfulness (Buttrose 1974; Mullins et al. 1992; Dry 2000; May 2004; Carmona et al. 2007b, 2008). The mechanism that controls fruitfulness and the strategy by which the above factors manipulate this mechanism are still not clear. Further study on bud reproductive development is therefore warranted.
Differentiation at the grapevine shoot apical meristem (SAM) leads to alternate production of leaf primordia and uncommitted lateral meristems (UCMs), while the SAM remains undifferentiated (Srinivasan and Mullins 1981; May 2004; Carmona et al. 2008). The UCM, also known as anlage, is the meristematic origin of both the tendril and the inflorescence. The UCM first divides to form two arms. If a tendril primordium is formed, there is no further branching. If an inflorescence is formed, the UCM proliferates to give multiple branch primordia, with each branch being a protuberance of undifferentiated meristematic tissue (Carmona et al. 2002).
At the apex of the actively growing shoot, UCMs differentiate only into tendrils. Within the latent bud, however, the first two to three UCMs on the primordial shoot have the potential to differentiate into inflorescences, while the following UCMs differentiate into tendrils (Pratt 1971, 1974; Srinivasan and Mullins 1981). This stage of primordial inflorescence differentiation within fruitful buds takes place from roughly anthesis to berry ripening and terminates at the end of the summer, when the buds enter a dormant phase (Srinivasan and Mullins 1976; Morrison 1991; Carmona et al. 2002). Development of flowers on the branches of the inflorescence primordia takes place during woody bud swelling and bud burst, at the end of the following winter (Carmona et al. 2002).
Based on the above, it appears that control of UCM fate may be in large part responsible for the degree of fruitfulness. Tendrils are generally considered to be reproductive organs representing immature inflorescences (Srinivasan and Mullins 1976; Morrison 1991; Carmona et al. 2002). The existence of intermediate hybrids that combine tendril and inflorescence structures, frequently observed under field conditions, supports this assumption. In addition, exogenous application of growth regulators can lead to the transformation of one shape into another. It was shown that cytokinins induce branching and development of inflorescence-like structures from in vitro-grown young tendrils. Moreover, when applied to shoot tips, cytokinin promoted the development of inflorescences rather than tendrils from the newly formed UCM (Srinivasan and Mullins 1978, 1980b). Gibberellic acid (GA) promoted UCM initiation but inhibited UCM differentiation into inflorescences, favoring tendril development (Srinivasan and Mullins 1980a). The notion that GA inhibits inflorescence differentiation was further supported by the phenotype of a gibberellin-insensitive grapevine mutant, in which inflorescences were produced along the main shoot where tendrils would normally be formed (Boss and Thomas 2002). The mechanism by which cytokinin and gibberellin regulate the final outcome of grapevine UCM development is unknown.
Since both tendril and inflorescence are considered reproductive organs that represent two sequential steps during inflorescence development, factors that control the progression of floral meristem development may take part in the final outcome of UCM differentiation and affect fruitfulness. In Arabidopsis, the master genes Leafy (LFY), Apetala1 (AP1), Flowering locus T (FT) and Terminal flower 1 (TFL1) serve as the backbone of the regulatory network that controls floral meristem formation and inflorescence development (Blázquez et al. 2006; Benlloch et al. 2007). Consequently, elucidating possible associations of their grapevine homologs to bud fruitfulness could shed light on the regulation of grapevine inflorescence development.
Identification and functional analyses have been carried out for the grapevine homologs of LFY, AP1, FT and TFL1. The first three were termed VFL, VAP1 and VvFT, respectively. Of the three Vitis TFL homologs, it was shown that VvTFL1A is the most closely related to both the Arabidopsis TFL1 and Snapdragon Centroradialis (CEN) genes (Carmona et al. 2002; Calonje et al. 2004; Joly et al. 2004; Boss et al. 2006; Sreekantan and Thomas 2006; Carmona et al. 2007a). A detailed summary of the findings is presented in Supplementary File S1 and Supplementary Table S1, as well as in recent reviews (Carmona et al. 2007b, 2008). In general, functional analyses demonstrated that VFL, VvTFL1A and VvFT are similar to those of their Arabidopsis homologs (Boss et al. 2006; Sreekantan and Thomas 2006; Carmona et al. 2007a; Supplementary File S1). Furthermore, VvTFL1A function was recently confirmed in grapevine as well (Fernandez et al. 2010). Spatial and temporal expression analyses of VFL, VAP1, VvTFL1A and VvFT have been carried out as well, using shoot tip, root, leaf, bud, inflorescence and tendril tissues: these revealed both expected and unexpected results relative to the Arabidopsis model (Supplementary File S1, Supplementary Table S1; Carmona et al. 2007b, 2008). However, their possible involvement in the control of grapevine fruitfulness has never been directly addressed.
To study this issue, we made use of the fruitfulness gradient that characterizes cane-pruned varieties such as cv. Thompson Seedless and cv. Sugarone. In these varieties, the buds that develop in the basal internodes of each shoot (basal buds) are much less fruitful than those in higher positions (top buds); hence, 10–15 nodes are left on each cane during winter pruning, to ensure maximum yield. As these buds originate from the same genetic background and are subjected to the same growing conditions, non-relevant variation is minimized. Since they both originate from the same primordial shoot in the latent bud, they also present a similar developmental stage. Thus, a comparison of the buds from these nodal positions, carried out here for the first time, may be instrumental in exposing differences related to fruitfulness regulation.
Such a comparison allowed associating the expression of the Arabidopsis TFL1 homolog VvTFL1A to fruitful node positions and that of the FT and LFY homologs to non-fruitful positions. It also revealed a significantly higher level of active cytokinins in fruitful positions. Combined with the documented induction of VvTFL1A expression in cultured tendrils in response to cytokinin, paralleling its transformation to an inflorescence-like structure, the data suggest the involvement of cytokinin-induced TFL1 homolog activity in the control of inflorescence differentiation. This scenario was supported by cytokinin-induced expression of a reporter gene fused to the VvTFL1A promoter in transgenic cv. Sugarone embryonic culture.
