Abstract
The nucleic acid binding protein Whirly1 of barley has been located to both chloroplasts and the nucleus of the same cell. Immunogold labelling furthermore showed that in vivo Whirly1 does not strictly co-localize with DNA in chloroplasts, while it is closely associated with DNA in the nucleus. High-resolution imaging of Whirly1-GFP and PEND-RFP fusion proteins revealed that only a minor part of Whirly1 co-localizes with nucleoids. The co-localization with nucleoids is in accordance with the detection of Whirly1 in a conventionally prepared fraction of the transcriptionally active chromosome (TAC). By further purification and enrichment of transcriptional activity Whirly1, however, was lost from the TAC fraction. Knockdown of Whirly1 in transgenic barley plants had neither impact on transcription of selected protein coding genes nor on genes coding for ribosomal RNAs or tRNAs. The results of RIP-chip experiments showed that barley Whirly1 as its maize orthologue associates with a set of intron containing plastid RNAs. Taken together, the results suggest that plastid-located Whirly1 functions primarily in RNA metabolism rather than as a DNA binding protein.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The single stranded DNA binding factor Whirly1 belongs to a small family of plant-specific proteins with two members in most angiosperms and three members in Arabidopsis thaliana (Desveaux et al. 2005; Krause et al. 2005, 2009). By in organello import and localization of GFP fusion proteins, Whirly1 has been shown to be translocated into plastids (Krause et al. 2005). Moreover, immunological analyses revealed that the protein is located in chloroplasts and the nucleus of the same cell (Grabowski et al. 2008).
Crystallographic analysis revealed that Whirly1 of potato (StWhy1) has a cyclic quaternary structure inspiring the name Whirly for this protein family. It has been proposed that StWhy1 binds as a tetramer to melted promoter regions, and might thus modulate transcription (Desveaux et al. 2002). In accordance with its binding to promoter sequences, in the nucleus Whirly1 has been shown to function as a transcription factor. Electrophoretic mobility shift assays indicated that nuclear Whirly1 in potato binds to the inverted repeat sequence of the elicitor response element (ERE) of the PR10a gene (Desveaux et al. 2000). Recently, chromatin immunoprecipitation revealed that the Whirly1 protein of A. thaliana together with Whirly3 in vivo can bind to the upstream region of the kinesin gene AtKP1 and repress its transcription (Xiong et al. 2009). In another study it had been shown that Whirly1 in the nucleus is also involved in telomere maintenance (Yoo et al. 2007).
The attachment of the Whirly1 protein to DNA seems to be mediated by the KGKAAL domain (Desveaux et al. 2002, 2005). Bimolecular complementation assays with partial Whirly1-YFP fusion constructs have shown that in vivo Whirly1 interacts with itself when it is located in the nucleus (Grabowski et al. 2008). This result is in accordance with a function as a DNA binding tetramer. When the Whirly1-YFP fusion proteins were, however, delivered to plastids, no interaction of Whirly1 with itself was detectable (Grabowski et al. 2008). If tetramer formation would be a prerequisite for DNA binding, it is unlikely that Whirly1 functions as a DNA binding protein in plastids.
Plastid-located Whirly1 has been detected in the fraction of the transcriptionally active chromosome (TAC) (Pfalz et al. 2006) which suggested association of the Whirly protein to plastid DNA. Binding to plastid DNA was confirmed by nucleic acid co-immunoprecipitation with an antibody directed towards maize Whirly1 (Prikryl et al. 2008). However, binding to DNA was uniformly distributed over the entire plastid genome making it rather unlikely that Whirly1 in plastids is a gene-specific transcription factor. Transcriptional activities of plastid genes specific for ribosomal RNA and tRNA-G showed no difference between wild type and maize Why1 mutants, further speaking against an involvement in transcription (Prikryl et al. 2008). Recently, it has been suggested that plastid-localized Whirly proteins are involved in maintenance of plastid genome stability by preventing accumulation of illegitimate recombination products (Maréchal et al. 2009).
In this report, immunogold labelling and high-resolution imaging of Whirly1-GFP fusion proteins were used to re-examine the association of Whirly1 with plastid nucleoids. Both methods showed that the majority of Whirly1 in barley chloroplasts is not associated with DNA. Furthermore, the protein was shown to get lost from the transcriptionally active chromosome during purification. Transgenic barley plants with a knockdown of Whirly1 were shown to have a wild-type-like appearance, and did not show changes in transcription of a representative set of genes. We furthermore showed that HvWhy1 in chloroplasts associates with a set of intron containing RNAs. In contrast to the situation in maize, a knockdown of Whirly1 in barley had, however, no obvious effect on chloroplast development.
