Abstract
Despite extensive research over the past years, regeneration from protoplasts has been observed in only a limited number of plant species. Protoplasts undergo complex metabolic modification during their isolation. The isolation of protoplasts induces reactive oxygen species (ROS) generation in Brassica napus leaf protoplasts. The present study was conducted to provide new insight into the mechanism of ROS generation in B. napus leaf protoplasts. In vivo localization of H2O2 and enzymes involved in H2O2 generation and detoxification, molecular antioxidant-ascorbate and its redox state and lipid peroxidation were investigated in the leaf and isolated protoplasts. Incubating leaf strips in the macerating enzyme (ME) for different duration (3, 6, and 12 h) induced accumulation of H2O2 and malondialdehyde (lipid peroxidation, an index of membrane damage) in protoplasts. The level of H2O2 was highest just after protoplast isolation and subsequently decreased during culture. Superoxide generating NADPH oxidase (NOX)-like activity was enhanced, whereas superoxide dismutase (SOD) and ascorbate peroxidase (APX) decreased in the protoplasts compared to leaves. Diaminobenzidine peroxidase (DAB-POD) activity was also lower in the protoplasts compared to leaves. Total ascorbate content, ascorbate to dehydroascorbate ratio (redox state), were enhanced in the protoplasts compared to leaves. Higher activity of NOX-like enzyme and weakening in the activity of antioxidant enzymes (SOD, APX, and DAB-POD) in protoplasts resulted in excessive accumulation of H2O2 in chloroplasts of protoplasts. Chloroplastic NADPH oxidase-like activity mediated perpetual H2O2 generation probably induced apoptotic-like cell death of B. napus leaf protoplasts as indicated by parallel DNA laddering and decreased mitochondrial membrane potential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability to regenerate protoplasts into plantlets provides a multitude of opportunities for crop improvement, including a system for protoplast fusion (somatic hybridization), somaclonal variation, and plant transformation. In addition, plant protoplasts are an efficient experimental model for physiological and molecular studies (Vasil 1987). Despite being provided with an osmoprotectant and optimal pH in the external medium, protoplasts isolated by enzymatic methods display recalcitrance and often exhibit a change in their cellular redox environment, which may initiate a cascade leading to programmed cell death (PCD). In higher plants, PCD plays a vital role in biogenesis and morphogenesis. Similar to apoptosis in animal cells, some biotic and abiotic stimuli trigger the death pathway in plants, resulting in mitochondrial dysfunction, ROS generation, and the activation of certain proteases (Greenberg 1996; Rogers 2006). In particular, PCD has been related to aleurone and root cap cell elimination, somatic embryogenesis, leaf and petal senescence, xylogenesis, reproduction (Greenberg 1996; Pennell and Lamb 1997), and aerenchyma formation (Drew et al. 2000). Moreover, PCD is enhanced in response to biotic and abiotic stresses (Greenberg 1996; Pennell and Lamb 1997; Beers and McDowell 2001; Lam et al. 2001). In plant cells, adverse environmental stresses can increase ROS levels due to perturbations of the cellular redox balance, leading to oxidative damage and subsequent cell death (Apel and Hirt 2004; Vacca et al. 2006; Gao et al. 2008). The production of ROS, especially H2O2, and mitochondrial dysfunction are necessary components and important indicators of PCD in response to various stimuli (Gao et al. 2008; Zhang and Xing 2008; Zhang et al. 2009b). H2O2 acts in a dose-dependent manner and synergistically with nitric oxide in hypersensitive cell death (Delledonne et al. 2001; Zago et al. 2006). It has also been reported that H2O2 levels are increased during senescence of the Phalaenopsis flower (a slowly senescing flower), due to elevated activity of xanthine oxidase, an enzyme capable of O2 ·− generation (Tewari et al. 2009).
Major sources of H2O2 in plant cells include the leakage of high-potential electrons from the saturated electron transport chains of chloroplasts and mitochondria, the Mehler reaction, a wide variety of limited substrate oxidases, NADPH oxidase (NOX), and type III peroxidase (Halliwell and Gutteridge 1999; Cheeseman 2007; Doyle et al. 2010). Peroxisomes are also included among the sites of intracellular H2O2 production (Cheeseman 2007). NADPH oxidase, a plasma membrane protein, oxidizes NADPH at the cytosolic surface and reduces O2 to O2 ·− at the outer surface of the plasma membrane (Sagi and Fluhr 2006; Zhang et al. 2009a), which spontaneously, or via superoxide dismutase (SOD), is dismutated to H2O2 (Cheeseman 2007). Recent studies have indicated that RBOH A and B (respiratory burst oxidase homologue, NOX genes) from Nicotiana benthamiana function in elicitor-induced NO and H2O2 generation and stomatal closure, but not in elicitor-induced free radical generation, hypersensitive cell death, or activation of the pathogenesis-related gene, PR1 (Zhang et al. 2009a).
Brassica napus leaf protoplasts initiate senescence during isolation and senesce thereafter without cell division (Watanabe et al. 1998, 2002a). B. napus leaf protoplasts die through PCD or apoptosis, which is similar to that observed in animal cells with a few morphological differences (Watanabe et al. 2002b). H2O2 has been reported to be involved in PCD (Zhang et al. 2009b; Doyle et al. 2010). ROS generation and antioxidant enzyme activities had been analyzed before (Papadakis et al. 2001). Yasuda et al. (2007) previously tried to inhibit ROS generation by including DPI (diphenyleneiodonium chloride) in a macerating enzyme (ME) solution but such an addition had no effect on H2O2 accumulation in the chloroplasts of protoplasts. DPI seems to be degraded or inactivated in MEs during the incubation period and does not lead to cell divisions of protoplasts. In the present study, we focused on the process of H2O2 generation in B. napus leaf tissue, chloroplasts and protoplasts and presented experimental evidence for the existence of DPI-sensitive NADPH oxidase-like activity and inhibition of H2O2 accumulation in the chloroplasts. Nonetheless, we also provided flow cytometric analysis of temporal changes in mitochondrial membrane potential as an index of apoptotic cell death in B. napus leaf protoplasts.