Materials and methods
Plant material
Grapevine lateral buds were sampled from shoots on vines growing in a commercial vineyard (Vitis vinifera L. cv. Sugarone) in the central plain of Israel. Sampling was begun at the beginning of April and buds were collected at weekly intervals for 10 weeks, in three successive years (2003–2005). A basal bud pool was established from buds originating from bud position 2 (from the base of the cane) that presented low average fertility (0.18 inflorescences per bud). A top bud pool was established from buds at positions 8–10 that presented high average fertility (0.72 inflorescences per bud). Leaves were collected from the same positions at the same time points.
Tendrils were collected from cvs. Sugarone Seedless commercial vineyards in the central plain of Israel from 20- to 25-node shoots at 1–2, 3–4, 5–6, 7–8 nodes from the shoot tip.
For tendril culture, tendrils positioned 1–2 nodes from the shoot apex were excised from a cv. Thompson Seedless lateral shoot of about 15 nodes with the help of a stereomicroscope. Tendrils were grown on 10-ml McCown Woody plant liquid medium (Duchefa Biochemie, Haarlem, The Netherlands). The medium for the cytokinin-treated tendrils was supplemented with 10 μM of N-benzyl-9-(2-tetrahydropyranyladenine) (PBA) (Duchefa Biochemie). Treated and control tendrils were grown on an orbital shaker (100 oscillations per minute) under conditions of 16-/8-h light/dark, 25°C. Tendrils were sampled after 7, 14 and 30 days under these conditions for further analysis.
Quantitative real-time PCR
Quantitative real-time PCR was performed as previously described (Halaly et al. 2008). Specific primers designed for VvTFL1A, VFL, VAP1 and VFT are described in Supplementary Table S2. For tendrils grown in liquid medium, normalization was carried out against the geometric mean of the amount of the reference genes Actin and glyceraldehyde 3-phosphate dehydrogenase transcript in each sample (Reid et al. 2006).
Fluorescence in situ hybridization
Tendrils positioned at 1–2 nodes from the shoot apex were collected from actively growing main shoots of cv. Thompson Seedless directly into Carnoy’s fixative (chloroform:ethanol:glacial acetic acid, 6:3:1, by vol.) and fixed overnight as previously reported (Gottlieb et al. 2006). After fixation, the samples were decolorized in 6% H2O2 in water for 15 min, pre-hybridized for 1–2 h and then hybridized overnight in hybridization buffer (50% formamide, 50% dextran sulfate, 4× SSC) containing 750 ng of fluorescent probe/ml. The full VvTFL1A gene including the 3′ UTR was used as the template. Labeling reactions of antisense and sense probes were performed using the MEGAscript® High-Yield Transcription Kit and the MEGAclear™ Purification Kit (both from Ambion, Austin, TX, USA), according to the manufacturer’s instructions, with fluorescein-12-UTP RNA labeling mix (Roche, Indianapolis, IN, USA).
After hybridization, samples were washed twice with 2× SSC/0.1% SDS for 15 min and soaked in 2× SSC. Samples were mounted and viewed under an Olympus IX81/FV500 confocal microscope.
Scanning electronic microscopy
Buds collected in 2005 and tendrils growing in liquid medium (see Plant Material) were fixed in FAA (ethanol:acetic acid:formaldehyde:H2O 5:0.5:1:3.5, by vol.). Buds were peeled from leaves, bracts and hairs under a stereomicroscope. Samples were then dehydrated in an ethanol dilution series and dried using CO2. The dried tissue was mounted on stubs with silver point, sputter-coated with gold and observed by scanning electron microscopy (SEM) (JSM-35C SEM, Jeol, Tokyo, Japan).
Cytokinin quantification
Cytokinins were extracted from ca 1 g of bud tissue (FW) according to Dobrev and Kaminek (2002). Frozen samples were ground in liquid nitrogen and extracted overnight with 10 ml of methanol:water:formic acid (15:4:1, by vol., pH ~2.5, −20°C). The following 14 deuterium-labeled standards were added (50 pmol of each): [2H5]Z, [2H5]Z9R, [2H5]Z7G, [2H5]Z9G, [2H5]ZOG, [2H5]Z9ROG, [2H6]iP, [2H6]iP9R, [2H6]iP7G, [2H6]iP9G, [2H3]DHZ, [2H3]DHZ9R, [2H3]DHZ9G, [2H7]DHZOG (Apex Organics, Honiton, UK). Reverse-phase and ion-exchange chromatography were used for sample purification.
HPLC–MS analysis was performed as described by Dobrev and Kaminek (2002) using a TSQ Quantum Ultra AM triple-quad high-resolution mass spectrometer (Thermo Electron, San Jose, CA, USA). Ternary gradient elution (water/acetonitrile/acetic acid) was applied. The mass spectrometer was operated in positive MS/MS mode (SRM = single reaction monitoring) with monitoring of two to four transitions for each compound. Detection limits of different cytokinins varied from 0.05 to 0.1 pmol/sample. Two independent experiments were carried out. Each sample was injected at least twice.
Transformation of cv. Sugarone callus with VvTFL1A promoter::GFP::GUS construct
A 1,565-bp fragment upstream of the first ATG of the VvTFL1A ORF was isolated from V. vinifera L. cv. Sugarone using the 5′-specific primer 5′-CACCAGAATCCTGTAGGAAT-3′ and the 3′-specific primer 5′-TGGACAAGACTAGAACTCTT-3′. These primers were designed based on the sequence between nucleotides 4,229 and 5,750 of the Vitis vinifera contig VV78X162920.4 (GenBank: AM452656.2). The fragment was cloned into the Gateway vector pKGWFS7,0 (VIB, University of Gent, Belgium) using the pENTR™ TOPO Cloning kit (Invitrogen, Carlsbad, CA, USA). The pVvTFL1A::GFP::GUS vector was introduced into Agrobacterium EHA105 and the resulting bacteria were used to transform V. vinifera cv. Sugarone embryonic callus according to Perl and Eshdat (2007). PCR-based validation of the presence of the pVvTFL1A::GFP::GUS insert in the transformed callus was carried out using specific primers for green fluorescent protein (GFP) and β-glucuronidase (GUS).