Materials and methods
Plant material
Barley (Hordeum vulgare L. cv. Steffi) seedlings used for preparation of TAC fractions were grown in a climate chamber for 7 days on vermiculite at 20°C in a light/dark regime of 16 h light and 8 h darkness. Transgenic barley plants (Hordeum vulgare L. cv. Golden Promise) with an RNAi knockdown of the Whirly1 gene were prepared as described by Hensel et al. (2008). Lines E1 and E9 were shown to have one insertion of the RNAi cassette each, while line E6 had two insertions (Supplemental Fig. S1). In the T4 generation, lines E1 and E9 were found to be homozygous, whereas the E6 line was heterozygous. Plants of the T4 generation from the three lines E1, E6, E9 and of one mock line were cultivated in a glass-house for 21 days on soil under long day conditions (16 h light/8 h dark) at maximal 20°C. For transcription and protein analyses fully mature third and developing fourth leaves were used. For preparation of protoplasts, 5–6-week-old tobacco (Nicotiana tabacum L. cv. Xanthi) plants grown on Murashige and Skoog medium were used.
Preparation of leaf segments and immunogold labelling
Small segments of barley leaves were fixed over night at 4°C in 4% (w/v) formaldehyde (freshly made from paraformaldehyde) in phosphate buffered saline solution (PBS), pH 7.4. After washing in PBS, the samples were dehydrated in a graded series of ice-cold ethanol and embedded in LR white resin (Plano, Marburg, Germany). Sections were cut with a diamond knife in a Leica Ultracut UCT ultramicrotome (Leica Microsystems GmbH, Wetzlar, Germany). Ultrathin sections were collected on pioloform-coated Au grids. After incubation with a primary antibody mixture of polyclonal anti-HvWhy1 (directed towards peptide P1, 1:10; Grabowski et al. 2008) from rabbit and monoclonal anti-DNA from mouse (1:50, clone AC-30-10, Chemicon/Millipore, Schwalbach, Germany) and washing, the sections were incubated with a mixture of the corresponding secondary antibodies (both diluted 1:50): anti-rabbit IgG, conjugated to 15 nm gold particles, and anti-mouse IgG, conjugated to 10 nm gold particles (British BioCell International Ltd, Cardiff, UK). Finally, the sections were poststained with satured uranyl acetate in water and observed in a Philips CM10 transmission electron microscope (TEM, Philips Scientifics, Eindhoven, The Netherlands). The obtained pictures were further evaluated with the CELL* Imaging Software for Life Microscopy (Soft Imaging System GmbH, Münster, Germany). After measuring the area of chloroplasts and nuclei on printed images, the number of HvWhy1- and DNA-associated gold particles per 2 μm2 section was calculated, and the distances between the HvWhy1- and DNA-associated particles were determined.
Transient expression of AtWhy1-GFP and PEND-RFP constructs in tobacco protoplasts
Protoplasts were isolated from tobacco leaves and were used for PEG-mediated transformation as described (Krause et al. 2005). AtWhy1-GFP and PEND-RFP constructs were already described by Krause et al. (2005) and Terasawa and Sato (2005), respectively. Distribution of GFP or RFP fusion proteins was inspected by confocal laser scanning microscopy and evaluated with the LAS FA SP5 software (Leica TCS SP5, Leica Microsystems CMS GmbH, Wetzlar, Germany).
Isolation of chloroplasts and preparation of stroma and thylakoid fractions
For immunological analysis, chloroplasts were prepared from primary foliage leaves of barley seedlings grown for 7 days by the procedure of Poulsen (1983). For fractionation of membranes and stroma, respectively, chloroplasts were osmotically lysed, and the membranes were enriched by centrifugation at 20,000×g for 10 min.
For run-on transcription assays, chloroplasts were prepared and purified on Percoll step gradients according to a standard procedure (Gruissem et al. 1986) modified as described (Krupinska 1992).
Isolation of transcriptionally active chromosome fractions (TAC-I and TAC-II)
The TAC-I fraction was prepared from barley chloroplasts by gel filtration on a Sepharose CL4B as described earlier (Suck et al. 1996). For further purification, the TAC-I fraction was subjected to precipitation by protamine sulphate and a second gel filtration on Sepharose CL4B column (Pharmacia, Freiburg, Germany) yielding TAC-II as described (Krause and Krupinska 2000). The transcriptional activity of TAC fractions was determined according to Krupinska and Falk (1994). One unit of transcriptional activity was defined as described by Suck et al. (1996) as 1 fmol radioactive-labelled UTP (α-32P) incorporated into in vitro elongated RNA chains during 30 min at 30°C. Incorporation of nucleotides was measured with DE81 filters according to Suck et al. (1996).
Immunological analysis of protein levels
Total protein extracts prepared from barley leaves were isolated according to Dehesh et al. (1986). For immunological analyses, proteins in fractions eluting from Sepharose column during preparation of TAC-I were precipitated with methanol and chloroform according to the method of Wessel and Flügge (1984). Protein concentrations were determined according to (Bradford 1976) using Roti-Nanoquant according to the manufacturer’s instructions (Roth, Karlsruhe, Germany).