Materials and methods
Plant material, protoplast isolation, and culture
Seeds of Brassica napus L. cv. Bronowski were obtained from the National Institute of Agrobiological Resources (Tsukuba, Japan). Plants were grown in a growth chamber under controlled light flux (50 μmol m−2 s−1), photoperiod (14 h), and temperature (day/night cycle of 25/20°C) conditions. Protoplasts were prepared from the leaves of 6- to 8-week-old plants (Watanabe et al. 1992). Sterilized B. napus leaves were cut into narrow strips and incubated in a macerating enzyme (ME) solution containing 5 mM Mes (pH 5.8) with 0.6 M sorbitol, 5 mM CaCl2, 0.5% cellulase Y-C (Kyowa Kasei, Osaka, Japan) and 0.03% pectolyase Y-23 (Kyowa Kasei), for different duration (3, 6 and 12 h). Incubating leaf strips in ME for 3 or 6 h did not remove cell wall completely and exhibited oxidant and antioxidant responses similar to those isolated by 12 h exposure in ME. Thus, protoplast isolated by 12 h exposure in ME was used for further study. For corresponding controls, leaf strips were incubated in the 5 mM Mes buffer (pH 5.8) with 0.6 M sorbitol and 5 mM CaCl2 (without ME). The isolated protoplasts (5 × 105) were cultured in Petri dishes in MS medium (Murashige and Skoog 1962) supplemented with 4.5 mM 2,4-dichlorophenoxyacetic acid (2,4-D), 2.2 mM benzyl adenine (BA), 2% sucrose, 1% glucose and 0.5 M sorbitol (pH 5.8). The bottom of each dish was layered with 1.5% agar to prevent the adhesion of protoplasts. The dishes were maintained in darkness at 25°C.
Confocal microscopic and flow cytometric examinations of the mitochondrial membrane potential
For the measurement of mitochondrial membrane potential, 10 μM 3,3′-dihexyloxacarbocyanine iodide (DiOC6, Molecular Probes, Eugene, OR, USA) was added to a protoplast suspension (5 × 105). After incubation at 25°C for 30 min, the protoplasts were washed twice with 10 mM phosphate buffer containing 0.6 M sorbitol, resuspended in the same buffer, and observed under a Leica confocal laser scanning system (Leica TCS-SP2; Leica Microsystems, Tokyo, Japan) using laser excitation at 488 nm and emission at 540 nm to visualize the fluorescent probe.
Protoplasts were analyzed using an FC500 flow cytometer (Beckman Coulter, Tokyo, Japan) to monitor changes in the mitochondrial membrane potential. For all flow cytometric analyses, unstained protoplasts, collected at the time of protoplast isolation or from the same culture time, were used as autofluorescence controls. The term ‘FL1’ with the channel number refers to the quantified fluorescence at 525 nm. The fluorescence intensity was plotted on a log scale with maximum and minimum fluorescence levels of 1,000 and 1, respectively. The peak height and area in the histograms represent the number of protoplasts. Linearity of the flow cytometer was ensured using Flow Check beads with the QC 1L Flow Check protocol (Beckman Coulter). To measure the mitochondrial membrane potential, samples were stained with 500 nM DiOC6 for 30 min in the dark and analyzed immediately. DiOC6 was excited with the 488-nm line of an argon ion laser, and the fluorescence signals were detected by the FL1 detector. At least 10,000 protoplasts were analyzed using the peak height or time versus the integral of the DiOC6 fluorescence signal. Data were analyzed using Cytomics FC500 RXP software (Beckman Coulter).
Genomic DNA fragmentation
Genomic DNA of protoplasts was isolated using a Floraclean plant genomic DNA isolation kit (BIO 101, Vista, CA, USA), according to the instruction manual. DNA was separated in a 2% agarose gel, stained with ethidium bromide, and photographed under a UV-transilluminator.
Localization of H2O2 production
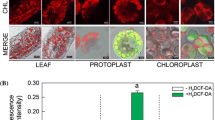
H2O2 production was measured by monitoring the fluorescence of dichlorofluorescein (DCF), which is the oxidation product of H2DCF (Allan and Fluhr 1997). As cell-permeant indicator of H2O2 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA; Molecular Probes) is non-fluorescent until the acetate groups are removed by intracellular esterases and oxidation occurs within the cell. The protoplasts were washed, resuspended in 0.6 M sorbitol in 5 mM Mes buffer (pH 5.8) and 5 mM CaCl2, and loaded with either 70 μM H2DCF-DA for 30 min in darkness. After washing the protoplasts with 0.6 M sorbitol in 5 mM Mes buffer and 5 mM CaCl2 the fluorescence signals from DCF and chlorophyll were observed at emission wavelengths of 494–559 and 680–730 nm, respectively, with an excitation wavelength of 488 nm in a Leica confocal laser scanning system (TCS-SP2; Leica). Microscope settings, for example, laser power (fixed by marking), the pinhole diameter, the objective lens, and the setting of the photomultipliers (fixed by noted digital values) were keep essentially same for individual set experiments. Excitation and emission bandwidth was saved as a file on OS for further operation with same settings. Experiment was repeated several times and relative fluorescence intensity of representative images was measured by selecting region of interest (whole cell) using ImageJ 1.42 software (http://rsb.info.nih.gov/ij/) and data was normalized against background.