Analyses of GFP signal and GUS activity in transformed callus
Transgenic calluses were maintained on MGN medium composed of MS medium supplemented with vitamins (Duchefa), 18 g/l maltose, 1 g/l casein enzymatic hydrolysate, 4.6 g/l glycerol and 1 mg/l 2-naphthoxyacetic acid (NOA), according to Perl and Eshdat (2007). To analyze the effect of cytokinins on expression from the candidate promoter region, the callus was transferred to regeneration medium without phytohormones, that allow embryogenesis. The callus was placed on six plates containing 1× MS medium supplemented with 30 g/l sucrose and 8 g/l plant agar. Three plates were supplemented with 2 mg/l zeatin (Duchefa) within the solid medium, while the others served as controls. Cells were examined twice a week by stereomicroscope Leica MZFLIII (Wetzlar, Germany) using excitation at 480/40 nm and emission >510 nm for GFP signal.
For fluorometric GUS assay, culture was harvested 3 weeks after transfer to MS medium, when differential GFP signal was observed between cytokinin-treated callus and controls. The assay was carried out as described previously (Jefferson et al. 1987) with modifications. Cell samples of 0.2 g were homogenized in extraction buffer (50 mM NaPO4, pH 7.0). Cells were precipitated, total protein was collected from the supernatant and protein concentration was determined using Protein Assay Dye reagent (Bio-Rad, Munchen, Germany) and bovine serum albumin (Sigma, St. Louis, MO, USA) for Bradford protein assay. For each sample, 100 mg of protein was mixed with 150 μl of sodium phosphate buffer and 150 μl of reaction buffer (50 mM NaPO4 pH 7.0, 10 mM EDTA pH 8, 0.1% laurylsarcosine and 0.1% Triton X-100) containing 2 mM MUG substrate (4-methylumbelliferyl β-d-glucuronide hydrate; Sigma). The mixture was incubated for 20 min at 37°C. The reaction was stopped with 0.2 M Na2CO3 and fluorescence was recorded using a TKO100 fluorometer (365 nm excitation/460 nm emission; Hoefer, San Francisco, CA, USA). Activity was determined using a MU (4-methylumbelliferone; Sigma)-fluorescence calibration curve. GUS activity was expressed as nM product/mg protein in 1 min.
Results
Fruitfulness gradient along the cane
To assess the fruitfulness of cv. Sugarone buds along the cane in the current experimental setup, the presence/absence of inflorescences in shoots emerging from buds at node positions 2–12 (starting from the base of the cane) was recorded (Fig. 1). Shoots emerging from buds at position 2 had an average 0.18 inflorescences per bud, while those emerging from buds at positions 7–11 had an average 0.72 inflorescences per bud. Thus, their comparison could indeed be instrumental in associating fruitfulness and the expression of its potential regulators.
Fruitfulness gradient along the cane. Presence/absence of inflorescences in shoots emerged from buds at node positions 2–12 (counted from the base of the cane) was recorded in 100 cv. Sugarone canes selected at random in the vineyard. The average number of inflorescences per bud position was calculated based on data collected in the same vineyard in three successive years
Shoot phenological development
Shoot development was monitored at weekly intervals in three successive growing seasons and expressed as number of nodes per shoot. About two to three nodes separated from the shoot apex every week, indicating a similar rate of development in the different years (Fig. S2a). Flowering started when 19 nodes, on average, had separated from the shoot tip. Full bloom was recorded 1–2 weeks later, depending on the year.
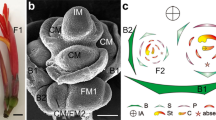
Young buds were sampled separately from the second node (basal, non-fruitful bud pool), and from nodes 8, 9 and 10 (top, fruitful bud pool) of the actively growing shoot. Sampling was begun in the first week of April, about 2 weeks before full bloom, corresponding to the estimated timing of inflorescence initiation within the bud (Lavee et al. 1967; Morrison 1991) and was carried out for 10 weeks. Samples from three successive years served as three biological replicates in the described analyses. SEM analysis was carried out on top buds that were sampled 2 weeks before full bloom (week 1), at full bloom (week 3), and 3 and 6 weeks after full bloom (week 6 and 9, respectively) (Fig. S2b). According to this analysis, UCM was first detected in buds sampled from a fruitful position 3 weeks after full bloom. About 3 weeks later, the UCM had already divided into outer and inner arms, supporting previous estimates that reproductive development within the bud occurs roughly between full bloom and veraison (Lavee et al. 1967; Morrison 1991; Carmona et al. 2008).
Effect of node position on expression of grapevine homologs of FT, AP1 and LFY
To find possible connections between fruitfulness and the expression of the homologs of the Arabidopsis positive regulator genes of flower meristem identity (FMI), VvFT, VFL and VAP1, we profiled their transcript levels in top and basal node positions, using cv. Sugarone.
In agreement with previous analyses (Sreekantan and Thomas 2006; Carmona et al. 2007a), we found that the level of VvFT transcript was barely detectable in young grapevine buds, similar to the situation in the SAM of Arabidopsis and rice (Takada and Goto 2003; Tamaki et al. 2007). In these and other photoperiodic plants, transcription of FT is upregulated in the leaves in response to photoperiodic and vernalization signals, and the FT protein is then transported from the leaf to the SAM, where it promotes the transition to flowering. A similar scenario has been described for tomato, a day-neutral plant (Giakountis and Coupland 2008 and references therein). In light of this, we analyzed the transcript levels of VvFT in leaves sampled from basal and top node positions. The level of VvFT transcript was higher in leaves that originated from non-fruitful, basal positions than in those that originated from fruitful, top positions, until the beginning of flowering (Fig. 2). At that developmental stage, VvFT expression in the top leaves increased, reaching the level in the basal buds. At later stages, VvFT transcript level decreased similarly in both top and basal leaves (Fig. 2).