Equal amounts of proteins were subjected to polyacrylamide gel electrophoresis employing a high concentration of Tris (Fling and Gregerson 1986), and were subsequently transferred onto nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany). Immunological analyses were carried out as described (Humbeck et al. 1996) using an ECL Western Blotting Detection Kit (GE Healthcare, Munich, Germany). For detection of HvWhy1 the antibody directed towards oligopeptide P2 (Grabowski et al. 2008) was used. Other antibodies used for immunological analysis were as follows: anti-MFP1-antibody (OSU91) directed towards nuclear-encoded MAR binding filament-like protein 1 (Jeong et al. 2003) and anti-TCP34 antibody directed towards tetratricopeptide-containing chloroplast protein of 34 kDa (Weber et al. 2006). A specific antibody directed towards the α-subunit of plastid-encoded RNA polymerase (RpoA) had been previously provided by Dr. Hans Kössel (Suck et al. 1996; Krause and Krupinska 2000).
Determination of chlorophyll content
Pigments were extracted from barley leaves with 80% (v/v) acetone. Absorbance of chlorophylls was measured at 663 and 646 nm with a spectrophotometer (Shimadzu, UV-2501PC, Duisburg, Germany), and concentrations were calculated using the formula given by Lichtenthaler (1987).
Run-on transcription assays with isolated chloroplasts
Run-on transcription assays were performed with chloroplasts derived from barley third and fourth leaves as described (Krupinska and Apel 1989). DNA filters carrying 23 barley plastid genes blotted in a series of three dilutions: 320 (1×), 80 (4×) and 20 (16×) were prepared according to Krupinska (1992). After hybridization, the radioactively labelled filters were exposed to a phosphor imaging plate (Fuji, Tokyo, Japan) and also to X-ray films (Hyperfilm MP, Amersham Biosciences, Freiburg, Germany). Radioactive signals measured by the image reader (FLA-5000, Fuji, Tokyo, Japan) were used for calculation of relative signal densities.
RNA co-immunoprecipitation assays
For chloroplast preparation and stroma extraction, 2-week-old barley plants were harvested and processed using conditions and procedures already established for maize (Schmitz-Linneweber et al. 2005). The antibody used to enrich HvWhy1 was described previously (directed against oligopeptide P2; Grabowski et al. 2008). Immunoprecipitation protocols, RNA labelling, hybridization of RNA on a maize chloroplast microarray and data analysis were carried out as reported previously (Schmitz-Linneweber et al. 2005). Control RIP-chip experiments were carried out using an antibody directed against the β-subunit of the chloroplast ATPase kindly provided by A. Barkan. Microarray probe positions and fluorescence data can be found in Supplemental Table S1.
RNA gel blot analysis of selected plastid genes
RNA was extracted from third and fourth leaves of barley plants. Agarose gels and blotting onto nictrocellulose filters and hybridization were performed with standard procedures. Probes specific for selected barley plastid genes were amplified by PCR using following primer: atpF (exon2) 5′-GATTTATTAGATAATCGAAAACA-3′ and 5′-TTATCTCTTCCATTCCAGGG-3′; atpF (intron) 5′-AGGGAGTCTGTGCGAGTT-3′ and 5′-AATGAAAGTAGATTATTTTGTAAG-3′; rpl16 (exon2) 5′-CTATATCTCTACTATCGAATAAATT-3′ and 5′-AACCCCAAAAGAACCAGATT-3′; rpl16 (intron) 5′-TCTCTAATTCCATAATATTTAAAA-3′ and 5′-CAACCTATTGCTTCGTATTG-3′. DNA fragments were radioactively labelled with α-32PdCTP (Hartmann Analytic, Braunschweig, Germany) using the HexaLabel Kit (MBI Fermentas, St. Leon, USA). After hybridization, filters were exposed to X-ray films (Hyperfilm MP, Amersham Biosciences, Freiburg, Germany).
Results
The majority of chloroplast-located Whirly1 in vivo is not associated with DNA
ZmWhy1 was immunologically detected in two fractions of chloroplast stroma separated by sucrose density gradients centrifugation. The smaller of the two peaks was sensitive to DNAse treatment, whereas the peak corresponding to larger particles was sensitive to RNAse treatment (Prikryl et al. 2008). To investigate the localization of barley Whirly1 in vivo, immunogold analyses with thin sections from barley leaves were performed. These analyses had shown that HvWhy1 is partly associated with thylakoids and is additionally found in the stroma (Grabowski et al. 2008). The results are in accordance with an association of part of Whirly1 to thylakoid-bound nucleoids. To further investigate whether thylakoid-associated HvWhy1 is in direct contact with DNA in vivo, immunogold labelling was performed with the antibody raised against the 15 amino acids oligopeptide P1 of the HvWhy1 protein (Grabowski et al. 2008) and a mouse monoclonal antibody directed towards DNA. Thin sections of primary foliage leaves of barley were simultaneously incubated with both antibodies, with small gold particles (10 nm) being used for DNA, and larger gold particles (15 nm) being used for detection of HvWhy1 (Fig. 1a, b). When the number of larger gold particles per area of the two compartments was counted, two times as much HvWhy1-associated gold particles were observed in the chloroplasts (a) than in the nucleus (b). In contrast, the density of DNA-associated small gold particles was higher in the nucleus than in the chloroplast. The average minimal distance between HvWhy1- and DNA-associated particles was twice as high in chloroplasts compared to the nucleus where frequently pairs and clusters consisting of both types of particles could be found (Fig. 1a, b; Supplemental Table S1). These results indicate that in the nucleus Whirly1 co-localizes with DNA, while in chloroplasts most of Whirly1 is not associated with DNA.