Chloroplast isolation from protoplasts
Chloroplasts were isolated as described by Kieselbach et al. (1998), with minor modifications. Briefly, the protoplasts were suspended in the chloroplast isolation medium containing 0.4 M sorbitol, 50 mM Hepes (pH 7.8), 1 mM MgCl2, 10 mM KCl, 1.0 mM EDTA, and 0.15% (w/v) bovine serum albumin. To release the chloroplasts, the protoplasts were broken by passage through 20-μm nylon net that was attached over the tip of a 5.0-ml disposable plastic syringe (without needle). The protoplasts were twice drawn into the syringe through the nylon net and dispersed. Microscopic observation revealed that this treatment resulted in the complete rupture of the protoplasts and the release the chloroplasts. The chloroplasts were sedimented by centrifugation of the protoplast homogenate at 1,000g for 1.5 min, and then washed with chloroplast isolation medium and extracted in enzyme extraction buffer. For purification, chloroplast suspension was loaded on 35% Percoll and centrifuged at 2,000g for 10 min. Pellet was re-suspended in the chloroplast isolation medium and centrifuged at 1,000g for 1.5 min to sediment chloroplasts.
Enzyme extraction, protein, and plasma membrane marker determination
Young leaves strips incubated in the 5 mM Mes buffer (pH 5.8) with 0.6 M sorbitol and 5 mM CaCl2 (without ME) were homogenized in 10 ml of chilled 50 mM potassium phosphate buffer (pH 7.0) containing 0.5% (w/v) insoluble polyvinylpolypyrrolidone and 1 mM phenylmethylsulfonylfluoride, using a chilled pestle and mortar on an ice bath. The homogenate was filtered through two-fold miracloth, followed by centrifugation at 15,000g for 10 min at 4°C. The supernatant was stored at 4°C and used for enzyme assays within 4 h. For the assay of APX activity, 5.0 mM ascorbic acid was also included in the extraction medium. Protoplast and chloroplast suspensions were homogenized in the same extraction medium using a Dounce homogenizer with a tight-fitting Teflon pestle, followed by centrifugation as described above. Protein concentrations were determined according to the method of Bradford (1976). Protoplast and chloroplasts extracts were assayed for vanadate-inhibited, Mg2+-ATPase and 5′-nucleotidase activities respectively after Truitt et al. (2004) and Schimmel et al. (1973).
Enzyme assays
NADPH oxidase-like (NOX-like, EC 1.6.3.1) enzyme activity was determined as described previously (Tewari et al. 2009). This modified assay is based on the reduction of 2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide sodium salt (XTT) by O2 ·− generated by the oxidation of NADPH. The reaction mixture contained 50 μl of enzyme extract, 0.3 mM XTT, 0.2 mM NADPH, 0.1 mM MgCl2, and 1.0 mM CaCl2 in 1 ml of 50 mM Tris–HCl (pH 7.4) except for where it stated otherwise. The reaction was initiated by the addition of NADPH, and the change in absorbance at 470 nm was recorded. NADPH oxidase-like activity was presented as unit (μmol XTT reduced min−1) mg−1 protein.
Superoxide dismutase (SOD, EC 1.15.1.1) activity was assayed by measuring its ability to inhibit the photochemical reduction of nitro-blue tetrazolium (NBT) at 560 nm. The reaction mixture (5.0 ml) contained 25 mM phosphate buffer (pH 7.8), 65 μM NBT, 2 μM riboflavin, enzyme extract, and 15 μl of N,N,N′,N′-tetramethylethylenediamine (TEMED; modified from Beauchamp and Fridovich (1971). The reaction mixture was exposed to light (350 μmol m−2 s−1) for 15 min. The amount of SOD corresponding to 50% inhibition of the reaction was defined as one unit of enzyme.
Catalase (CAT, EC 1.11.1.6) activity was measured after Beers and Sizer (1952). Briefly, 25 μl of the extract was added to 3 ml of 5 mM H2O2 in 50 mM potassium phosphate (pH 7.0). The decrease in absorbance at 240 nm was monitored for 3 min, and the linear part of the curve was used to quantitate the rate of decrease by using an extinction coefficient of H2O2 at 240 nm of 0.0435 mM−1 cm−1. CAT activity was presented as unit (μmol H2O2 decomposed min−1) mg−1 protein.
Ascorbate peroxidase (APX, EC 1.11.1.11) was measured in a 3.0 ml reaction mixture containing 50 mM phosphate buffer (pH 7.0), 0.5 mM AsA, 0.1 mM H2O2, 0.1 mM EDTA, and a suitable quantity of enzyme extract. The blanks included reactions without tissue extract and without H2O2. The change in absorbance at 290 nm was measured every 15 s (Nakano and Asada 1981). The activity of APX was expressed as unit (μmol AsA oxidized min−1) mg−1 protein).
Native PAGE and visualization of enzymes on native gels
Enzyme isoforms were separated by polyacrylamide gel electrophoresis in a discontinuous gel under non-denaturing conditions, as described by Hames (1990). Native gels were run at constant current (30 mA) at 4°C. NOX, SOD, and guaiacol peroxidase (POD) were visualized on native gels as described previously (Tewari et al. 2008). NADPH oxidase-like activity was visualized on a 12.5% native gel by soaking the gel in a reaction mixture containing 50 mM Tris–HCl (pH 7.4), 0.2 mM NBT, 0.1 mM MgCl2, 1 mM CaCl2, and 0.2 mM NADPH in the absence and presence of 200 U of SOD (O2 ·− scavenger) and 100 μM DPI (NOX inhibitor).
