Abstract
In this study, two WRKY genes were isolated from Erysiphe necator-resistant Chinese wild Vitis pseudoreticulata W. T. Wang ‘Baihe-35-1’, and designated as VpWRKY1 (GenBank accession no. GQ884198) and VpWRKY2 (GenBank accession no. GU565706). Nuclear localization of the two proteins was demonstrated in onion epidermal cells, while trans-activation function was confirmed in the leaves of ‘Baihe-35-1’. Expression of VpWRKY1 and VpWRKY2 was induced rapidly by salicylic acid treatment in ‘Baihe-35-1’. Expression of VpWRKY1 and VpWRKY2 was also induced rapidly by E. necator infection in 11 grapevine genotypes; the maximum induction of VpWRKY1 was greater in E. necator-resistant grapevine genotypes than in susceptible ones post E. necator inoculation. Furthermore, ectopic expression of VpWRKY1 or VpWRKY2 in Arabidopsis enhanced resistance to powdery mildew Erysiphe cichoracearum, and enhanced salt tolerance of transgenic plants. VpWRKY2 also enhanced cold tolerance of transgenic plants. In addition, the two proteins were shown to regulate the expression of some defense marker genes in Arabidopsis and grapevine. The data suggest that VpWRKY1 and VpWRKY2 may underlie the resistance in transgenic grapevine to E. necator and tolerance to salt and cold stresses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Abiotic and biotic stresses cause major losses in crop productivity worldwide. The responses of plants to these stresses are regulated by multiple signaling pathways (Singh et al. 2002). A considerable number of genetic and molecular approaches have demonstrated that stress-responsive signaling pathways in plants involve a complex network of multiple components including receptors, kinases, phosphatases, and transcription factors (Gutterson and Reuber 2004). These studies have shown that plants are capable of extensive, highly dynamic, and temporal reprogramming of their transcriptome to generate a defense response. Regulation for adaptive plasticity is mainly achieved by the enforcement of a network of various transcription factors (Pandey and Somssich 2009).
WRKY proteins comprise a large family of transcription factors (Ulker and Somssich 2004) potentially involved in the regulation of transcriptional reprogramming responsible for plant immune responses (Eulgem and Somssich 2007). This family is defined by the conserved amino acid sequence WRKY together with a novel zinc-finger-like motif. WRKY proteins are classified into three groups based on the number of WRKY domains and the type of zinc-finger-like motif (Eulgem et al. 2000). Those with two WRKY domains belong to group I, whereas most proteins with one WRKY domain belong to group II or III. Group I and group II members have the finger motif C2–H2 (C–X4–5–C–X22–23–H–X–H). Group II members are further divided into five distinct subgroups (IIa–e) based on ten additional conserved motifs. Instead of a C2–H2 pattern, group III WRKY proteins contain a C2–HC finger motif (C–X7–C–X23–H–X1–C) (Eulgem et al. 2000).
Grape is an important fruit crop, but cultivated grapevine (Vitis vinifera) is susceptible to many pathogens. For instance, powdery mildew caused by Erysiphe necator (Schw.) Burr. is an economically important disease that infects green tissues of vines, and causes significant losses in yield and reduces berry quality (Fung et al. 2008). Wild species are often a valuable source of resistance to crop pathogens (Pavlousek 2007). In recent years, there has been a new trend of generating novel varieties of V. vinifera by introducing disease resistance gene(s) from resistant species into susceptible species (Bisson et al. 2002). Resistance genes Run1 and Ren1 against powdery mildew and Rpv1, Rpv2 and Rpv3 against downy mildew have been isolated from resistant grapevine species (Bellin et al. 2009; Coleman et al. 2009). Although two WRKY transcription factors genes (VvWRKY1 and VvWRKY2) isolated from susceptible V. vinifera L. cv. Cabernet Sauvignon have been shown to enhance resistance to fungal pathogens in transgenic tobacco plants (Marchive et al. 2007; Mzid et al. 2007; Guillaumie et al. 2010), transcription factors from powdery mildew-resistant grapevine species have not yet been studied.
In a previous study, Chinese wild grapevine genotype Vitis pseudoreticulata W. T. Wang ‘Baihe-35-1’ was identified as resistant to E. necator (Wang et al. 1995). The goal of the present research was to characterize two genes encoding transcription factors, VpWRKY1 and VpWRKY2, isolated from Chinese wild V. pseudoreticulata W. T. Wang ‘Baihe-35-1’. We focused on the roles of VpWRKY1 and VpWRKY2 in resistance to biotic and abiotic stresses. Expression of VpWRKY1 and VpWRKY2 post E. necator inoculation and signaling molecule treatments were determined by qRT-PCR. In addition, expression of some SA- and JA/ET-dependent defense marker genes was tested in transgenic Arabidopsis and grapevine. We also analyzed resistance of transgenic Arabidopsis plants to Erysiphe cichoracearum, salt and cold stresses.
Materials and methods
Plant materials
Grapevines were grown in grape germplasm resources orchard of Northwest A&F University, Yangling, China (34°20′N, 108°24′E). Eleven grapevine genotypes were tested in this study including five E. necator-resistant and six E. necator-susceptible genotypes. The E. necator-resistant genotypes were Chinese wild V. pseudoreticulata W. T. Wang ‘Baihe-35-1’, ‘Baihe-13’, ‘Baihe-13-1’, ‘Guangxi-1’, and ‘6-12-6’ (a cross between Chinese wild V. pseudoreticulata W. T. Wang ‘Baihe-35-1’ and V. vinifera L. cv. Carignane). The six E. necator-susceptible grapevine genotypes were V. vinifera L. cv. Carignane, Chinese wild V. pseudoreticulata W. T. Wang ‘Guangxi-2’, ‘Hunan-1’, ‘Shangnan-2’, ‘Baihe-35-2’, and ‘6-12-2’ (another cross between Chinese wild V. pseudoreticulata W. T. Wang ‘Baihe-35-1’ and V. vinifera L. cv. Carignane). The susceptibility index and resistance ratings to E. necator of the 11 grapevine genotypes are listed in Supplementary Table S1. In vitro cultivation of grapevines used for transient experiments was performed as described by Guan et al. (2010).
Arabidopsis thaliana L. ecotype Col-0 was used for over-expression experiments and was grown in a chamber at 22°C in long-day conditions (16 h of light and 8 h of dark). Onion (Allium cepa L.) was purchased from a local market.
Biotic and abiotic treatments
Grapevine E. necator was maintained as described by Guan et al. (2010). When shoots of vines were 25–35 cm in length, the third to fifth fully expanded young grapevine leaves beneath the apex were selected for biotic and abiotic treatments. Inoculation by E. necator was performed on the selected leaves under field conditions as described by Wang et al. (1995), and was repeated three times on three independent plants for each species. Leaves sprayed with sterile water were used as negative control. Inoculated leaves were then covered with plastic bags for 12 h to maintain humidity. Field leaves were collected 0, 6, 12, 24, 48, 72, 96 and 120 h post inoculation (hpi), and immediately frozen in liquid nitrogen for further study.