Effect of leaf position on the expression of VvFT. The level of VvFT transcript in leaves collected from basal (triangle) and top (square) positions for 10 weeks in 2003 was analyzed by quantitative real-time PCR using a standard curve. The level of expression was normalized against the amount of Actin transcript in each sample and relative expression (RE) values were established. Values for each date were obtained from two biologically independent samples and two repeats of RT-PCR for each sample, followed by two technical replicates for each real-time PCR. Standard error bars are indicated
In Arabidopsis, low expression of LFY is evident in leaf primordia, but after transition to reproductive growth, it is upregulated in the SAM. Shortly thereafter, AP1 is expressed in the transition meristem and it is assumed to be regulated by the LFY product. AP1 is also expressed in the first and second whorls of the flower and regulates floral organ formation (Blázquez et al. 2006; Turck et al. 2008).
Analysis of the transcript levels of VFL and VAP1, the grapevine homologs of LFY and AP1, respectively, was carried out throughout young bud development, using the above-described buds sampled from basal and top node positions. Comparative analysis of VFL temporal expression profiles revealed significantly higher transcript levels in the basal buds, before or at full bloom, and lasting until the end of the analyzed period. The higher transcript level in basal buds was consistently observed over three consecutive seasons (Fig. 3a and Fig. S3a).
Effect of bud position on the expression of VFL and VvTFL1A. The relative expression (RE) levels of VFL (a) and VvTFL1A (b) transcript in basal (triangle) and top (square) buds collected for 9–10 weeks in 2003 were analyzed as described in Fig. 2. Standard error bars are indicated. Results of similar analysis of buds collected at 2004 and 2005 are presented in Fig. S3
Similar analysis revealed that consistent with previous reports (Calonje et al. 2004), VAP1 transcript levels in both basal and top buds were undetectable or very low (at least 100-fold lower, compared with levels in mature woody buds) during the analyzed period in the three seasons. It was thus impossible to detect a consistent difference in VAP1 transcript level between top and basal buds (Fig. S4).
Effect of bud position on expression of grapevine homolog of TFL1
In Arabidopsis, TFL1 expression is upregulated in the inner cells of the SAM after transition, but in contrast to LFY and AP1, it represses transformation of the reproductive SAM into flower meristem, which thereby retains its meristematic identity, leading to increased branching. This, in turn, increases inflorescence size and the final number of flowers (Conti and Bradley 2007; Turck et al. 2008).
The effect of bud position on the transcription of VvTFL1A, the grapevine homolog of the Arabidopsis TFL1 gene (Carmona et al. 2007a), was analyzed throughout young bud development, as described above. VvTFL1A transcript levels in the top buds were significantly higher than in the basal buds before and during flowering. Later, the level in the top buds decreased and showed no significant difference from the level in the basal buds. The higher transcript level in top buds was consistently observed over the three analyzed seasons (Fig. 3b and Fig. S3b).
Based on the above findings, which ran counter to our initial assumption that expression of positive regulators will appear in fruitful buds, it was hypothesized that the development of the inflorescence in fruitful buds is a function of delayed acquisition of flower meristem characteristics by the UCM, positively regulated by VvTFL1A. Such a delay might allow extended branching of the UCM to form inflorescence structures. According to this hypothesis, decreased levels of positive regulators and increased levels of negative regulators might result in the transformation of a tendril into an inflorescence.
Effect of cytokinin-induced tendril apex branching on expression of VvTFL1A, VFL and VAP1
Using both rooted cuttings and explants in culture, it has been shown that application of exogenous cytokinin to either the shoot tip or tendrils less than 7 mm in length transforms cv. Thompson Seedless tendrils into inflorescences (Srinivasan and Mullins 1978, 1980b). To reveal possible relations between transformation of the tendril apex to an inflorescence-like structure and the expression of FMI genes, we monitored changes in cv. Thompson Seedless tendril morphology and transcript levels of VvTFL1A, VFL and VAP1 in parallel, following application of cytokinin to isolated young tendrils grown in culture.
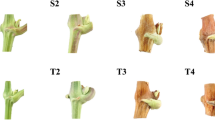
Seven days after cytokinin application, the treated tendrils were wider and flatter than the controls. At that time, the tip of the treated tendrils formed three separate meristematic fingers, as opposed to the control tendril in which the fingers were connected and curved (Fig. 4). After 14 days, the treated tendrils were much wider than the controls, and in a few of them, an inflorescence structure was visible. SEM analysis of control and cytokinin-treated tendrils carried out at this stage revealed inflorescence-containing floral branches covered by a bract below the treated tendril tips. This inflorescence did not contain flower organs on the floral branches (Fig. 4). In the control tendrils, no inflorescence could be seen. After 30 days, treated tendrils were either forming an inflorescence structure or divided at the tip into many meristematic branches (Fig. 4).
Analysis of the effect of cytokinin on cultured tendril phenotype. Tendrils were sampled from one to two nodes below the shoot tip of 15-node lateral shoots of cv. Thompson Seedless and were cultured on liquid McCown Woody plant medium. Cytokinin-treated cultures were supplemented with a final concentration of 10-μM PBA. Tendrils were examined with a stereomicroscope after 7, 14 and 30 days. Control and PBA-treated tendrils were analyzed after 14 days by SEM
Transcript levels of VvTFL1A, VFL and VAP1 were analyzed in treated and control tendrils after 14 days (Fig. 5). In the treated tendrils, VvTFL1A expression was upregulated to almost 14-fold that of the control (Fig. 5a) and that of VAP1 was 5-fold lower than in controls (Fig. 5b). There was no difference in VFL transcript level between the treated and control tendrils in response to exogenous cytokinin (Fig. 5c).