Subcellular localization of the Whirly1 protein and DNA. Immunogold labelling of the HvWhy1 protein and DNA in chloroplasts (a) and nuclei (b) in ultrathin sections from barley leaves. Under these staining conditions the thylakoid membranes appear lighter than the stroma. HvWhy1-associated gold particles have a size of 15 nm, and DNA-associated particles have a size of 10 nm. Some clusters of particles are highlighted by circles. When they include only small particles, the circle is of a full-line; in case of clusters of large particles a hatched line circle and in case of clusters consisting of both types of particles a circle of a complex line was used. The number gold particles associated with Whirly1 and DNA, respectively, as wells as values for distances between HvWhy1- and DNA-associated gold particles are presented in Supplemental Table S1. G granum, S starch. Scale bars 500 nm. Distribution of the AtWhy1-GFP (c) and AtPEND-RFP (d) fluorescent signals in chloroplasts of tobacco protoplasts. The red fluorescence of the RFP protein was converted into a blue colour for better contrast. e Merged image of the fluorescent signals of the AtWhy1-GFP and AtPEND-RFP proteins. Scale bar 5.7 μm
The intraplastidic localization of Whirly1 fused to GFP was re-examined after transient transformation of protoplasts employing a high-resolution confocal microscope. As described previously (Krause et al. 2005) GFP was located to speckles throughout the chloroplast (Fig. 1c). Some of the speckles had a yellow colour indicating association with the red fluorescing thylakoids. Most of the speckles were, however, green. For comparison, a PEND-RFP construct was used for co-transformation with the Whirly1-GFP fusion construct. The plastid envelope DNA binding (PEND) protein is known to bind to plastid DNA, and therefore, can be used for visualization of plastid nucleoids (Terasawa and Sato 2005). The signals obtained with the PEND-RFP protein showed similar speckled patterns as the GFP signals of the Whirly1-GFP protein. For better visualization, the red signals of RFP were converted into blue signals (Fig. 1d, e). In comparison to GFP, the RFP speckles were, however, more discrete and located in close proximity to the thylakoids (Fig. 1d). Merged images obtained for the two constructs revealed that most of the Whirly1-GFP fusion protein was not associated with the PEND-RFP fusion protein (Fig. 1e).
Whirly1 is a component of TAC-I, but not of highly purified TAC-II from barley chloroplasts
Plastid DNA of barley contains four elicitor response elements (ERE) which in the nucleus have been shown to be binding motifs for Whirly1 (Desveaux et al. 2000, 2002) and 12 similar elements with either one nucleotide exchanged or one nucleotide missing (Saski et al. 2007). Though the major fraction of Whirly1 in chloroplasts is not associated with DNA, a part of the protein pool could specifically bind to plastid DNA and might regulate transcription. Transcriptional activity in plastids can be found in a soluble fraction called soluble plastid RNA polymerase (sRNAP) and in a membrane-associated fraction called transcriptionally active chromosome (TAC) which is an elongation complex (Igloi and Kössel 1992). The TAC consists of both plastid DNA and nascent RNA chains as well as proteins attached to DNA or to RNA and being involved in posttranscriptional processes of plastid gene expression (Igloi and Kössel 1992; Lakhani et al. 1993; Krause and Krupinska 2000).
Whirly1 and 3 in Arabidopsis thaliana have been detected in a membrane derived fraction enriched in TAC (Pfalz et al. 2006). To examine whether Whirly1 in chloroplasts of barley is an intrinsic DNA binding protein of TAC, an antibody raised against barley Whirly1 (Grabowski et al. 2008) was used for Western blot assays with fractions collected during isolation of TAC-I by gel filtration. Immunological analysis of these fractions clearly showed that HvWhy1 co-elutes from the column with plastid DNA as shown by staining with ethidium bromide (Fig. 2).