Arabidopsis powdery mildew E. cichoracearum was identified in Col-0 plants. Fungal isolate was purified via single colony inoculation of clean Col-0 plants for five consecutive generations. The isolate was then maintained on live Col-0 plants at 22°C (16 h light, 8 h dark) in a separate growth chamber for generation of fresh inocula. E. cichoracearum inoculation was conducted on leaves of selected 6-week-old T2 transgenic and wild type plants as described by Xiao et al. (1997). Visual scoring of disease reaction phenotypes was done 12 days post inoculation (dpi) as described previously (Xiao et al. 2005). Spore count of the most susceptible leaves was determined as following: leaf samples were collected and weighed, and placed in 50-ml tubes containing 40 ml of sterilized dH2O and 0.02% of Tween 20, after which the tubes were stirred vigorously for 60 s using a vortex mixer. The resulting spore suspension was diluted 1:10 with 0.02% Tween 20 solution. Spores were then counted under a dissecting microscope in a large area using a hemocytometer to get a more reliable spore density estimate.
To test if VpWRKY1 and VpWRKY2 expression is induced by plant defense signaling molecules, 100 μM salicylic acid (SA) (Wang and Li 2006), 50 μM methyl jasmonate (MeJA) (Repka et al. 2004) and 0.5 g/l ethephon (Eth) (Belhadj et al. 2008) were sprayed on selected leaves of ‘Baihe-35-1’ under field conditions. Three leaves were selected from three independent plants for each treatment at 0, 3, 6, 12, 24, 48, 72 and 96 h post treatment (hpt). Samples were immediately frozen in liquid nitrogen.
To determine the salt tolerance of the transgenic plants, T2 transgenic Arabidopsis seeds were sowed in MS medium with and without 150 mM NaCl. Germination rate was calculated based on the presence of green cotyledons 10 days after sowing. Cold treatment was accomplished by transferring 2-week-old seedlings grown in MS media to a 4°C refrigerator for 6 h, after which they were returned to the growth chamber. Fresh weight was determined 1 week after the cold treatment.
RNA isolation and cDNA library construction
Total RNA of Arabidopsis was extracted as described by Ulker et al. (2007), whereas total RNA of grapevines was extracted using improved SDS/phenol method (Zhang et al. 2003) at 0, 6, 12, 24, 48, 72, 96 and 120 h post E. necator infection of ‘Baihe-35-1’ leaves. cDNA library was constructed as described by Xu et al. (2009) with an equal amount of mRNA pooled at each time point. All sequences in this study were determined by Generay Biotech Co. Ltd (Shanghai, China).
Full length cloning and sequence analysis
Rapid amplification of cDNA ends (RACE) was conducted using BD SMART RACE cDNA Amplification Kit (Clontech, Mountain View, CA, USA), and cDNA synthesized from E. necator-infected leaves of ‘Baihe-35-1’. Specific primers were designed based on the WRKY EST sequences (GenBank accession no. GR883935 and GR883939). Primers GSP1: 5′ TTCACGATGACGGTTATGCCTGGCG for 3′ RACE and GSP2: 5′ TGCACTCCATTGTGCTCATGGTGGC for 5′ RACE were used to obtain the full length of VpWRKY1, while primers GSP3: 5′ CATTTCCAAAGGCTAACAGTGAA for 3′ RACE and GSP4: 5′ ATACATTCACTGTTAGCCTTTGG for 5′ RACE were used to obtain the full length of VpWRKY2. RACE results were compiled using Seqman. Nuclear-localization signals were predicted by PSORT WWW Server (http://psort.ims.u-tokyo.ac.jp/) and the phylogenetic tree was constructed using the CLUSTALW2 program (http://www.ebi.ac.uk/Tools/clustalw2/index.html). Sequence alignment was performed using DNAMAN.
Subcellular localization
Coding sequences of VpWRKY1 and VpWRKY2 without the termination codon were introduced into pCAMBIA1302 vector to generate 35S::VpWRKY1–GFP and 35S::VpWRKY2–GFP. Sequenced plasmids were delivered into onion epidermal cells using PDS-1000/He gene gun at 1,100 psi as described by Mare et al. (2004), and then cultured in MS media in darkness at 22°C for 18 h. After cultivation, GFP visualization at excitation wavelength 480 ± 20 nm and emission wavelength 510 ± 20 nm was conducted using a Zeiss confocal microscope (LSM510; Carl Zeiss Thornwood, NY, USA).
Trans-activation assay
Primers 5′ CTATTAGGAGGAGTTGGTTG and 5′ CTCATGGTGGCGTCTGTG were designed based on previously released whole genome sequences of V. vinifera (Jaillon et al. 2007; Velasco et al. 2007) and the cDNA sequence of VpWRKY1 to clone the VpWRKY1 promoter. The promoter fragment was amplified from the genomic DNA of ‘Baihe-35-1’. Sequence analysis showed that VpWRKY1 promoter (GenBank accession no. GU565705) was enriched in W-boxes (TGAC). Minimal-100 CaMV35S promoter (m35S) was inserted upstream of the GUS gene of pC0390GUS (Xu et al. 2010) to generate an m35S-GUS construct (Fig. 3a). The 140-bp fragment containing three W-boxes (A/TGAC/A, −215 to −354 bp) from VpWRKY1 promoter was amplified by PCR, and then inserted upstream of m35S to generate W-box-m35S-GUS reporter construct (Fig. 3a). The gusA of pCAMBIA1301 was replaced with the coding region of VpWRKY1 or VpWRKY2 to generate over-expression constructs p1301-VpWRKY1 and p1301-VpWRKY2, respectively (Fig. 3a). Agrobacterium strain GV3101 harboring recombinant plasmids was transformed in vitro into 6-week-old plantlet leaves of V. pseudoreticulata W. T. Wang ‘Baihe-35-1’ via Agrobacterium-mediated transient assay. GUS staining was performed at 3 dpi as described by Xu et al. (2010).
Gene expression analysis by semi-quantitative RT-PCR and qRT-PCR
First-strand cDNA was synthesized from 1 μg of DNase-treated total RNA using PrimeScript™ RTase (TaKaRa Biotechnology, Dalian, Liaoning, China). Semi-quantitative RT-PCR was performed at 94°C for 3 min, 25 cycles at 94°C for 30 s, 60°C for 30 s and 72°C for 30 s, followed by final elongation at 72°C for 5 min. qRT–PCR was conducted using SYBR green (Takara Biotechnology) on an IQ5 real time PCR machine (Bio-Rad, Hercules, CA, USA). Each reaction was done in triplicates with a reaction volume of 25 μl. Cycling parameters were 95°C for 30 s, 40 cycles of 95°C for 5 s, and 60°C for 30 s. To analyze the quality of the dissociation curves, the following program was added after 40 PCR cycles: 95°C for 15 s, followed by a constant increase from 60 to 95°C. Grapevine VpGAPDH or Arabidopsis tubulin was amplified as internal control. Primers used for qRT-PCR are listed in Supplementary Table S2. Each relative expression level was analyzed with IQ5 software using the normalized-expression method. A one-side paired t test using SigmaPlot 11.0 (Ashburn, VA, USA) was performed to assess significant differences between the negative control and the treatment.