Superoxide dismutase (SOD) activity was visualized after Beauchamp and Fridovich (1971) by soaking the gel in 2.45 mM NBT for 20 min, followed by immersion in a solution containing 28.0 mM TEMED, 0.028 mM riboflavin, and 36 mM potassium phosphate buffer (pH 7.8). The gel was then placed on a clean glass plate and illuminated under cool fluorescent light for 2–3 min.
Guaiacol POD was separated in a 12.5% native gel and was visualized by soaking the gel in a reaction mixture containing 20 ml of 0.1 M phosphate buffer (pH 7.0), 4 ml of 0.01% (v/v) H2O2, and 4.0 ml of 0.5% (w/v) guaiacol for 20 min. For the detection of DAB-POD activity gel was stained in a reaction mixture containing 0.1 M sodium acetate buffer (pH 5.5) containing 1 mM 3,3-diaminobenzidine (DAB) and 0.03% H2O2 (Wang et al. 2009). All gels were rinsed with distilled water and then imaged.
Ascorbate, dehydroascorbate and lipid peroxidation
Fresh leaf tissue (250 mg) was homogenized in 2.0 ml of 10% (w/v) trichloroacetic acid, followed by centrifugation at 10,000g for 5 min. Total ascorbate, which was determined after the reduction of dehydroascorbate (DHA) to AsA by dithiothreitol (DTT) and then the neutralization of the excess DTT by N-ethylmaleimide, and AsA in the supernatant were measured as the Fe+2-bipyridyl complex (A max at 525 nm) formed from the reduction of Fe+3 by AsA, according to the method of Law et al. (1983). The DHA content was calculated from the difference between total ascorbate and AsA. Lipid peroxidation was determined by method of Heath and Packer (1968) in terms of malondialdehyde (MDA) content by thiobarbituric acid (TBA) reaction. The amount of TBA reactive substance (TBARS) was calculated from the difference in absorbance at 532 and 600 nm using extinction coefficient of 155 mM−1 cm−1.
Statistical analysis
All results are means of experimental replicates (at least n = 6) of protoplast/chloroplast images or protoplast/chloroplast preparations as specified in captions. The data were analyzed by analysis of variance (ANOVA) and tested for significance by Bonferroni t test using Sigma-stat software.
Results
Temporal effect of exposure of ME on oxidative metabolism
Incubating leaf strips in ME for 3 or 6 h did not dissolve cell wall completely as revealed by non-circular cell shape (Fig. 1a). Moreover, irrespective of exposure duration (3, 6, or 12) in ME, cells/protoplasts showed an increase in H2O2 (Fig. 1a, b) and MDA accumulation (Fig. 1c), higher NOX-like enzyme activity and lower SOD and APX (Fig. 2a, b, d) activities. Macerating enzyme did not cause any significant change in CAT activity (Fig. 2c). Therefore, we performed experiments on B. napus leaf protoplasts isolated by 12 h exposure in macerating enzyme.
Hydrogen peroxide (H2O2) generation and lipid peroxidation in B. napus leaf protoplasts/cells obtained by differential exposure duration in macerating enzyme (ME). Protoplasts were prepared and stained as described in “Materials and methods”. a Cell/Protoplasts were isolated at different time intervals and either not stained (Blank) or loaded with 70 μM H2DCF-DA. Images obtained in green, red and transmission channels were merged and presented. b Quantitative analysis of the relative DCF fluorescence intensity from representative images. c Lipid peroxidation in B. napus leaf strips (−Macerating enzyme) or isolated cell/protoplasts (+Macerating enzyme) obtained by differential exposure duration with ME. Bars represent mean ± SE of ROIs (region of interest i.e. whole cell) of different protoplasts. Bars having different letters are significantly different by Bonferroni t test (P < 0.05)
NADPH oxidase, NOX (a), superoxide dismutase, SOD (b), catalase (c) and ascorbate peroxidase, APX (d) activities in the B. napus leaf strips (−Macerating enzyme) or protoplasts/cells (+Macerating enzyme) obtained by differential exposure duration (0, 3, 6, 12 h). Vertical bars represent the mean ± SE (n = 6). Bars having different letters are significantly different by Bonferroni t test (P < 0.05)
B. napus leaf protoplasts undergo apoptotic-like cell death
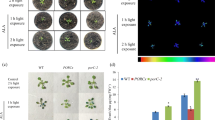
B. napus leaf protoplasts undergo apoptotic cell death as indicated by decreased mitochondrial membrane potential and DNA fragmentation. DiOC6 (a carbocyanine dye accumulates in active mitochondria) (Fig. 3a) was used to monitor the mitochondrial membrane potential by flow cytometry (Fig. 3b). The mitochondrial membrane potential decreased with culture time. Only 8.4% of protoplasts were detected in the lower fluorescence intensity region (T) after 10 h of culture, whereas more than 82% of protoplasts were detected in the same region after 60 h. No DNA fragmentation was detected in the protoplasts cultured for 10 h; however, DNA fragmentation was induced during 10–24 h of culture, and advanced further with culture period, 48 h (Fig. 3c).
Mitochondrial membrane potential of B. napus leaf protoplasts. a DiOC6-stained protoplasts showing DiOC6-specific fluorescence image, chlorophyll fluorescence image, and transmission image under a confocal laser scanning microscope. b Flow cytometric analysis of the changes in mitochondrial membrane potential of B. napus leaf protoplasts during culture. T and U regions represent the percentages of cells showing non-specific fluorescence and DiOC6-specific fluorescence (mitochondrial membrane potential), respectively, at the time points indicated. Non-stained protoplasts served as the blank. c Agarose gel analysis for nuclear DNA fragmentation in B. napus leaf protoplasts cultured for 10, 24, and 48 h
Hydrogen peroxide generation in protoplasts
H2O2 was generated excessively in the protoplasts during isolation. H2O2 signal was very weak in the transverse sections of leaf tissue used for protoplasts isolation (Supplementary Fig. S1). The highest DCF-fluorescence, an index of H2O2 accumulation, was detected in the protoplasts isolated by 6 or 12 h exposure in ME on the day of protoplast isolation (Fig. 1a, b). The level of DCF-fluorescence although declined gradually with culture time but decrease was non-significant (Fig. 4a, b). When 100 μM DPI was added to the protoplast or chloroplast suspension before the addition of 70 μM H2DCF-DA, an inhibition of DCF fluorescence was observed in both chloroplasts and protoplasts (Fig. 5 a, b).