HvWhy1 co-elutes from the Sepharose CL4B column with plastid DNA. After gel filtration 20 μl of every tenth fraction was loaded on an agarose gel containing ethidium bromide. The plastid DNA was present in fractions 10–30. The staining revealed some additional bands in the fractions 50–90, which could represent free plastid RNAs or tRNAs complexes. Additionally, proteins of the same fractions were analysed by polyacrylamide gel electrophoresis, transferred to nitrocellulose membrane and immunologically analysed with an antibody directed against the peptide P2 of barley Whirly1 protein. As a loading control, the Coomassie brilliant blue staining of proteins having molecular weights in the range from 20 to 40 kDa is shown
Chloroplast membranes were used as a starting material for preparation of TAC (Hallick et al. 1976; Krause and Krupinska 2000). Accordingly, Whirly1 was immunologically detected in a chloroplast membrane fraction (Fig. 3). Similar to already identified TAC components such as RpoA (α-subunit of the plastome-encoded RNA polymerase; Suck et al. 1996) and TCP34 (tetratricopeptide-containing chloroplast protein of 34 kDa, Weber et al. 2006), the abundance of Whirly1 was higher in the stroma fraction than in the membrane fraction (Fig. 3). Such a distribution of TAC proteins between membrane fraction and stroma is in accordance with the concept that TAC is a membrane-bound elongation complex, and the components involved in transcription are also found in the soluble fraction of the plastid RNA polymerase (sRNAP) (Igloi and Kössel 1992).
Detection of the HvWhy1 protein in the TAC-I but not in the TAC-II fraction. SDS-PAGE was performed with 15 μg of total protein extract from barley leaves (TP), chloroplasts (CP), chloroplast membranes (M) and stroma (S), respectively. Aliquots of the transcriptionally active chromosome TAC-I and TAC-II fractions having the same transcriptional activity (17,000 U) corresponding to about 20 and 7 μg protein, respectively, were analysed in addition. Immunoreactions were performed with antibodies directed against HvWhy1, RpoA (α-subunit of plastome-encoded RNA polymerase), MFP1 (MAR binding filament-like protein 1) and TCP34 (tetratricopeptide-containing chloroplast protein of 34 kDa). As a loading control, Coomassie brilliant blue staining of the gel in the range of proteins with molecular weights from 20 to 50 kDa is shown
To examine whether the presence of Whirly1 in the TAC fraction correlates with the transcriptional activity of the fraction, we compared its level in TAC-I with the level in the TAC-II extract which has been purified from TAC-I by precipitation and a second gel filtration (Krause and Krupinska 2000). Transcriptional activity based on protein content increased threefold during purification of TAC. Aliquots of TAC-I and TAC-II fractions having the same transcriptional activity but differing in protein content were loaded onto a gel. While the level of the α-subunit of RpoA was similar between TAC-I and TAC-II, the level of the HvWhy1 protein was clearly decreased in TAC-II compared to TAC-I (Fig. 3). For comparison, further proteins known to be TAC components were analysed for their distribution between TAC-I and TAC-II. The level of MAR binding filament-like protein 1 (MFP1), similar to the level of Whirly1, decreased during purification of TAC-I, while the level of TCP34 increased (Fig. 3). While the level of RpoA correlated with the transcriptional activity of TAC fractions, the levels of TCP34 and MFP1 did not correlate with transcriptional activity of the fractions. These results indicate that neither these two proteins nor Whirly1 in chloroplasts are part of the functional core of the transcriptional apparatus of plastids.
Plastid gene transcription is not affected in transgenic barley plants with a knockdown of Whirly1
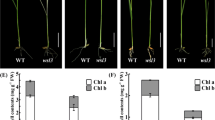
To investigate whether Whirly has an effect on transcription in chloroplasts, run-on transcription assays were performed with chloroplasts from three independent barley Whirly1 RNAi knockdown lines and from a mock control line (Supplemental Fig. S1). To examine the expression level of the Whirly1 protein in the RNAi lines and in control plants, protein extracts prepared from leaf material were immunologically analysed. In the homozygous lines E1 and in E9 Whirly1 was almost undetectable, whereas in the heterozygous E6 line Whirly1 level was about 10–20% in comparison to the control plants (Fig. 4a). In contrast to the ivory and pale green phenotypes of Whirly1 maize mutants (Prikryl et al. 2008), leaves of barley Whirly1 knockdown showed a wild-type-like appearance as also reported for Arabidopsis Whirly1 T-DNA insertion mutants (Yoo et al. 2007; Maréchal et al. 2009). Accordingly, the chlorophyll content of the Whirly1 knockdown lines was similar to that of the mock line used as a control (Fig. 4b).
Characterization of the barley Whirly1 RNAi lines. a Levels of the Whirly1 protein in the knockdown lines and in the mock plants. Equal amounts of protein from leaves from RNAi lines (E1, E6 and E9) and mock plants were separated by polyacrylamide gel electrophoresis and after transfer immunologically analysed for the presence of the HvWhy1 protein. As a loading control, the Coomassie blue staining of the large subunit of Rubisco and two subunits of the ATPase having molecular weight of about 55 kDa are shown. b Chlorophyll content of chloroplasts prepared from leaves of the RNAi lines. c Hybridization patterns obtained with the run-on transcripts isolated from chloroplasts. 32P-labelled transcripts were hybridized to identical blots containing DNA fragments representing 23 plastid genes and the control plasmid DNA pBluescript in three dilutions (1×, 4× and 16×). d Ratios of the relative transcriptional activities of selected plastid genes determined by run-on transcription assays with chloroplasts from barley Whirly1 RNAi lines (E1, E6 and E9) and mock plants
Run-on transcription assays were performed with mature third and fourth leaves. Radiolabelled run-on transcripts were hybridized with DNA dot-blot filters carrying a representative selection of 23 plastid gene-specific probes as reported earlier (Krupinska and Falk 1994). Visually, no obvious differences were observed between hybridization signal intensities obtained with the control line and the transgenic lines, respectively (Fig. 4c). Ratios of relative transcriptional activities of selected plastid genes calculated from values obtained by densitometric scanning of the autoradiograms as described previously (Krupinska and Falk 1994) were almost identical (Fig. 4d).