Effect of cytokinin on expression of VvTFL1A, VAP1 and VFL in cv. Thompson Seedless cultured tendrils. Tendrils were cultured as described in Fig. 6 and sampled from control and cytokinin-treated cultures 14 days after onset of culture for total RNA extraction. Relative expression (RE) of VvTFL1A (a), VAP1 (b) and VFL (c) in tendrils grown in the absence (gray bar) or presence (black bar) of 10-μM PBA. Expression analysis was as in Fig. 2
Spatial analysis of VvTFL1A in young tendrils
Fluorescence in situ hybridization (FISH) conducted with young tendrils suggested that VvTFL1A expression is restricted to the meristimatic tissue in the apex of the tendril arms. Closer inspection revealed VvTFL1A transcript in both the cytosolic and nuclear regions of proliferating cells (Fig. S5).
Effect of cytokinin on transcription from the VvTFL1A promoter
Cv. Sugarone transgenic embryogenic callus containing the pVvTFL1A::GFP::GUS transgene was transferred from callus maintenance medium to regeneration medium, with or without 2 mg/l zeatin in the growth medium. The cultures were examined twice a week by stereomicroscope. Strong signal of green fluorescence from GFP was observed in defined meristematic aggregates in the regenerating callus exposed to zeatin about 3 weeks after transfer to the regeneration medium (Fig. 6b). At the same time, controls, which were not exposed to zeatin, presented low GFP signal (Fig. 6a). Cultures were harvested after 2 and 3 weeks, total proteins were extracted and GUS activity was measured. In agreement with the GFP signal, GUS activity was low and similar in control and treated cells after 2 weeks on regeneration medium (expressed as nM product/mg protein in 1 min). However, significant difference was observed after 3 weeks, where GUS activity was three times higher in the cultures exposed to zeatin than in the controls (Fig. 6c). Similar analysis of nontransgenic calli revealed that exogenous cytokinin application increased endogenous TFL1A transcript level as well in a similar stage of development, while VFL transcript level of treated cells was not different from that of control (data not shown).
Effect of cytokinin on transcription from VvTFL1A promoter. Vitis vinifera cv. Sugarone embryonic callus carrying a pVvTFL1A::GFP::GUS transgene was grown on regeneration medium with or without zeatin for 3 weeks. Fluorescent signal of GFP was recorded in control (a) and zeatin-treated embryogenic cultures (b). (c) Analysis of β-glucuronidase (GUS) activity in control (gray) and zeatin-treated (black) embryogenic calli. Bars represent standard error, n = 4 (control), n = 5 (cytokinin-treated cells)
Effect of bud position on endogenous cytokinin levels
The effect of bud position on the level of endogenous cytokinins was determined throughout young bud development. Levels of both the cytokinin biosynthetic precursors [trans-zeatin riboside phosphate, N 6-(∆2-isopentenyl)adenosine phosphate, dihydrozeatin riboside phosphate; Fig. 7a] and the active cytokinins [sum of trans-zeatin, N 6-(∆2-isopentenyl)adenine, dihydrozeatin and their corresponding ribosides; Fig. 7b] were significantly higher in top than in the basal buds at all analyzed time points.
The content of active cytokinins and their precursors in top and basal buds during their development. a Cytokinin phosphates [trans-zeatin riboside phosphate, N 6-(∆2-isopentenyl)adenosine phosphate, dihydrozeatin riboside phosphate] content in top (purple) and basal (green) buds. b Content of total active cytokinins [trans-zeatin, N 6-(∆2-isopentenyl)adenine, dihydrozeatin and the corresponding ribosides] in top buds (dark purple) and basal buds (dark green), zeatin fraction (trans-zeatin and its riboside) in top buds (light purple) and basal buds (light green). Similar results were obtained from independent measurements in another year. Bars represent standard error
The most pronounced difference was observed before full bloom (weeks 1–3), during inflorescence initiation. At this time, levels of cytokinin phosphates [mainly of trans-zeatin riboside phosphate and N 6-(∆2-isopentenyl) adenosine phosphate, which represented about 55 and 35% of the phosphates, respectively, at these stages] were sevenfold (week 1) and fourfold (week 3) higher in the top buds compared to the basal buds. During the same period, a threefold higher content of active cytokinins (mainly of trans-zeatin and its riboside, which accounted for two-thirds of the active cytokinins) was observed in top buds compared to the levels in basal buds. During flowering (weeks 4, 5), the differences between top and basal buds became smaller, but the levels of active cytokinins in the top buds were still at least twofold higher than in the basal ones. After flowering (week 6), the differences were minimized. It is important to note that levels of both cytokinin biosynthetic precursors and active cytokinins in top buds sampled as late as week 4 were at least twofold higher than that of basal bud in its youngest stage (week 1).
A relatively high concentration of cytokinin O-glucosides was observed during the entire tested period, similar in the top and basal buds. Irreversible cytokinin inactivation (cytokinin N-glycosylation) was very low in grapevine, with no distinct trend observed between bud positions (data not shown).
Discussion
Since tendril and inflorescence represent sequential steps during reproductive meristem differentiation, factors that control the progression of floral meristem development may take part in the final outcome and affect fruitfulness (see simplified hypothetical model in Fig. 8). Thus, we initially attempted to relate the degree of bud fruitfulness to the expression of grape homologs of the Arabidopsis master regulators of inflorescence and flower development.
A hypothetical model for differentiation of the UCM. Orange triangle represents the degree of UCM branching. Dark green circles represent the SAM and light green circles the UCM branches. Stages 1 and 2 are common to both tendril and inflorescence development: 1 differentiation of UCM from SAM; 2 first and second cycle of UCM branching. TP represents a transition point in which the final fate of UCM is determined based on hormonal balance and interplay between positive and negative regulators of FMI. According to the hypothetical model, high GA and high FT level favor early FMI and lead to termination of branching, resulting in tendril formation. High cytokinin level upregulates VvTFL1A, which delays FMI and increases branching and thus lead to cluster formation
Analyses of VvTFL1A and VFL transcript levels during flowering, the timing of inflorescence differentiation within the buds, revealed higher VFL transcript level in the non-fruitful buds, while VvTFL1A level was higher in the fruitful buds. These findings were contrary to our initial assumption that the level of positive regulators would be higher in fruitful buds.