Hydrogen peroxide (H2O2) generation in B. napus leaf protoplasts during culture. Protoplasts were prepared, cultured, and stained as described in “Materials and methods”. a Protoplasts were harvested at different time intervals and either not stained (Blank) or loaded with 70 μM H2DCF-DA. Images obtained in green, red and transmission channels were merged and presented. b Quantitative analysis of the relative DCF fluorescence intensity from representative images. Bars represent mean ± SE of ROIs (region of interest i.e. whole cell) of different protoplasts. Bars having different letters are significantly different by Bonferroni t test (P<0.05)
Effect of a NADPH oxidase inhibitor (100 μM DPI) on hydrogen peroxide (H2O2) generation in B. napus leaf chloroplasts and protoplasts. Chloroplasts and protoplasts were prepared and stained as described in “Materials and methods”. a Chloroplasts and protoplasts were either not stained (Blank) or loaded with 70 μM H2DCF-DA in the absence or presence of 100 μM DPI. b Quantitative analysis of the relative DCF-fluorescence intensity (an index of in vivo H2O2 generation) from representative images. Bars represent mean ± SE of ROIs (region of interest i.e. whole chloroplast or cell) of different chloroplasts/protoplasts. Bars having different letters are significantly different by Bonferroni t test (P < 0.05)
NADPH oxidizing, DPI-sensitive NOX-like enzyme is involved in H2O2 generation
The inhibition of DCF fluorescence (Fig. 5) and NOX-like enzyme activity (Fig. 6a, d) in the presence of 100 μM DPI (NOX inhibitor) revealed NOX-like enzyme-mediated generation of O2 ·−, which subsequently dismutated and accumulated as H2O2 in the chloroplasts and protoplasts. The NOX-like enzyme-mediated generation of O2 ·− in chloroplasts was confirmed by the visualization of NOX-like enzyme activity on a native gel in the presence of 200 U of SOD (Fig. 6c). The activity of the NOX-like enzyme isoforms disappeared almost completely in the presence of SOD and 100 μM DPI (Fig. 6c, d). To rule out the possibility of plasma membrane contamination in chloroplasts fractions, we prepared chloroplasts as described in the “Materials and methods”, also purified with 30% Percoll gradient and monitored for marker enzymes of plasma membrane; vanadate sensitive ATPase and 5′-nucleotidase. None of these plasma membrane enzymes activities were detected in chloroplast fraction but it showed presence of NOX-like enzyme activity (Fig. 6e).
NADPH oxidase-like (NOX-like) activity of leaf (L), protoplasts (P) and chloroplasts isolated from protoplasts (PC) measured in cuvette (a) or in native gels in the absence (b) or presence of 200 U of SOD (c) or 100 μM DPI (d). NOX-like activity in leaf (L) and Percoll purified chloroplasts (PPC) (e). Superoxide dismutase (SOD) (f) and guaiacol peroxidase (G-POD) (g) and 3,3-diaminobenzidine peroxidase (DAB-POD) (h) activities in native gels. Extracts of leaf (L), protoplasts (P), and chloroplasts isolated from protoplasts (PC) of B. napus were separated by native PAGE. Leaf extract of Petunia hydrida was considered as positive control for G-POD. Enzyme activities were visualized using standard protocols as described in “Materials and methods”
Ineffective cellular antioxidant system provides poor protection against oxidative stress
The activity of the antioxidant enzymes SOD was lower in the protoplasts compared with the activities in the leaf extract of B. napus (Fig. 2b). Seven SOD isoforms were detected in the leaf and protoplast extracts on native gels; however, the intensities of the SOD isoforms in the protoplasts were lower than those in the leaf extract (Fig. 6f). The activity of APX was lower in protoplasts compared to leaf (Fig. 2d). No G-POD isoforms were detected in the leaf extract, protoplasts, or chloroplasts of B. napus on a native gel (Fig. 6g), although two isoforms of G-POD were detected in the leaf extract of Petunia hybrida (which considered to be a positive control for G-POD) on the same gel (Fig. 6g). Activity of DAB-POD was reduced in protoplasts and chloroplasts did not show DAB-POD activity (Fig. 6h). These reductions in the activities of APX and DAB-POD and activation of NOX-like enzyme resulted in the accumulation of H2O2 and lipid peroxidation. While protoplasts isolated by 3 h exposure in ME did not show any significant change in ascorbate concentration, the protoplasts isolated by 6 or 12 h exposure in ME enhanced AsA concentration compared to the corresponding control leaves (Fig. 7a, b). The AsA/DHA ratio was higher in the protoplasts isolated by 12 h exposure in ME solution (Fig. 7b).
Concentrations of ascorbic acid (AsA) and total ascorbate (TAsc) (a), and the AsA/DHA ratio (b) in the leaf strips (−Macerating enzyme) or cells/protoplasts obtained by differential exposure period with ME (+Macerating enzyme) of B. napus plants. Vertical bars represent the mean ± SE (n = 6). Bars for an individual parameter having different letters are significantly different by Bonferroni t test (P < 0.05)
Discussion
Mitochondrial dysfunction is characteristic of apoptotic cells (Zhang et al. 2009b). The ROS generated initially in chloroplasts may induce mitochondrial dysfunction. The loss of mitochondrial membrane potential (mitochondrial dysfunction) accompanied by DNA fragmentation in the B. napus leaf protoplasts suggests that the protoplasts underwent apoptosis during culture.