Barley Whirly1 associates with at least five chloroplast RNAs containing group II introns
To further examine whether HvWhy1 in like manner as ZmWhy1, might interact with plastid RNA and its association with TAC-I might be related to a function in posttranscriptional processes, the specific antibody raised towards the HvWhy1 protein was used for RIP-chip experiments with chloroplast stromal extracts isolated from barley leaves. To verify precipitation of the HvWhy1 protein, a control Western blot experiment was performed with aliquots from input, supernatant and pellet protein fractions obtained during the immunoprecipitation protocol (Fig. 5b). A protein of about 24 kDa detected in HvWhy1 immunoprecipitation pellets (IPs) corresponds in size to Why1 and is enriched in the pellet (P) fraction. A fraction of the protein remained in the supernatant (S). An additional band at the size of about 50 kDa appeared on the blot, and most probably represents some unspecific cross-reaction of the antibody.
Association of Whirly1 with chloroplast transcripts. a RIP-chip analysis of HvWhy1. The enrichment ratios (FCy5:FCy3) were normalized between two assays involving anti-HvWhy1 antibody and two assays involving AtpB antibody. The median normalized values for replicate spots from the AtpB data were subtracted from those from Whirly1 data and plotted according to fragment number. Fragments are numbered according to chromosomal position. The data used to generate this figure are provided in Supplemental Table S2. b Western analysis to verify precipitation of HvWhy1. Aliquots of fractions from HvWhy1 and AtpB immunoprecipitations were separated by SDS-PAGE and blotted onto a nylon membrane. The blot was cut into two halves to allow independent probing of samples from HvWhy1 and AtpB immunoprecipitations (cut site indicated by an arrow). The two halves of the immunoblot were probed with the antibodies used in the corresponding IPs. I 5% input (stroma) from IP, S 10% of supernatant fraction from IP, P 10% of pellet fraction from IP. IgGs in the pellet fractions are detected by the secondary antibody used to probe the immunoblot
Nucleic acids purified from the immunoprecipitation pellet were used as the experimental sample and labelled with the red fluorescing dye Cyanine 5 (Cy5), whereas nucleic acids prepared from the supernatant were used as a reference and labelled with the green fluorescing dye Cyanine 3 (Cy3). The labelled RNAs were then combined and hybridized to a microarray representing the complete plastid genome of maize (Schmitz-Linneweber et al. 2005). Only peaks three times above the median value of 0.10 of all differential signals on the array were considered further. By this approach, six prominent peaks corresponding to atpF, rps16, rpl16, petB and both parts of the trans-spliced rps12 transcripts could be detected (Fig. 5a; Supplemental Table S2). With the exception of rps16 and petB, these transcripts were also found to associate with the Whirly1 protein of maize (Prikryl et al. 2008). All transcripts co-immunoprecipitated with HvWhy1 have introns. In contrast to ZmWhy1, HvWhy1 did not co-precipitate with transcripts from rpoC, rps14, petD, orf99/173, trnR and ndhA genes (Fig. 5a). With the exception of petD and ndhA, these transcripts do not have introns. To investigate whether the association of HvWhy1 with RNA species containing introns has an impact on processing of the transcripts, Northern blot analyses were performed with RNA prepared from third and fourth leaves of transgenic barley lines showing a knockdown of Whirly1 (E1, E6 and E9) and from mock control plants. To confirm equal loading, the RNA separated by agarose gel electrophoresis was stained by ethidium bromide. In contrast to the ZmWhy1 mutants (Prikryl et al. 2008), but in accordance with the wild-type-like appearance of the barley Whirly1 knockdown plants (Maréchal et al. 2009), abundance of ribosomal RNA was not affected by the knockdown of Whirly1 (Fig. 6a). Hybridization with atpF-specific probes revealed that the abundance of atpF transcripts was increased in the Whirly1 knockdown plants compared to mock plants. A comparison between the hybridization pattern obtained with an intron-specific probe and an exon-specific probe, respectively, showed that specifically the abundance of spliced RNA was decreased in Whirly1 knockdown plants (Fig. 6a, b). In contrast, unspliced precursor transcripts overaccumulated in leaves of the transgenic plants in comparison to the wild type. This result is in accordance with the finding that in ZmWhy1 mutants the ratio of spliced to unspliced atpF transcripts is lower than in wild-type plants (Prikryl et al. 2008). The findings suggest that Whirly1 in barley as well as in maize affects the splicing of atpF transcripts. Another transcript immunoprecipitated by the antibody specific for HvWhy1 was rpl16. Northern blot analyses with probes specific for an exon and the intron, respectively, did not show significant differences in splicing (Fig. 6c, d). This result is also in accordance with the results obtained with RNA from ZmWhy1 mutants (Prikryl et al. 2008).