Generation of transgenic Arabidopsis, and transient over-expression and silencing of VpWRKY1 and VpWRKY2 in grapevine
Agrobacterium GV3101 harboring over-expression construct p1301-VpWRKY1 or p1301-VpWRKY2 was used for Arabidopsis transformation via the floral dip method (Clough and Bent 1998). Arabidopsis transformants were selected based on hygromycin B resistance on MS plates. Semi-quantitative RT-PCR was used to select T2 lines with the highest expression level of VpWRKY1 or VpWRKY2. The fourth to seventh rosette leaves of 6-week-old Arabidopsis plants were selected for qRT-PCR analysis. Fragment of VpWRKY1 from 131 to 488 bp was amplified as sense and antisense fragments, and inserted into pKANNIBAL (Wesley et al. 2001) to generate the VpWRKY1 silencing construct. The same procedure was done on the fragment of VpWRKY2 from 24 to 323 bp to generate the VpWRKY2 silencing construct. Transient over-expression and silencing of VpWRKY1 and VpWRKY2 in ‘Baihe-35-1’ leaves was performed as described by Xu et al. (2010). qRT-PCR was used to analyze expression of defense marker genes at 3 days post infiltration.
Results
Cloning and sequence analysis of VpWRKY1 and VpWRKY2
To identify candidate genes from Chinese wild Vitis and facilitate the molecular breeding of resistant varieties, a cDNA library was constructed from E. necator-inoculated ‘Baihe-35-1’ leaves and sequenced. Among more than 4,300 sequences in the library, two ESTs (GenBank accession no. GR883935 and GR883939) with single copy for each were found to contain conserved WRKY domain(s). By using RACE technique, full lengths of the two WRKY genes were obtained and designated as VpWRKY1 (GenBank accession no. GQ884198) and VpWRKY2 (GenBank accession no. GU565706). Full length of VpWRKY1 cDNA was 1,157 bp, encoding a polypeptide of 322 amino acids (Supplementary Fig. S1), while VpWRKY2 was 1,607 bp, encoding a polypeptide of 499 amino acids (Supplementary Fig. S2). Sequence analysis showed that VpWRKY1 contains one WRKY domain, one C2–HC zinc-finger motif (C–X7–C–X23–H–X1–C) and one predicted nuclear-localization signal (KRRK) (Supplementary Fig. S1), whereas VpWRKY2 contains two WRKY domains, one C2H2 zinc-finger motif (C–X4–C–X23–H–X–H), and four putative nuclear-localization signals (Supplementary Fig. S2). The phylogenetic tree based on the classification method of Eulgem et al. (2000) showed that VpWRKY1 belongs to group III and VpWRKY2 belongs to group I of the WRKY superfamily (Supplementary Fig. S3). AtWRKY70 from Arabidopsis is the most closely related gene to VpWRKY1 (Supplementary Fig. S3). Multiple alignments of the amino acid sequences of this two proteins and reported VvWRKY1 and VvWRKY2 indicated that they share an overall 22–45% sequence similarity. However, the deduced amino acid sequence of VpWRKY1 has 100% similarity with the predicted protein acc no. XP 002272504 from V. vinifera with the corresponding gene located in chromosome 8, while VvWRKY2 shares 99% similarity with the predicted protein acc no. XP 002276194 from V. vinifera with the corresponding gene located in chromosome 11. The corresponding gene of VvWRKY1 is located in chromosome 17, whereas the location of the corresponding gene of VvWRKY2 is unknown.
Expression of VpWRKY1 and VpWRKY2 are induced rapidly by E. necator and some plant defense signaling molecules
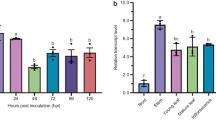
Results of qRT-PCR showed that both VpWRKY1 and VpWRKY2 were induced rapidly by E. necator in Chinese wild V. pseudoreticulata W. T. Wang ‘Baihe-35-1’, from which they were originally isolated (Fig. 1a, b). To determine whether VpWRKY1 and VpWRKY2 respond to E. necator in different grapevine genotypes, qRT-PCR was conducted on the ten other grapevine genotypes. Findings indicated that expression of VpWRKY1 and VpWRKY2 was induced by E. necator infection in all 11 grapevine genotypes tested (Fig. 1a, b). Expression levels of VpWRKY1 and VpWRKY2 peaked at 6–12 hpi, and then decreased to original levels at 96–120 hpi in all genotypes. Maximum induction of VpWRKY1 was observed in E. necator-resistant genotype ‘Baihe-35-1’ at 12 hpi (Fig. 1a).
Expression profiles of VpWRKY1 and VpWRKY2. a VpWRKY1 was induced by E. necator in eleven grapevine genotypes, the five grapevines with VpWRKY1 induction levels of more than fourfold are all resistant to E. necator infection (solid lines), while the six genotypes with VpWRKY1 induction levels lower than fourfold are all susceptible to E. necator infection (dotted lines). b VpWRKY2 was induced by E. necator in five resistant (solid lines) and six susceptible grapevine genotypes (dotted lines). Expression profiles of VpWRKY1 and VpWRKY2 in response to SA (c), MeJA (d), and Eth (e) treatments in leaves of Chinese wild V. pseudoreticulata W. T. Wang ‘Baihe-35-1’. VpGAPDH was used as internal control for qRT-PCR and fold expressions indicate expression level in treated leaves of each genotype compared with the negative control, which was set to 1. Asterisks indicate a significant difference (P < 0.05) in VpWRKY1 expression. Mean values and SDs were obtained from three technical and three biological replicates
Though there were differential levels of basal expression of VpWRKY1 or VpWRKY2 between the genotypes (Supplementary Fig. S4, S5), they are not consistent with maximum induction levels (Fig. 1a, b) and degree of disease resistance of these genotypes (Supplementary Table S1). However, all E. necator-resistant grapevine genotypes had a maximum VpWRKY1 induction of more than fourfold, while susceptible genotypes had a maximum VpWRKY1 induction less than fourfold (Fig. 1a). Therefore, maximum VpWRKY1 induction levels correlate well with the degree of disease resistance of the 11 grapevine genotypes. Although VpWRKY2 was induced by E. necator in all genotypes tested, the maximum induction of VpWRKY2 does not correlate with disease resistance levels of the grapevine genotypes (Fig. 1b).