The occurrence of the highest DCF fluorescence, an index of H2O2 generation (Kristiansen et al. 2009), in B. napus leaf protoplasts on the first day of their isolation suggests that the ME solution probably induced a form of metabolic redox disturbance during protoplast isolation, and this probably induced excessive ROS generation and oxidative stress. The observed increase in the generation of O2 ·− and H2O2 in B. napus leaf protoplasts is consistent with the findings of Ishii (1987) and Papadakis et al. (2001). H2DCF-DA has also been reported to react with O2 ·− (Allan and Fluhr 1997; Kristiansen et al. 2009); however, it should also be taken into account that observed intense fluorescence in B. napus protoplasts might be an outcome of reactivity of both O2 ·− and H2O2 with fluorescence probe (H2DCF-DA). Superoxide itself is highly reactive and rapidly dismutated to H2O2. Therefore, it generally leads to the low persistence of O2 ·− in chloroplast. The inhibition of DCF fluorescence in chloroplasts or protoplasts upon incorporation of 100 μM DPI, an inhibitor of NADPH oxidase (NOX), before the addition of H2DCF-DA suggests NOX-like enzyme-mediated generation of O2 ·− in chloroplasts, which subsequently dismutated to H2O2 and accumulated in the chloroplasts. Thus, H2O2 is produced by spontaneous or SOD-mediated dismutation (to a lower extent) in B. napus leaf protoplasts. Extent of H2O2 accumulation in the protoplasts isolated by 6 or 12 h exposure in the ME was almost similar. They also exhibited a similar response for the activities of NOX-like enzyme, APX and SOD. Excessive accumulation H2O2 in protoplasts suggests a response similar to wounding or pathogenic infection leading to apoptosis-like cell death (Zago et al. 2006; Chaki et al. 2009). Commercially available ME (cellulase and pectolyase) used for the isolation of protoplasts either contain some elicitor (because they obtained from fungal sources) or that generated in the process of cell wall dissolution which might have triggered oxidative burst (ROS generation) in the protoplasts.
Antisense knockouts and gene silencing experiments in tobacco as well as an analysis of Arabidopsis mutants have confirmed the role of NOX as a source of O2 ·− (Simon-Plas et al. 2002) in the oxidative burst that regulates cell death (Torres et al. 2002; Yoshioka et al. 2003). H2O2 is a relatively stable product of spontaneous or SOD-catalyzed dismutation of O2 ·−. Therefore, this study focused on H2O2 instead of O2 ·−. In gel localization revealed higher NOX-like activity, particularly in the B. napus chloroplasts and protoplasts compared with the leaf, suggests the involvement of a NOX-like enzyme in O2 ·− generation in the chloroplasts. The activity of NOX-like enzyme isoforms disappeared completely in the presence of SOD (200 U) or DPI (50 μM). These observations suggest the existence of a NOX-like protein in B. napus leaf chloroplasts. The most promising candidate in the chloroplasts, which may exhibit NOX-like enzyme activity is small ferredoxin:NADP+ oxidoreductase (FNRs). It has been reported previously that small FNR isoforms of cyanobacterium Synechocystis sp. strain PCC6803, (FNRs ≈ 34 kDa) which corresponds to plastid FNR, is a NADPH oxidase (Thomas et al. 2006; Korn et al. 2009). Moreover, there is a report that supports the existence of NOX in chloroplasts: the hydrogen peroxide generated by a Mn2+-dependent enzyme in the chloroplasts of Euglena has been shown to be involved in the decarboxylation of glyoxylate (Yokota et al. 1983). However, no report is yet available on NADPH oxidase-like activity in the chloroplasts.
The reduced activities of the antioxidant enzymes SOD and POD in the protoplasts and chloroplasts compared with the leaf extract suggest a weakening of antioxidant protection in B. napus protoplasts. The decreased DAB-POD activity and lack of G-POD probably caused an over-accumulation of H2O2 in the B. napus leaf protoplasts due to a lower antioxidant potential of the cells. SOD is an O2 ·−-dismutating antioxidant enzyme and is present in various cellular compartments such as chloroplasts, mitochondria, and cytosol (Mittler et al. 2004). SOD generally scavenges the O2 ·− generated under normal conditions and dismutates O2 ·− into H2O2 (Mittler et al. 2004). H2O2 can diffuse across the membrane or move through water channels (Bienert et al. 2007) and is scavenged generally by the participation of various antioxidant enzymes such as catalase, non-specific peroxidase, ascorbate peroxidase, and glutathione peroxidase (Cheeseman 2007). The superoxide anion, generated by excessive NOX-like activity in the chloroplasts, was dismutated to H2O2, spontaneously and/or via chloroplastic SOD activity. However, H2O2 was not scavenged by the available cellular antioxidants because of the undetectable G-POD and reduced DAB-POD activities in B. napus protoplasts. An ineffective antioxidant system as seen in B. napus protoplasts, may be characteristic of apoptosis of protoplasts. Previously, Watanabe et al. (2002a) have reported a high protease activity and excessive production of ethylene gas in apoptotic protoplasts of B. napus. Papadakis et al. (2001) have been reported reduced activity of the cellular antioxidants in the apoptotic protoplasts. They also reported a lower AsA/AsA+DHA ratio in non-totipotent protoplasts compared with the leaf, indicating the accumulation DHA. However, our observations with B. napus protoplasts are not in agreement with the findings of Papadakis et al. (2001), as B. napus protoplasts displayed enhanced AsA, lower DHA, and a higher AsA/DHA ratio. Generally, AsA has been considered as an efficient free radical scavenger (Arrigoni and De Tullio 2002); however, AsA exerts pro-oxidant effects as well (Podmore et al. 1998; Arrigoni and De Tullio 2002). AsA exerts pro-oxidant effects in vitro, usually by interactions with transition metal ions to promote its oxidation, accompanied by H2O2 production (Halliwell 2001). Moreover, an enhanced apoptotic-like cell death has been reported in the cell culture of Arabidopsis exposed to heat stress by ascorbic acid or sodium ascorbate treatment (Doyle et al. 2010).