The transcript level of the grapevine homolog of FT appears to be barely detectable in grapevine buds (current analysis; Sreekantan and Thomas 2006; Carmona et al. 2007a), similar to the situation in Arabidopsis and rice SAM, where the protein is mainly transported from the leaves (Takada and Goto 2003; Tamaki et al. 2007). It is therefore assumed that the VvFT protein is transported from the leaves to the adjacent developing buds, as described in Arabidopsis, tomato and rice (Giakountis and Coupland 2008 and references therein), and that its transcript levels in leaves originating from fruitful and non-fruitful positions might be informative. Indeed, the level of VvFT transcript was significantly higher in leaves collected from the basal non-fruitful positions, compared with those from the top fruitful positions, until the beginning of flowering, in agreement with the higher level of VFL in non-fruitful buds.
Combining these findings with results from previous expression studies in grapevine and Arabidopsis, the possibility was raised that in the fruitful buds, a higher level of VvTFL1A might maintain the meristematic identity of the UCM for a longer time and allow further branching. According to the suggested scenario, a branched inflorescence meristem is gradually produced, whose branches ultimately acquire FMI and yield a higher number of floral meristems relative to the tendril meristem.
To further explore the possibility that VvTFL1A expression may be involved in the control of grapevine inflorescence differentiation, we asked whether it is affected by cytokinin, a positive regulator of grapevine inflorescence differentiation. For this analysis, we exploited the demonstrated positive effect of cytokinin on transformation of cultured tendrils into inflorescences (Srinivasan and Mullins 1978, 1980b), and associated between cytokinin application, acquisition of a branched inflorescence appearance and remarkable upregulation of VvTFL1A expression, as well as downregulation of VAP1 expression. Cytokinin-mediated inflorescence regeneration from Arabidopsis callus was similarly accompanied by TFL1 induction (Guan et al. 2006). These data support the hypothesis that VvTFL1A expression may be involved in the control of grapevine inflorescence differentiation. They also suggest that a transition point exists during tendril development, which may be delayed by cytokinin, due to the modification it exerts on the developmental program that naturally leads to VvTFL1A downregulation and VvAP1 upregulation. Such a delay might negatively affect early acquisition of FMI and facilitate excess branching, which transforms the tendril into an inflorescence-like structure. The existence of such a transition point may be supported by the ability to transform young tendrils, but not ‘mature’ ones, into inflorescence-like structures. It is also supported by the expression profile of VvTFL1A in the tendrils of cv. Sugarone (Fig. S6) and the reiterated reproductive meristem (RRM) mutant (Fernandez et al. 2010), which declined as the tendrils matured. VvTFL1A downregulation and VvAP1 upregulation, during tendril development and during completion of inflorescence development at bud break were shown for other grapevine varieties (Carmona et al. 2002; Calonje et al. 2004). This has been confirmed in the current study (data not shown) and serves as additional support for the assumption raised above.
Based on the above, it was assumed that the increased level of VvTFL1A expression in the fruitful bud is the result of a higher level of active cytokinins. Higher level of active cytokinin and their precursors in the top buds, compared with the basal buds, during the stages of inflorescence differentiation, in particular, and throughout a major period of bud development, in general, supported this rationale. The twofold higher level of active cytokinin and their precursors in top buds sampled at week 4 compared to basal buds that were sampled a month earlier may support the assumption that difference in position, rather than the younger stage of top buds, may explain the difference in cytokinin content.
Positive regulation of VvTFL1A expression by cytokinin was further confirmed by upregulation of GFP and GUS activity in transgenic embryogenic culture carrying GFP and GUS reporter genes under the control of the VvTFL1A promoter in the presence of cytokinin. It is important to note that GFP expression was confined to defined meristematic aggregates during regeneration of non-synchronic embryogenic culture. This may suggest that additional factors other than cytokinin are involved in the regulation of transcription from the promoter of VvTFL1A and are developmentally regulated. A similar situation was reported in Arabidopsis regenerating callus (Guan et al. 2006).
Taken together, the results of the current analysis suggest that the positive effect of cytokinin on fruitfulness may be mediated by its ability to induce expression of VvTFL1A, which controls branching and timing of acquisition of flower meristem characteristics. This scenario is in full agreement with the recent finding that transposon-driven increase of the VvTFL1Aa allele expression level (without affecting developmental timing of expression), in a RRM somatic variant of cv. Carignan, is correlated with increased branching of the cluster (Fernandez et al. 2010).
Such a scenario implies a higher degree of conservation between grapevine and Arabidopsis than previously considered (Boss et al. 2003; Carmona et al. 2007b, 2008), since Arabidopsis TFL1 enhances inflorescence branching, delays FMI acquisition and is regulated by cytokinin (Ratcliffe et al. 1998; Guan et al. 2006). It should be noted that VvTFL1Aa shows higher homology to the snapdragon CEN gene, compared to the Arabidopsis TFL1 (Carmona et al. 2007a). While CEN is induced only during the reproductive phase and control inflorescence indeterminacy, TFL1 expression also is found during the vegetative phase and prolongs it (Bradley et al. 1996, 1997). However, when development of a reproductive meristem is discussed, both CEN and TFL1 play a role in regulating inflorescence branching, and such function was confirmed for VvTFL1Aa both in Arabidopsis and grapevine (Carmona et al. 2007a; Fernandez et al. 2010). It appears that poplar presents similar conservation in the control of flowering and inflorescence architecture, based on the function of its FT, LFY and CEN/TFL homologs. In the current context, it is important to note that poplar CEN/TFL homologs are significantly upregulated during inflorescence differentiation, their absence leads to a decreased number of flowers, and they have a positive role in maintaining the indeterminacy of the inflorescence apex (Mohamed et al. 2010 and references therein). Interestingly, over-expression of rice and maize CEN/TFL1 also lead to an increase in the number of secondary branches on the rice panicle and the maize tassel (Nakagawa et al. 2002; Danilevskaya et al. 2010). These findings may imply conservation of CEN/TFL1-like function in inflorescence development between dicots and monocots.