To determine whether VpWRKY1 and VpWRKY2 were induced by defense signaling molecules, the selected leaves of Chinese wild V. pseudoreticulata W. T. Wang ‘Baihe-35-1’ were treated with SA, MeJA or Eth. Basal VpWRKY1 transcript level in ‘Baihe-35-1’ was not significantly induced by Eth and MeJA, but was slightly induced by SA at 3 hpt (Fig. 1c, d, e). In contrast, VpWRKY2 was induced rapidly by all three signaling molecules with SA as the strongest inducer (Fig. 1c, d, e).
VpWRKY1 and VpWRKY2 proteins are localized in the nucleus
Sequence analysis of the two genes revealed that their proteins contain putative nuclear-localization signal(s) (Supplementary Fig. S1, S2). To investigate the subcellular localization of VpWRKY1–GFP and VpWRKY2–GFP proteins, plasmids 35::VpWRKY1–GFP, 35::VpWRKY2–GFP, and negative control 35::GFP were transiently transformed into onion epidermal cells by particle bombardment. Results showed that the fusion proteins VpWRKY1–GFP and VpWRKY2–GFP targeted the nucleus of onion epidermal cells (Fig. 2). In contrast, control GFP was observed throughout the whole cell (Fig. 2). These indicate that VpWRKY1 and VpWRKY2 are nuclear proteins, which are consistent with their roles as transcription factors.
VpWRKY1 and VpWRKY2 function as potential transcriptional activators
Since reported WRKY factors have shown high binding affinity to W-box (TGAC), which is regarded as the pivotal sequence in numerous defense genes promoters (Ulker and Somssich 2004; Eulgem and Somssich 2007), transient co-expression was performed in a homologous system to test the effects of VpWRKY1 and VpWRKY2 on reporter gene expression. Leaves co-transformed with reporter and over-expression constructs were stained dark blue, leaves transformed with only W-box–m35S–GUS showed a slight blue background, and the leaves transformed with only m35S–GUS or co-transformed with m35S–GUS and over-expression constructs did not stain blue (Fig. 3b). Results imply that VpWRKY1 and VpWRKY2 can activate GUS expression by binding to the 140-bp promoter fragment that contains three W-boxes.
Trans-activation of VpWRKY1 and VpWRKY2. a Schematic diagram of reporter and over-expression constructs (1301-VpWRKY1 and 1301-VpWRKY2) used for transient transformation of grapevine leaves. b GUS staining of two representative leaves transformed with constructs (n = 20). GUS staining was performed 3 days after transformation with m35S-GUS alone or co-transformed with m35S-GUS and over-expression constructs (top row). GUS staining was performed 3 days after transformation with W-box-m35S-GUS alone or co-transformed with W-box-m35S-GUS and over-expression constructs (bottom row). Fully expanded leaves from 6-week-old in vitro plantlets of Chinese wild grape clone V. pseudoreticulata ‘Baihe-35-1’ were used. Similar staining results were obtained in three biological experiments
Ectopic over-expression of VpWRKY1 and VpWRKY2 in Arabidopsis results in enhanced resistance to E. cichoracearum
To analyze the biological function of VpWRKY1 and VpWRKY2, the coding sequences of the two genes were transformed into Arabidopsis under the control of 35S promoter. T2 transgenic Arabidopsis were generated with the lines VpWRKY1-T2-4 (W1-4) and VpWRKY1-T2-13 (W1-13) exhibiting the greatest expression of VpWRKY1, and VpWRKY2-T2-2 (W2-2) and VpWRKY2-T2-7 (W2-7) exhibiting the greatest expression of VpWRKY2, as determined by semi-quantitative RT-PCR (Fig. 4a). Therefore, W1-4 and W1-13, W2-2 and W2-7 were selected for further studies. These transgenic Arabidopsis lines exhibited enhanced resistance to E. cichoracearum at 12 dpi compared to wild type (Fig. 4b). To quantify the E. cichoracearum resistance, the number of spores on the most susceptible leaf of each plant was determined. The number of E. cichoracearum spores was significantly decreased in the four transgenic lines at 12 dpi compared with the wild type (Fig. 4c).
VpWRKY1 (W1) and VpWRKY2 (W2) over-expression enhanced E. cichoracearum resistance of Arabidopsis. Six-week-old T2 lines were used for resistance and expression tests. a Semi-quantitative RT-PCR analysis of VpWRKY1 and VpWRKY2 expression in wild type and transgenic lines. The tubulin gene was amplified as control. b Disease reaction phenotypes of representative wild type and transgenic Arabidopsis at 12 dpi. c The ten most susceptible leaves from ten seedlings from each genotype at 12 dpi were pooled and the number of spores per milligram of fresh tissue was determined. Similar results were obtained in two biological experiments. Asterisks indicate a significant difference (P < 0.05). Expression of defense marker genes AtPR1 (d), AtPR10 (e), AtNPR1 (f), AtCOR1 (g), and AtPDF1.2 (h) in non-inoculated transgenic Arabidopsis under normal conditions. Fold changes indicate relative expression levels in transgenic lines compared with the wild type, which was set as 1. Mean values ± SD were obtained from three technical and three biological replicates
Since VpWRKY1 and VpWRKY2 are induced by defense signaling molecules, the defense response of the selected lines was analyzed by comparing the expression of SA- and JA/ET-dependent Arabidopsis defense marker genes AtPR1, AtPR10, AtNPR1, AtCOR1, and AtPDF1.2 with 35S::VpWRKY1, 35S::VpWRKY2, and wild type controls under normal conditions. qRT-PCR showed that the amount of AtPR10 and AtNPR1 transcripts were much higher in the two VpWRKY1 transgenic lines compared with the wild type (Fig. 4e, f). Transcription of AtPR10 was up-regulated only in the VpWRKY1 transgenic Arabidopsis lines under normal conditions (Fig. 4f). In contrast, AtPR1 transcription was dramatically down-regulated in all the four transgenic Arabidopsis lines compared with the wild type (Fig. 4d). Transcripts of AtCOR1 and AtPDF1.2 were down-regulated only in VpWRKY2 transgenic Arabidopsis lines (Fig. 4g, h). These results suggest that SA-dependent Arabidopsis defense marker genes are likely regulated by transcription factors VpWRKY1 and VpWRKY2, while JA/ET-dependent Arabidopsis defense marker genes are likely regulated only by VpWRKY2.