In summary, the isolation of protoplasts using macerating enzymes is a stress-inducing process and results in the accumulation of H2O2 in chloroplasts due to the activation of NOX-like activity. The inactivation of DAB-POD and lack of guaiacol POD activity appears to be another cause of sustained H2O2.accumulation. The sustained generation of H2O2 induced the apoptotic cell death of B. napus leaf protoplasts. Therefore, promising approach would be to suppress NOX-mediated ROS generation to control apoptotic cell death of protoplasts. Inhibition of NOX-like enzyme-mediated generation of H2O2 may be implied in the regulation of apoptotic-like death of B. napus protoplasts. However, further experiments are required to reach on this conclusion.
Abbreviations
- APX:
-
Ascorbate peroxidase
- AsA:
-
Ascorbic acid
- DAB-POD:
-
3,3-Diaminobenzidine peroxidase
- DHA:
-
Dehydroascorbic acid
- DiOC6 :
-
3,3′-Dihexyloxacarbocyanine iodide
- DPI:
-
Diphenyleneiodonium chloride
- G-POD:
-
Guaiacol peroxidase
- H2DCF-DA:
-
2′,7′-Dichlorodihydrofluorescein diacetate
- ME:
-
Macerating enzyme
- NOX:
-
NADPH oxidase
- PCD:
-
Programmed cell death
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- XTT:
-
2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide sodium salt
References
Allan AC, Fluhr R (1997) Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell 9:1559–1572
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Arrigoni O, De Tullio MC (2002) Ascorbic acid: much more than just an antioxidant. Biochim Biophys Acta 1569:1–9
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and assays applicable to acrylamide gels. Anal Biochem 44:276–287
Beers EP, McDowell JM (2001) Regulation and execution of programmed cell death in response to pathogens, stress and developmental cues. Curr Opin Plant Biol 4:561–567
Beers RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Bienert GP, Moller ALB, Kristiansen KA, Schulz A, Moller IM, Schjoerring JK, Jahn TP (2007) Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem 282:1183–1192
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chaki M, Fernández-Ocaña AM, Valderrama R, Carreras A, Esteban FJ, Luque F, Gómez-Rodríguez MV, Begara-Morales JC, Corpas FJ, Barroso JB (2009) Involvement of reactive nitrogen and oxygen species (RNS and ROS) in sunflower-mildew interaction. Plant Cell Physiol 50:265–279
Cheeseman JM (2007) Hydrogen peroxide and plant stress: a challenging relationship. Plant Stress 1:4–15
Delledonne M, Zeier J, Marocco A, Lamb C (2001) Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA 98:13454–13459
Doyle SM, Diamond M, McCabe PF (2010) Chloroplast and reactive oxygen species involvement in apoptotic-like programmed cell death in Arabidopsis suspension cultures. J Exp Bot 61:473–482
Drew MC, He C-J, Morgan PW (2000) Programmed cell death and aerenchyma formation in roots. Trends Plant Sci 5:123–127
Gao CJ, Xing D, Li LL, Zhang LR (2008) Implication of reactive oxygen species and mitochondrial dysfunction in the early stages of plant programmed cell death induced by ultraviolet-C overexposure. Planta 227:755–767
Greenberg JT (1996) Programmed cell death: a way of life for plants. Proc Natl Acad Sci USA 93:12094–12097
Halliwell B (2001) Vitamin C and genomic stability. Mutation Res 475:29–35
Halliwell B, Gutteridge J (1999) Free radicals in biology and medicine. Oxford University Press, Oxford
Hames BD (1990) One dimensional polyacrylamide gel electrophoresis. In: Hames BD, Rickwood D (eds) Gel electrophoresis of protein. Oxford University Press, UK, pp 1–87
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast, I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:180–198
Ishii S (1987) Generation of active oxygen species during enzymic isolation of protoplasts from oat leaves. In Vitro Cell Dev Biol 23:653–658
Kieselbach T, Hagman Å, Andersson B, Schröder WP (1998) The thylakoid lumen of chloroplasts: isolation and characterization. J Biol Chem 273:6710–6716
Korn A, Ajlani G, Lagoutte B, Gall A, Sétif P (2009) Ferredoxin:NADP+ oxidoreductase association with phycocyanin modulates its properties. J Biol Chem 284:31789–31797
Kristiansen KA, Jensen PE, Møller IM, Schulz A (2009) Monitoring reactive oxygen species formation and localisation in living cells by use of the fluorescent probe CM-H2DCFDA and confocal laser microscopy. Physiol Plant 136:369–383
Lam E, Kato N, Lawton M (2001) Programmed cell death, mitochondria and the plant hypersensitive response. Nature 411:848–853
Law MY, Charles SA, Halliwell B (1983) Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts, the effect of hydrogen peroxide and of paraquat. Biochem J 210:899–903
Mittler R, Vanderauwera S, Gollery M, van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplast. Plant Cell Physiol 22:867–880
Papadakis AK, Siminis CI, Roubelakis-Angelakis KA (2001) Reduced activity of antioxidant machinery is correlated with suppression of totipotency in plant protoplasts. Plant Physiol 126:434–444
Pennell RI, Lamb C (1997) Programmed cell death in plants. Plant Cell 9:1157–1168