Impact of barley Whirly1 on the abundance of the atpF and rpl16 transcripts. RNA gel blot analysis of three RNAi lines and mock plants. Total RNA isolated from 4-week-old barley plants was loaded onto a denaturing agarose gel and after transfer onto a nitrocellulose membrane incubated with probes for a exon and b intron of the atpF gene, respectively and c exon and d intron of the rpl16 gene, respectively. As a loading control, the ethidium bromide staining of the plastidic ribosomal 16S rRNA and the cytoplasmic 18S rRNA is shown. Maps of the polycistronic transcription units containing the barley atpF and rpl16 genes are shown. Coding regions are indicated by shaded boxes and the introns by dashed line. The regions used for hybridization probes are marked
Discussion
Whirly proteins have been described as DNA binding proteins involved in regulation of transcription and telomere maintenance in the nucleus (Desveaux et al. 2005; Yoo et al. 2007; Krause et al. 2009). Very recently, it has been furthermore proposed that plastid-located Whirly1 and Whirly3 in A. thaliana are involved in maintenance of plastid genome stability (Maréchal et al. 2009). Such an organellar function is in accordance with the binding of Whirly proteins to DNA. Considering, however, that Whirly1 was shown to bind to DNA as a tetramer (Desveaux et al. 2002), it is unlikely that Whirly1 functions in chloroplasts as a DNA binding protein because bimolecular fluorescence complementation did not reveal interaction of Whirly1 with itself in plastids while the interaction in the nucleus was clearly detectable (Grabowski et al. 2008). In Arabidopsis thaliana plastidic Whirly proteins were found in the proteome of TAC (Pfalz et al. 2006), and in maize ZmWhy1 was shown to co-sediment with DNA complexes (Prikryl et al. 2008). Association of a protein with the TAC fraction does, however, not necessarily mean that the protein is a DNA binding protein. TAC has a rather complex protein composition and includes also proteins binding to RNA (Igloi and Kössel 1992; Lakhani et al. 1993; Krause and Krupinska 2000). Our new data on in vivo localization of barley Whirly1 together with the results on immunoprecipitation of Whirly1-associated RNA species suggest that plastid Whirly1 is rather an RNA binding protein than a DNA binding protein.
Co-localization with DNA in vivo was investigated by immunogold labelling with the Whirly1- and a DNA-specific antibody. In comparison to the nucleus, where Whirly1 was found in close contact with DNA, in chloroplasts rarely an association of Whirly1 with DNA was found. This result is in accordance with our previous findings that Whirly1 in the nucleus interacts with itself allowing the formation of DNA binding tetramers while in plastids no such interaction was detectable (Grabowski et al. 2008). The results on immunolocalization of Whirly1 in chloroplasts are somehow contradictory to our earlier attempts to localize Whirly1 in chloroplasts by transient transformation of protoplasts with a Whirly1-GFP fusion construct (Krause et al. 2005). GFP fusion proteins were detected in speckles throughout the chloroplasts, and these speckles were discussed to potentially represent nucleoids. We therefore re-examined the localization of Whirly1-GFP fusion proteins in comparison to PEND-RFP fusion proteins by high-resolution confocal laser scanning microscopy. By this approach we could demonstrate that only few of the previously observed Why1-GFP speckles might represent nucleoids which were now clearly visualized by the PEND-RFP fusion protein in the same protoplasts expressing the Whirly1-GFP construct (Fig. 1c, d).
By proteome analysis, Whirly1 of Arabidopsis thaliana was detected in a fraction containing the transcriptionally active chromosome of chloroplasts (Pfalz et al. 2006). By immunological analysis, the distribution of Whirly1 between stroma and membrane fractions was shown to be similar to that of other TAC components, e.g. RpoA and TCP34. Our immunological analysis furthermore confirmed that Whirly1 is contained in a conventionally prepared TAC fraction (Fig. 2). When, however, this TAC fraction (TAC-I) was further purified to give TAC-II which has a higher specific transcriptional activity based on protein content as described by Krause and Krupinska (2000), only traces of the protein could be detected (Fig. 3). In contrast to the α-subunit of RpoA, the abundance of Whirly1 therefore does not correlate with transcriptional activity of the fractions. This result suggests that Whirly1 in chloroplasts is not part of the functional core of the transcriptional apparatus.
To further examine whether Whirly1 might affect transcription in plastids, run-on transcription assays were performed with barley Whirly1-RNAi plants having reduced levels of Whirly1. In comparison to Prikryl et al. (2008) who tested transcription of two ribosomal RNA genes and one trn gene, we extended the analyses to genes from different functional groups including genes encoding proteins of the photosynthetic apparatus. Although Whirly1 was almost undetectable in the homozygous lines, no changes in relative transcriptional activities of a representative set of genes were observed between transgenic lines and control lines.