Ectopic over-expression of VpWRKY1 and VpWRKY2 in Arabidopsis results in enhanced tolerance to abiotic stress(es)
About 7% wild type Arabidopsis seeds were able to germinate in MS media with 150 mM NaCl, whereas germination rates of VpWRKY1 and VpWRKY2 transgenic seeds were significantly higher than that of the wild type, and transgenic seedlings grew very well under the same condition (Fig. 5a, b). In addition, cold tolerance test revealed that the two VpWRKY2 transgenic Arabidopsis seedlings grew to almost twice the size of wild type seedlings 1 week after cold treatment (Fig. 5c), biomass of the two VpWRKY2 transgenic lines was significantly higher than the wild type 1 week after cold treatment (Fig. 5d), whereas the two VpWRKY1 transgenic lines did not show any difference in growth compared with the wild type (data not shown). These indicate that over-expression of VpWRKY2 enhanced tolerance of transgenic seedlings to both salinity and cold stresses, while over-expression of VpWRKY1 only enhanced tolerance of transgenic seedlings to salinity stress.
VpWRKY1 and VpWRKY2 enhanced tolerance to abiotic stresses in transgenic Arabidopsis plants. a VpWRKY1 and VpWRKY2 enhanced salt tolerance in Arabidopsis. Seedlings were grown in MS medium with and without 150 mM NaCl. Phenotypes were scored 10 days after sowing. b Quantitative analysis of germination rate. Average germination rates and standard errors were calculated using results of three replicated experiments (n = 50). c VpWRKY2 enhanced cold tolerance. Seedlings were grown on MS medium before and after cold treatment (4°C for 6 h). Seedlings grown on MS medium for 2 weeks before cold treatment are shown in top row. Seedlings grown on MS medium 1 week after cold treatment are shown in bottom row. d Quantitative analysis of fresh weights 1 week after cold treatment. Average fresh weights and standard errors were calculated using results of three replicates (n = 50)
VpWRKY1 and VpWRKY2 regulate expression of grapevine defense marker genes in a transient transformation assay
The roles of VpWRKY1 and VpWRKY2 in the regulation of grapevine defense marker genes in a homologous system were studied using transient over-expression and silencing assay. qRT-PCR results showed that over-expression of VpWRKY2 resulted in increased VpPR1 transcripts, and silencing of VpWRKY1 reduced VpPR1 transcripts (Fig. 6a, b). In contrast, over-expression of VpWRKY1 enhanced expression level of VpPR10, and silencing of VpWRKY2 resulted in decreased expression of VpPR10 (Fig. 6a, b). Moreover, over-expression of VpWRKY1 or VpWRKY2 enhanced accumulation of VpNPR1, while silencing of VpWRKY1 or VpWRKY2 reduced VpNPR1 transcripts (Fig. 6a, b). Results indicate that VpWRKY1 and VpWRKY2 play a role in regulating the expression of the defense marker genes in homologous system.
Expression of grapevine defense marker genes VpPR1, VpPR10, and VpNPR1 when VpWRKY1 and VpWRKY2 are transiently over-expressed (a) and silenced (b) in grapevine leaves. VvGAPDH was used as internal control, and fold expression indicates expression levels in transiently over-expressed or silenced leaves compared with expression in GV3101 infiltrated leaves, which was set as 1. Mean values and SDs were obtained from three technical and three biological replicates
Discussion
Plant WRKY transcription factors are a superfamily of regulatory proteins forming a network of genes that regulate plant responses to variable environmental conditions (Pandey and Somssich 2009). In grape, 43 assembled WRKY genes have been predicted from susceptible V. vinifera using PlantGDB independent from the released whole genome sequences of V. vinifera (Jaillon et al. 2007; Velasco et al. 2007; Guo et al. 2008), and two WRKY transcription factor genes (VvWRKY1 and VvWRKY2) isolated from susceptible V. vinifera have been studied (Marchive et al. 2007; Mzid et al. 2007; Guillaumie et al. 2010). In the present study, both VpWRKY1 and VpWRKY2 were isolated from E. necator-resistant Chinese wild V. pseudoreticulata W. T. Wang ‘Baihe-35-1’. Sequence analysis showed that VpWRKY1 and VpWRKY2 belong to group III and group I of the WRKY superfamily, respectively (Supplementary Fig. S3). Both VpWRKY1 and VpWRKY2 proteins were shown to localize in the nucleus of onion epidermal cells (Fig. 2). Trans-activation assays showed that both proteins obviously activate GUS expression in a homologous system by binding to a 140-bp promoter fragment that contains three W-boxes. However, leaves transformed with only W-box-m35S-GUS also present with a slight blue background (Fig. 3b), suggesting that the endogenous WRKY proteins may be responsible for the background GUS expression by binding to the 140-bp fragment.
The response to pathogenic attack requires large-scale transcriptional reprogramming of WRKY genes (Pandey and Somssich 2009), and most of these processes have been proven to be induced by pathogens (Eulgem and Somssich 2007). In a previous report, powdery mildew-induced transcriptional change of VaWRKY30 was observed in the powdery mildew-susceptible V. vinifera only, and not in the powdery mildew-resistant V. aestivalis (Fung et al. 2008). However, in the current study, expression of VpWRKY1 and VpWRKY2 can rapidly be induced by E. necator in their genotype of origin (‘Baihe-35-1’) and ten other grapevine genotypes (Fig. 1b, c). In particular, expression levels of VpWRKY1 correlate well with the degree of resistance to E. necator of all 11 grapevine genotypes (Fig. 1a), whereas VpWRKY2 does not exhibit such correlation (Fig. 1b). E. necator-induced expression patterns provide preliminary evidence for the role of VpWRKY1 and VpWRKY2 in regulating powdery mildew resistance in grapevine. The deduced amino acid sequences for VpWRKY1 and VpWRKY2 show very high similarities with the predicted proteins from susceptible V. vinifera, whereas the differences in the post-inoculation expression levels between powdery mildew-resistant V. pseudoreticulata and powdery mildew-susceptible V. vinifera might be attributed to the different regulatory mechanisms controlled by cis-regulatory elements in the promoter region (Xu et al. 2010).
To investigate the role of VpWRKY1 and VpWRKY2 in powdery mildew resistance, the two genes were over-expressed in Arabidopsis Col-0 and the resistance of the transgenic plants was tested against E. cichoracearum, a fungal biotroph that is virulent to Arabidopsis Col-0. Results showed that VpWRKY1 and VpWRKY2 enhanced resistance to E. cichoracearum (Fig. 4b, c). Similarly, AtWRKY3, AtWRKY4, AtWRKY18, AtWRKY33, AtWRKY53 and AtWRKY70 in Arabidopsis also have been shown to enhance resistance to pathogens (Xu et al. 2006; Knoth et al. 2007; Lai et al. 2008; Pandey and Somssich 2009). In rice, OsWRKY13, OsWRKY31, OsWRKY45, OsWRKY53 and OsWRKY71 positively contribute to resistance to pathogens (Chujo et al. 2007; Liu et al. 2007; Qiu et al. 2007; Zhang et al. 2008; Qiu and Yu 2009). Expression of a Medicago truncatula WRKY (W109669) can enhance the response to the tobacco mosaic virus in transgenic tobacco plants (Naoumkina et al. 2008). In addition, over-expression of WRKY transcription factor genes isolated from susceptible V. vinifera leads to enhanced resistance to tobacco mildew (Marchive et al. 2007). However, the closest homolog of VpWRKY1 (Supplementary Fig. S3), AtWRKY70, acts as a negative regulator of disease resistance in Arabidopsis (Ulker et al. 2007). This may be attributed to the different roles that WRKY genes play in decreasing or enhancing susceptibility toward pathogens (Eulgem and Somssich 2007). Results of this study suggest that VpWRKY1 and VpWRKY2 play a positive role in Arabidopsis powdery mildew resistance.