Within the framework of the current discussion, we suggest an educated guess, schematically described in Fig. 8, for which we do not have direct empirical support. Based on the assumption that inflorescence differentiation in fruitful buds is the result of a delay in FMI acquisition and intense branching, we speculate that in non-fruitful buds, UCMs are exposed to strong floral stimuli at the time of inflorescence initiation, which mediate early acquisition of FMI and limited branching. The result is a tendril phenotype that shares similarities with the terminal flower phenotype in Arabidopsis (Shannon and Meeks-Wagner 1991). We suppose that this hypothetical scenario describes the developmental process taking place in the shoot apex as well.
At this point, this guess may be supported by the higher level of positive regulators (VvFT and VFL) in non-fruitful nodal positions. It may also be supported by the transient existence of VvTFL1A in the developing tendril, accompanied by an early increase in VFL and later induction of VAP1 expression (Calonje et al. 2004; current study), which may be involved in inhibition of further branching as exemplified in Arabidopsis (Simon et al. 1996; Ratcliffe et al. 1999). Localization of VAP1 transcript exclusively in the tendril arms, maximal expression of VAP1 in the oldest tendril (Calonje et al. 2004) and inhibition of VAP1 expression during cytokinin-induced branching of cultured tendrils also support the assumption that tendril formation is the result of early acquisition of FMI.
The inhibitory effect of GA on grapevine inflorescence development and the increased fruitfulness reported in the absence of GA stimulus (Coombe 1967; Sugiura et al. 1976; Boss and Thomas 2002; Carmona et al. 2007b, 2008) may support the suggested hypothesis as well. This is due to a possible inductive effect of GA, a strong floral stimulus in Arabidopsis, on floral meristem differentiation, which leads to early branching termination, causing tendril development instead of inflorescences, as suggested by the hypothetical model described in Fig. 8.
In this regard, it should be mentioned that it is not yet known why the suggested acquisition of FMI in the tendril apex does not regularly lead to flower formation. Nevertheless, tendrils with a flower on their tip, resembling the Arabidopsis tfl1 mutant (Shannon and Meeks-Wagner 1991; Ratcliffe et al. 1998), are often seen in the field. Such a phenotype supports the proposed hypothetical model (see Fig. 8) and raises the possibility that interactions between genetic, environmental and hormonal parameters may exert an additional level of control over flower organs differentiation.
Abbreviations
- AP1:
-
Apetala1
- CEN:
-
Centroradialis
- FMI:
-
Flower meristem identity
- FT:
-
Flowering locus 1
- GA:
-
Gibberellic acid
- GFP:
-
Green fluorescent protein
- GUS:
-
Beta-glucoronidase
- LFY:
-
Leafy
- RRM:
-
Reiterated reproductive meristem
- SAM:
-
Shoot apical meristem
- TFL1:
-
Terminal flower 1
- UCM:
-
Uncommitted meristem
- Vv:
-
Vitis vinifera
References
Benlloch R, Berbel A, Serrano-Mislata A, Madueño F (2007) Floral initiation and inflorescence architecture: a comparative view. Ann Bot 100:659–676
Blázquez MA, Ferrandiz C, Madueño F, Parcy F (2006) How floral meristems are built. Plant Mol Biol 60:855–870
Boss PK, Thomas MR (2002) Association of dwarfism and floral induction with a grape ‘green revolution’ mutation. Nature 416:847–850
Boss PK, Buckeridge EJ, Pool A, Thomas MR (2003) New insights into grapevine flowering. Funct Plant Biol 30:593–606
Boss PK, Sreekantan L, Thomas MR (2006) A grapevine TFL1 homologue can delay flowering and alter floral development when overexpressed in heterologous species. Funct Plant Biol 33:31–41
Bradley D, Carpenter R, Copsey L, Vincent C, Rothstein S, Coen E (1996) Control of inflorescence architecture in Antirrhinum. Nature 379:791–797
Bradley D, Ratcliffe O, Vincent C, Carpenter R, Coen E (1997) Inflorescence commitment and architecture in Arabidopsis. Science 275:80–83
Buttrose MS (1974) Climatic factors and fruitfulness in grapevines. Hortic Abstr 44:319–326
Calonje M, Cubas P, Martinez-Zapater JM, Carmona MJ (2004) Floral meristem identity genes are expressed during tendril development in grapevine. Plant Physiol 135:1491–1501
Carmona MJ, Cubas P, Martinez-Zapater JM (2002) VFL, the grapevine FLORICAULA/LEAFY ortholog, is expressed in meristematic regions independently of their fate. Plant Physiol 130:68–77
Carmona MJ, Calonje M, Martínez-Zapater JM (2007a) The FT/TFL1 gene family in grapevine. Plant Mol Biol 63:637–650
Carmona MJ, Cubas P, Calonje M, Martínez-Zapater JM (2007b) Flowering transition in grapevine (Vitis vinifera L.). Can J Bot 85:701–711
Carmona MJ, Chaib J, Martínez-Zapater JM, Thomas MR (2008) A molecular genetic perspective of reproductive development in grapevine. J Exp Bot 59:2579–2596
Conti L, Bradley D (2007) TERMINAL FLOWER1 is a mobile signal controlling Arabidopsis architecture. Plant Cell 19:767–778
Coombe BG (1967) Effects of growth retardants on Vitis vinifera L. Vitis 6:278–287
Danilevskaya ON, Meng X, Ananiev EV (2010) Concerted modification of flowering time and inflorescence architecture by ectopic expression of TFL1-like genes in maize. Plant Physiol 153:238–251