Podmore ID, Griffiths HR, Herbert KE, Mistry N, Mistry P, Lunec J (1998) Vitamin C exhibits pro-oxidant properties. Nature 392:559
Rogers HJ (2006) Programmed cell death in floral organs: How and why do flowers die? Ann Bot 97:309–315
Sagi M, Fluhr R (2006) Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol 141:336–340
Schimmel SD, Kent C, Bischoff R, Vagelos PR (1973) Plasma membranes from cultured muscle cells: isolation procedure and separation of putative plasma-membrane marker enzymes. Proc Natl Acad Sci USA 70:3195–3199
Simon-Plas F, Elmayan T, Blein JP (2002) The plasma membrane oxidase NtrbohD is responsible for AOS production in elicited tobacco cells. Plant J 31:137–147
Tewari RK, Kim S, Hahn EJ, Paek KY (2008) Involvement of nitric oxide-induced NADPH oxidase in adventitious root growth and antioxidant defence in Panax ginseng. Plant Biotechnol Rep 2:113–122
Tewari RK, Kumar P, Kim S, Hahn EJ, Paek KY (2009) Nitric oxide retards xanthine oxidase-mediated superoxide anion generation in Phalaenopsis flower: an implication of NO in the senescence and oxidative stress regulation. Plant Cell Rep 28:267–279
Thomas JC, Ughy B, Lagoutte B, Ajlani G (2006) A second isoform of the ferredoxin:NADP+ oxidoreductase generated by an in-frame initiation of translation. Proc Natl Acad Sci USA 103:18368–18373
Torres MA, Dangl JL, Jones JDG (2002) Arabidopsis gp91(phox) homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99:517–522
Truitt CL, Wei HX, Pare PW (2004) A plasma membrane protein from Zea mays binds with the herbivore elicitor volicitin. Plant Cell 16:523–532
Vacca RA, Valenti D, Bobba A, Merafina RS, Passarella S, Marra E (2006) Cytochrome c is released in a reactive oxygen species-dependent manner and is degraded via caspase-like proteases in tobacco Bright-Yellow 2 cells en route to heat shock induced cell death. Plant Physiol 14:208–219
Vasil IK (1987) Developing cell and tissue culture systems for the improvement of cereal and grass crops. J Plant Physiol 128:193–218
Wang C, Zhang SH, Wang PF, Hou J, Zhang WJ, Li W, Lin ZP (2009) The effect of excess Zn on mineral nutrition and antioxidative response in rapeseed seedlings. Chemosphere 75:1468–1476
Watanabe M, Watanabe Y, Shimada N (1992) Colorimetric estimation of leaf-protoplast potential-to-divide by use of 2, 3, 5-triphenyl tetrazolium chloride. J Plant Physiol 44:111–114
Watanabe M, Kawasaki H, Itho Y, Watanabe Y (1998) Senescence development of Brassica napus leaf protoplasts during isolation and subsequent culture. J Plant Physiol 152:487–493
Watanabe M, Setoguchi D, Uehara K, Ohtsuka W, Watanabe Y (2002a) Apoptotic-like cell death of Brassica napus leaf protoplasts. New Phytol 156:417–426
Watanabe M, Suzuki K, Kawasaki H, Watanabe Y (2002b) Differential responses of Brassica napus and Petunia hybrida to leaf protoplast isolation stress. Physiol Plant 114:645–651
Yasuda K, Watanabe Y, Watanabe M (2007) Generation of intracellular reactive oxygen species during the isolation of Brassica napus leaf protoplasts. Plant Biotechnol 24:361–366
Yokota A, Kawabata A, Kitaoka S (1983) Mechanism of glyoxylate decarboxylation in the glycolate pathway in Euglena gracilis Z: participation of Mn2+-dependent NADPH oxidase in chloroplasts. Plant Physiol 71:772–776
Yoshioka H, Numata N, Nakajima K, Katou S, Kawakita K, Rowland O, Jones JDG, Doke N (2003) Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. Plant Cell 15:706–718
Zago E, Morsa S, Dat JF, Alard P, Ferrarini A, Inze′ D, Delledonne M, Van Breusegem F (2006) Nitric oxide- and hydrogen peroxide-responsive gene regulation during cell death induction in tobacco. Plant Physiol 141:404–411
Zhang LR, Xing D (2008) Methyl jasmonate induces production of reactive oxygen species and alterations in mitochondrial dynamics that precede photosynthetic dysfunction and subsequent cell death. Plant Cell Physiol 49:1092–1111
Zhang H, Fang Q, Zhang Z, Wang Y, Zheng X (2009a) The role of respiratory burst oxidase homologues in elicitor-induced stomatal closure and hypersensitive response in Nicotiana benthamiana. J Exp Bot 60:3109–3122
Zhang L, Li Y, Xing D, Gao C (2009b) Characterization of mitochondrial dynamics and subcellular localization of ROS reveal that HsfA2 alleviates oxidative damage caused by heat stress in Arabidopsis. J Exp Bot 60:2073–2091
Acknowledgments
This work was financially supported by Japan Society for the Promotion of Science (JSPS) as a postdoctoral fellowship (P08413) to RKT and grant-in-aid for scientific research. Authors are thankful to Prof. Esso Nishino, Chiba University, for his help in preparation of microtome sections of leaf.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tewari, R.K., Watanabe, D. & Watanabe, M. Chloroplastic NADPH oxidase-like activity-mediated perpetual hydrogen peroxide generation in the chloroplast induces apoptotic-like death of Brassica napus leaf protoplasts. Planta 235, 99–110 (2012). https://doi.org/10.1007/s00425-011-1495-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-011-1495-8