Plant defense responses to microbial attack is regulated through a complex network of signaling pathways that involve SA, JA, and Eth (Glazebrook 2005), and WRKY genes are often induced by these signaling molecules (Eulgem et al. 2000). In this study, both VpWRKY1 and VpWRKY2 were induced rapidly by SA treatment and E. necator infection in E. necator-resistant Chinese wild V. pseudoreticulata W. T. Wang ‘Baihe-35-1’ (Fig. 1a, b, c). Similarly, 43 out of 72 WRKY genes tested in Arabidopsis were induced by SA treatment or bacterial infection (Dong et al. 2003). Studies on WRKY genes predominantly indicate an involvement in the SA signaling pathway (Wang et al. 2009). However, SA, JA, and Eth defense signaling pathways do not function independently, but exhibit complicated cross-talk and interaction including synergism and antagonism, during defense response (Glazebrook 2005). In the present study, VpWRKY2 was also shown to be induced rapidly by signaling molecules MeJA and Eth in ‘Baihe-35-1’, whereas VpWRKY1 was unaffected by MeJA or Eth (Fig. 1d, e). Though these are not conclusive evidence for synergistic and/or antagonistic relationships among the three signaling molecules during VpWRKY2 expression, results suggest that VpWRKY2 is involved in a more complex defense signaling network than VpWRKY1 in grapevine. Transcription factors are essential components of the defense signaling pathways, since they regulate the expression of defense-related marker genes (Eulgem 2005). In this study, over-expression of VpWRKY1 and VpWRKY2 were shown to be capable of regulating the expression of SA-dependent maker genes AtPR1 and AtNPR1 in transgenic Arabidopsis plants (Fig. 4d, f). However, another SA-dependent maker gene, AtPR10, was up-regulated only in VpWRKY1 transgenic Arabidopsis plants and not in VpWRKY2 transgenic Arabidopsis (Fig. 4e). This may be because AtPR10 cannot be regulated by VpWRKY2 in transgenic Arabidopsis plants. Among the three SA-dependent defense marker genes, only AtPR1 was down-regulated in all four transgenic lines (Fig. 4d), which concurs with previous reports that AtPR1 appears to function negatively in disease resistance in Arabidopsis (Ulker et al. 2007; Savitch et al. 2007). Moreover, expression of AtCOR1 and AtPDF1.2 was down-regulated in VpWRKY2 transgenic lines, suggesting that VpWRKY2 acts as a repressor of some JA/Eth-dependent genes in Arabidopsis. This is in agreement with previous studies stating that AtWRKY70 acts as a repressor of JA/Eth response genes AtCOR1 and AtPDF1.2 (Li et al. 2004; Ulker et al. 2007). Therefore, VpWRKY1 and VpWRKY2 may enhance resistance to E. cichoracearum through repression of some defense genes that function negatively and increasing the expression of other genes that regulate positively in disease resistance.
Defense marker genes are up- or down-regulated in transgenic Arabidopsis plants, possibly as a result of the transcriptional output of individual downstream target genes that are either positively or negatively affected by WRKY proteins (Journot-Catalino et al. 2006). Heterologous expression in Arabidopsis has shown that VpWRKY1 and VpWRKY2 regulate the expression of defense marker genes. To evaluate the effect of VpWRKY1 and VpWRKY2 in a homologous system, the transient expression system was utilized. The results confirmed that VpWRKY1 and VpWRKY2 regulate some defense marker genes in a homologous system thus suggesting that these defense marker genes in Arabidopsis and grapevine are regulated by VpWRKY1 or VpWRKY2. In this study, AtPR1 expression was decreased in VpWRKY2 transgenic Arabidopsis plants, while VpPR1 was increased in VpWRKY2 transgenic grapevine leaves. What may be the reason which causes the different expression models of AtPR1 and VpPR1 in both VpWRKY2 transgenic plants? First, though they are both PR1 proteins, deduced amino acid sequences of the two PR1 proteins only share 57% similarity. Second, there is a difference in promoter sequences of PR1 genes between Arabidopsis and grapevine, which may result in VpWRKY2 protein specific binding to different cis-elements of the PR1 promoters. Third, other factors that interact with VpWRKY2 protein may be involved in enhancing or inhibiting the expression of different PR1 genes in grapevine or Arabidopsis.
WRKY proteins specifically interact with the W-box, a major class of cis-element in the context of promoters of pathogen- or elicitor-responsive genes, such as pathogenesis-related proteins, receptor protein kinases, or WRKY transcription factors (Journot-Catalino et al. 2006; Xu et al. 2006; Pandey and Somssich 2009; Wang et al. 2009). Thus, this study analyzed the 1-kb sequence upstream of the translation start sites of all eight defense marker genes that were selected based on the released whole genome sequences of V. vinifera in NCBI and Arabidopsis in TAIR. Results revealed that the promoters of these eight defense marker genes are enriched with W-boxes, containing three to six W-boxes within the 1-kb sequences upstream of VpPR1, VpPR10, VpNPR1, AtPR1, AtPR10, AtNPR1, AtCOR1, and AtPDF1.2 were found. These indicate that VpWRKY1 and VpWRKY2 may enhance resistance to E. cichoracearum by regulating the expression of defense marker genes. Their ability to regulate the expression of grapevine defense marker genes provides important insights into the molecular basis of VpWRKY1 and VpWRKY2 in grapevine powdery mildew resistance.
Another important role of WRKY genes is the enhancement of tolerance to abiotic stresses. Numerous studies have reported that expression of WRKY genes in plants is induced by abiotic stresses (Rizhsky et al. 2002; Ulker and Somssich 2004; Marchive et al. 2007; Jing et al. 2009). In the present study, VpWRKY1 and VpWRKY2 transgenic Arabidopsis plants are more capable of adapting to the salt stress during germination (Fig. 5a, b). Similarly, recent reports have revealed that Arabidopsis WRKY2 mediates seed germination, and OsWRKY08 improves osmotic stress tolerance of transgenic Arabidopsis (Jiang and Yu 2009; Yu et al. 2009). Moreover, VpWRKY2 transgenic Arabidopsis plants have enhanced cold tolerance in growing seedlings (Fig. 5c, d). These findings concur with an earlier report that over-expression of soybean GmWRKY21 in Arabidopsis can enhance tolerance to cold stress (Zhou et al. 2008). Therefore, results of the present study confirm that grapevine WRKY also functions in regulating tolerance to abiotic stresses.