Dobrev PI, Kaminek M (2002) Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J Chromatogr A950:21–29
Dry PR (2000) Canopy management for fruitfulness. Aust J Grape Wine Res 6:109–115
Fernandez L, Torregrosa L, Segura V, Bouquet A, Martinez-Zapater JM (2010) Transposon-induced gene activation as a mechanism generating cluster shape somatic variation in grapevine. Plant J 61:545–557
Giakountis A, Coupland G (2008) Phloem transport of flowering signals. Curr Opin Plant Biol 11:687–694
Gottlieb Y, Ghanim M, Chiel E, Gerling D, Portnoy V, Steinberg S, Tzuri G, Horowitz AR, Belausov E, Mozes-Daube N, Kontsedalov S, Gershon M, Gal S, Katzir N, Zchori-Fein E (2006) Identification and localization of a Rickettsia sp. in Bemisia tabaci (Homoptera: Aleyrodidae). Appl Environ Microbiol 72:3646–3652
Guan CM, Zhu SS, Li XG, Zhang XS (2006) Hormone-regulated inflorescence induction and TFL1 expression in Arabidopsis callus in vitro. Plant Cell Rep 25:1133–1137
Halaly T, Pang X, Batikoff T, Crane O, Keren A, Venkateswari J, Ogrodovitch A, Sadka A, Lavee S, Or E (2008) Similar mechanisms might be triggered by alternative external stimuli that induce dormancy release in grape buds. Planta 228:79–88
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Joly D, Perrin M, Gertz C, Kronenberger J, Demangeat G, Masson JE (2004) Expression analysis of flowering genes from seedling-stage to vineyard life of grapevine cv. Riesling. Plant Sci 166:1427–1436
Lavee S, Regev U, Samish RM (1967) The determination of induction and differentiation in grape vines. Vitis 6:1–13
May P (2004) Flowering and fruitset in grapevines. Lythrum Press, Adelaide
Mohamed R, Wang CT, Ma C, Shevchenko O, Dye SJ, Puzey JR, Etherington E, Sheng X, Meilan R, Strauss SH, Brunner AM (2010) Populus CEN/TFL1 regulates first onset of flowering, axillary meristem identity and dormancy release in Populus. Plant J 62:674–688
Morrison JC (1991) Bud development in Vitis vinifera L. Bot Gaz 152:304–315
Mullins MG, Bouquet A, Williams LE (1992) Biology of the grapevine. Cambridge University Press, UK
Nakagawa M, Shimamoto K, Kyozuka J (2002) Overexpression of RCN1 and RCN2, rice TERMINAL FLOWER/CENTRORADIALIS homologs, confers delay of phase transition and altered panicle morphology in rice. Plant J 29:743–750
Perl A, Eshdat Y (2007) Grapes. In: Pua EC, Davey MR (eds) Biotechnology in agriculture and forestry—transgenic crops V, vol 60. Springer-Verlag, Berlin, pp 189–208
Pratt C (1971) Reproductive anatomy in cultivated grapes—a review. Am J Enol Vitic 22:92–109
Pratt C (1974) Vegetative anatomy in cultivated grapes—a review. Am J Enol Vitic 25:131–150
Ratcliffe OJ, Amaya I, Vincent CA, Rothstein S, Carpenter R, Coen ES, Bradley DJ (1998) A common mechanism controls the life cycle and architecture of plants. Development 125:1609–1615
Ratcliffe OJ, Bradley D, Coen ES (1999) Separation of shoot and floral identity in Arabidopsis. Development 126:1109–1120
Reid KE, Olsson N, Schlosser J, Peng F, Lund ST (2006) An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biol 6:27
Shannon S, Meeks-Wagner DR (1991) A mutation in the Arabidopsis TFL1 gene affects inflorescence meristem development. Plant Cell 3:877–892
Simon R, Igeno MI, Coupland G (1996) Activation of floral meristem identity genes in Arabidopsis. Nature 384:59–62
Sreekantan L, Thomas MR (2006) VvFT and VvMADS8, the grapevine homologues of the floral integrators FT and SOC1, have unique expression patterns in grapevine and hasten flowering in Arabidopsis. Funct Plant Biol 33:1129–1139
Srinivasan C, Mullins MG (1976) Reproductive anatomy of the grape-vine (Vitis vinifera L.): origin and development of anlage and its derivatives. Ann Bot 38:1079–1084
Srinivasan C, Mullins MG (1978) Control of flowering in the grapevine (Vitis vinifera L.). Plant Physiol 61:127–130
Srinivasan C, Mullins MG (1980a) Effects of temperature and growth regulators on formation of anlagen, tendrils and inflorescences in Vitis vinifera L. Ann Bot 45:439–446
Srinivasan C, Mullins MG (1980b) Flowering in Vitis: effects of genotype on cytokinin-induced conversion of tendrils into inflorescences. Vitis 19:293–300
Srinivasan C, Mullins MG (1981) Physiology of flowering in the grapevine—a review. Am J Enol Vitic 32:47–61
Sugiura A, Utsunomiya N, Tomana T (1976) Induction of inflorescence by CCC application on primary shoots of grapevines. Vitis 15:88–95
Takada S, Goto K (2003) TERMINAL FLOWER2, an Arabidopsis homolog of HETEROCHROMATIN PROTEIN1, counteracts the activation of FLOWERING LOCUS T by CONSTANS in the vascular tissues of leaves to regulate flowering time. Plant Cell 15:2856–2865
Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K (2007) Hd3a protein is a mobile flowering signal in rice. Science 316:1033–1036
Turck F, Fornara F, Coupland G (2008) Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu Rev Plant Biol 59:573–594
Author information
Authors and Affiliations
Corresponding author
File S1. Expression summary. Summary of published expression analyses of VFL, VAP1, VvTFL1A and VvFT in grapevine
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Crane, O., Halaly, T., Pang, X. et al. Cytokinin-induced VvTFL1A expression may be involved in the control of grapevine fruitfulness. Planta 235, 181–192 (2012). https://doi.org/10.1007/s00425-011-1497-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-011-1497-6