Despite the similar expression patterns of the two genes in post E. necator infection of grapevine and the similar roles in enhancing resistance of transgenic Arabidopsis plants to E. cichoracearum infection, the two WRKY genes differ in some other characteristics. First, VpWRKY1 can activate reporter genes in yeast, while VpWRKY2 is unable to do (Supplementary Fig. S6); second, MeJA and Eth can induce VpWRKY2 expression in grapevine, whereas they cannot induce VpWRKY1. Third, weak VpWRKY2 expression can be detected in roots while VpWRKY1 cannot be detected in roots of ‘Baihe-35-1’ under natural field conditions (Supplementary Fig. S7); fourth, VpWRKY1 regulates expression of AtPR10, while VpWRKY2 cannot, and VpWRKY2 regulates AtCOR1 and AtPDF1.2, while VpWRKY1 cannot; fifth, VpWRKY2 can enhance cold tolerance of transgenic Arabidopsis plants, while VpWRKY1 cannot. There may be additional differences between these two WRKY genes, suggesting that they perform different functions in plant defense and development.
In summary, the two transcription factor genes, VpWRKY1 and VpWRKY2, isolated from grapevine powder mildew-resistant Chinese wild V. pseudoreticulata, were induced rapidly by E. necator infection in 11 grapevine genotypes. The increase in induction of VpWRKY1 correlates with the level of resistance of the genotypes. Over-expression of VpWRKY1 and VpWRKY2 in Arabidopsis can increase resistance to powdery mildew and regulate the expression of some defense marker genes in transgenic Arabidopsis plants. The two genes can also regulate the expression of some grapevine defense marker genes in a transient transformation assay. Results suggest that VpWRKY1 and VpWRKY2 may participate in the resistance of transgenic grapevine to E. necator. In addition, these two genes may enhance tolerance of transgenic grapevine to abiotic stresses. Further studies using a stable homologous expression system will confirm the role of these two WRKY transcription factors in grapevine defense mechanisms.
Abbreviations
- qRT-PCR:
-
Quantitative reverse transcriptase-polymerase chain reaction
- VpWRKY1 :
-
Vitis pseudoreticulata WRKY1
- VpWRKY2 :
-
Vitis pseudoreticulata WRKY2
- SA:
-
Salicylic acid
- MeJA:
-
Methyl jasmonate
- Eth:
-
Ethephon
- Hpi:
-
Hours post inoculation
- dpi:
-
Days post inoculation
- hpt:
-
Hours post treatment
- EST:
-
Expressed sequence tag
References
Belhadj A, Telef N, Cluzet S, Bouscaut J, Corio MF, Merillon M (2008) Ethephon elicits protection against Erysiphe necator in grapevine. J Agric Food Chem 56:5781–5787
Bellin D, Peressotti E, Merdinoglu D, Wiedemann-Merdinoglu S, Adam-Blondon AF, Cipriani G et al (2009) Resistance to Plasmopara viticola in grapevine ‘Bianca’ is controlled by a major dominant gene causing localized necrosis at the infection site. Theor Appl Genet 120:163–176
Bisson LF, Waterhouse AL, Ebeler SE, Walker MA, Lapsley JT (2002) The present and future of the international wine industry. Nature 418:696–699
Chujo T, Takai R, Akimoto-Tomiyama C, Ando S, Minami E, Nagamura Y et al (2007) Involvement of the elicitor-induced gene OsWRKY53 in the expression of defense related genes in rice. Biochim Biophys Acta 1769:497–505
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Coleman C, Copetti D, Cipriani G, Hoffmann S, Kozma P, Kovacs L et al (2009) The powdery mildew resistance gene REN1 co-segregates with an NBS-LRR gene cluster in two Central Asian grapevines. BMC Genet 10:89
Dong J, Chen C, Chen Z (2003) Expression profiles of Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol Biol 51:21–37
Eulgem T (2005) Regulation of the Arabidopsis defense transcriptome. Trends Plant Sci 10:71–78
Eulgem T, Somssich IE (2007) Networks of WRKY transcription factors in defense signaling. Curr Opin Plant Biol 10:366–371
Eulgem T, Rushton PJ, Robatzek S, Somssich IE (2000) The WRKY superfamily of plant transcription factors. Trends Plant Sci 5:199–206
Fung RWM, Gonzalo M, Fekete C, Kovacs LG, He Y, Marsh E et al (2008) Powdery mildew induces defense-oriented reprogramming of the transcription in a susceptible but not in a resistance grapevine. Plant Physiol 146:236–249
Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43:205–227
Guan X, Zhao HQ, Xu Y, Wang YJ (2010) Transient expression of glyoxal oxidase from the Chinese wild grape Vitis pseudoreticulata can suppress powdery mildew in a susceptible genotype. Protoplasma. doi:10.1007/s00709-010-0162-4
Guillaumie S, Mzid R, Mechin V, Leon C, Hichri I, Destrac-Irvine A et al (2010) The grapevine transcription factor WRKY2 influences the lignin pathway and xylem development in tobacco. Plant Mol Biol 72:215–234
Guo AY, Chen X, Gao G, Zhang H, Zhu QH, Liu XC et al (2008) PlantTFDB: a comprehensive plant transcription factor database. Nucleic Acids Res 36:966–969
Gutterson N, Reuber TL (2004) Regulation of disease resistance pathways by AP2/ERF transcription factors. Curr Opin Plant Biol 7:465–471
Jaillon O, Aury JM, Noel B, Policriti A, Clepet C, Casagrande A et al (2007) The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449:463–468
Jiang W, Yu D (2009) Arabidopsis WRKY2 transcription factor mediates seed germination and postgermination arrest of development by abscisic acid. BMC Plant Biol 9:96
Jing S, Zhou X, Song Y, Yu D (2009) Heterologous expression of OsWRKY23 gene enhances pathogen defense and dark-induced leaf senescence in Arabidopsis. Plant Growth Regul 58:181–190
Journot-Catalino N, Somssich IE, Roby D, Kroj T (2006) The transcription factors WRKY11 and WRKY17 act as negative regulators of basal resistance in Arabidopsis thaliana. Plant Cell 18:3289–3302
Knoth C, Ringler J, Dangl JL, Eulgem T (2007) Arabidopsis WRKY70 is required for full RPP4-mediated disease resistance and basal defense against Hyaloperonospora parasitica. Mol Plant Microbe Interact 20:120–128
Lai Z, Vinod KM, Zheng Z, Fan B, Chen Z (2008) Roles of Arabidopsis WRKY3 and WRKY4 transcription factors in plant responses to pathogens. BMC Plant Biol 8:68
Li J, Brader G, Palva ET (2004) The WRKY70 transcription factor: a node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 16:319–331
Liu X, Bai X, Wang X, Chu C (2007) OsWRKY71, a rice transcription factor, is involved in rice defense response. J Plant Physiol 164:969–979
Marchive C, Mzid R, Deluc L, Barrieu F, Pirello J, Gauthier A et al (2007) Isolation and characterization of a Vitis vinifera transcription factor, VvWRKY1, and its effect on responses to fungal pathogens in transgenic tobacco plants. J Exp Bot 58:1999–2010
Mare C, Mazzucotelli E, Crosatti C, Francia E, Stanca AM, Cattivelli L (2004) Hv-WRKY38: a new transcription factor involved in cold- and drought-response in barley. Plant Mol Biol 55:399–416
Mzid R, Marchive C, Blancard D, Deluc L, Barrieu F, Corio-Costet MF et al (2007) Overexpression of VvWRKY2 in tobacco enhances broad resistance to necrotrophic fungal pathogens. Physiol Plant 131:434–447
Naoumkina M, He X, Dixon R (2008) Elicitor-induced transcription factors for metabolic reprogramming of secondary metabolism in Medicago truncatula. BMC Plant Biol 8:132
Pandey SP, Somssich IE (2009) The role of WRKY transcription factors in plant immunity. Plant Physiol 150:1648–1655
Pavlousek P (2007) Evaluation of resistance to powdery mildew in grapevine genetic resources. J Cent Eur Agric 8:105–114
Qiu Y, Yu D (2009) Over-expression of the stress-induced OsWRKY45 enhances disease resistance and drought tolerance in Arabidopsis. Environ Exp Bot 65:35–47
Qiu D, Xiao J, Ding X, Xiong M, Cai M, Cao Y et al (2007) OsWRKY13 mediates rice disease resistance by regulating defense related genes in salicylate- and jasmonate-dependent signaling. Mol Plant Microbe Interact 20:492–499
Repka V, Fischerova I, Silharova K (2004) Methyl jasmonate is a potent elicitor of multiple defense responses in grapevine leaves and cell-suspension culture. Biol Plant 48:273–283
Rizhsky L, Liang H, Mittler R (2002) The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol 130:1143–1151
Savitch LV, Subramaniam R, Allard GC, Singh J (2007) The GLK1 ‘regulon’ encodes disease defense related proteins and confers resistance to Fusarium graminearum in Arabidopsis. Biochem Biophys Res Commun 359:234–238
Singh KB, Foley RC, Onate-Sanchez L (2002) Transcription factors in plant defense and stress responses. Curr Opin Plant Biol 5:430–436
Ulker B, Somssich IE (2004) WRKY transcription factors: from DNA binding towards biological function. Curr Opin Plant Biol 7:491–498
Ulker U, Shahid MM, Somssich IE (2007) The WRKY70 transcription factor of Arabidopsis influences both the plant senescence and defense signaling pathways. Planta 226:125–137
Velasco R, Zharkikh A, Troggio M, Cartwright DA, Cestaro A, Pruss D et al (2007) A high quality draft consensus sequence of the genome of a heterozygous grapevine variety. PLoS ONE 2:e1326
Wang LJ, Li SH (2006) Thermotolerance and related antioxidant enzyme activities induced by heat acclimation and salicylic acid in grape (vitis vinifera L.) leaves. Plant Growth Regul 48:137–144
Wang YJ, Liu Y, He PC, Chen J, Lamicanra O, Lu J (1995) Evaluation of foliar resistance to Uncinula necator in Chinese wild Vitis species. Vitis 34:159–164
Wang Z, Zhu Y, Wang L, Liu X, Liu Y, Phillips J et al (2009) A WRKY transcription factor participates in dehydration tolerance in Boea hygrometrica by binding to the W-box elements of the galactinol synthase (BhGolS1) promoter. Planta 230:1155–1166
Wesley SV, Helliwell CA, Smith NA et al (2001) Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J 27:581–590
Xiao S, Ellwood S, Findlay K, Oliver RP, Turner JG (1997) Characterization of three loci controlling resistance of Arabidopsis thaliana accession Ms-0 to two powdery mildew diseases. Plant J 12:757–768
Xiao S, Calis O, Patrick E, Zhang G, Charoenwattana P, Muskett P et al (2005) The atypical resistance gene, RPW8, recruits components of basal defence for powdery mildew resistance in Arabidopsis. Plant J 42:95–110
Xu XP, Chen CH, Fan BF, Chen ZX (2006) Physical and functional interactions between pathogen-induced Arabidopsis WRKY18, WRKY40, WRKY60 transcription factors. Plant Cell 18:1310–1326
Xu Y, Zhu Z, Xiao Y, Wang Y (2009) Construction of a cDNA library of Vitis pseudoreticulata native to China inoculated with uncinula necator and the analysis of potential defence-related expressed sequence tags (ESTs). Safr J Enol Vitic 30:65–71
Xu W, Yu Y, Ding J, Hua Z, Wang Y (2010) Characterization of a novel stilbene synthase promoter involved in pathogen- and stress-inducible expression from Chinese wild Vitis pseudoreticulata. Planta 231:475–487
Yu S, Jing S, Yu D (2009) Overexpression of the stress-induced OsWRKY08 improves osmotic stress tolerance in Arabidopsis. Chin Sci Bull 54:4671–4678
Zhang JJ, Wang YJ, Wang XP (2003) An improved method for rapidly extracting total RNA from Vitis. J Fruit Sci 20:178–181 (in Chinese with English abstract)
Zhang J, Peng Y, Guo Z (2008) Constitutive expression of pathogen inducible OsWRKY31 enhances disease resistance and affects root growth and auxin response in transgenic rice plants. Cell Res 18:508–521
Zhou QY, Tian AG, Zou HF, Xie ZM, Lei G, Huang J et al (2008) Soybean WRKY-type transcription factor genes, GmWRKY13, GmWRKY21, GmWRKY54, confer differential tolerance to abiotic stresses in transgenic Arabidopsis plants. Plant Biotechnol J 6:486–503
Acknowledgments
We thank Dr. Zhongchi Liu (Department of Cell Biology and Molecular Genetics, University of Maryland, USA) for critical review and comments of the manuscript and Courtney Hollender (Department of Cell Biology and Molecular Genetics, University of Maryland, USA) for the help of revising this manuscript (language). This work was supported by the National Natural Science Foundation of China for “Expression and Functional Analysis of Transcription Factor Genes Isolated from Powdery Mildew-resistant Chinese Wild Grape” (Grant No. 30771493).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, H., Xu, Y., Xiao, Y. et al. Expression and functional analysis of two genes encoding transcription factors, VpWRKY1 and VpWRKY2, isolated from Chinese wild Vitis pseudoreticulata . Planta 232, 1325–1337 (2010). https://doi.org/10.1007/s00425-010-1258-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-010-1258-y