Nucleoids of human mitochondria were shown to have a layered structure with a central core as the site of transcription and replication and a peripheral zone (Bogenhagen et al. 2008). The results of our study suggest that Whirly1 is not an intrinsic component of the plastid nucleoid core. During association with the periphery of the nucleoids it might, however, be involved in posttranscriptional processes. By RNA co-immunoprecipitation and chip hybridization, several RNA species were identified as binding partners of ZmWhy1 (Prikryl et al. 2008). Some of these such as atpF contain introns, and Northern blot analyses showed that the abundance of unspliced atpF transcripts was enhanced in Whirly1 mutants (Prikryl et al. 2008). Using the same methodological approach, we identified several of the plastid RNA species binding to ZmWhy1 also to be bound to HvWhy1, e.g. atpF and rpl16. While HvWhy1 was found to bind to intron containing RNAs only, the ZmWhy1 protein was shown to bind also to transcripts devoid of introns such as rpoC, rps14, trnR and orf99/173. Furthermore, we showed here that the rps16 and the petB mRNAs are putative barley-specific ligands of Whirly1. These differences in RNA ligands might reflect differences in the properties of the two Whirly1 proteins. However, we cannot rule out that usage of the maize microarray for analysis of barley transcripts might have prevented detection of certain transcripts.
The differences in RNA species identified as binding partners of Whirly1 in maize and barley could be responsible for the differences in the phenotype of Whirly1 mutants of maize and barley Whirly1 knockdown plants. While maize mutants are severely disturbed in chloroplast development, the transgenic barley plants lacking Whirly1 have a similar chlorophyll content as the control plants. The barley plants thereby resemble the Whirly1 T-DNA insertion mutants described for Arabidopsis thaliana (Yoo et al. 2007; Maréchal et al. 2009). RNA analyses moreover showed that the abundance of plastidic ribosomal RNAs was neither affected by the knockdown of Whirly1 in barley nor by the T-DNA insertion in the Arabidopsis Whirly1 (Maréchal et al. 2009). Among the transcripts precipitated in maize and not in barley is the rpoC transcript. It is well known that plants with disrupted rpoB/C operon are disturbed in chloroplast development (De Santis-Maciossek et al. 1999). Because the rpoB/C operon has no intron, binding of Whirly1 to the transcript could be related to other processing events which might be affected in maize Whirly1 mutants.
Taken together, our analyses showed that Whirly1 predominantly is a stromal protein. Only a minor fraction was found to be loosely associated with the transcriptionally active chromosome where it could be involved in posttranscriptionally processes as suggested also by binding of HvWhy1 to plastid RNA. Our data demonstrate that Whirly1 in barley is not involved in maturation and abundance of ribosomal RNA. Similarly as in maize it was, however, shown to associate with a subset of intron containing plastid RNAs such as atpF and rpl16. Though in case of the atpF transcript splicing might be affected by Whirly1, this is not the case with the rpl16 transcript, both in barley and in maize (Prikryl et al. 2008). This suggests that binding of Whirly1 to intron containing RNA species not necessarily has consequences for splicing. At least in maize Whirly1 was shown to bind also to transcripts not having introns such as rpoC, rps14, orf99/173 and trnR-ACG/orf23. This suggests that Whirly1 could also play a role in other processing events which maybe affected in the Whirly1 maize mutants.
As already discussed by Maréchal et al. (2009) plastidic Whirly proteins fulfil a variety of functions depending on the cellular context and even species. As the expression of the Whirly1 gene in barley is under developmental control (Krupinska et al., unpublished), it is likely that the function of Whirly1 in plastids depends on the developmental stage of the organelle. As long as the developmental stage of the plastids investigated is not precisely defined, it is hard to compare results from studies with different plant material. Taking advantage of the developmental gradient of cells in a monocot leaf (Mullet 1988; Krupinska and Falk 1994), in future studies the intraplastidic distribution and functionality of plastidic Whirly1 at different stages of development will be investigated.
Abbreviations
- TAC:
-
Transcriptionally active chromosome
- RIP-chip:
-
RNA co-immunoprecipitation and chip hybridization
- sRNAP:
-
Soluble RNA polymerase
References
Bogenhagen DF, Rousseau D, Burke S (2008) The layered structure of human mitochondrial DNA nucleoids. J Biol Chem 283:3665–3675
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
De Santis-Maciossek G, Kofer W, Bock A, Schoch S, Maier RM, Wanner G, Rüdiger W, Koop HU, Herrmann RG (1999) Targeted disruption of the plastid RNA polymerase genes rpoA, B and C1: molecular biology, biochemistry and ultrastructure. Plant J 18:477–489
Dehesh K, Klaas M, Häuser I, Apel K (1986) Light-induced changes in the distribution of the 36000-Mr polypeptide of NADPH-protochlorophyllide oxidoreductase within different cellular compartments of barley (Hordeum vulgare L.). I. Localization by immunoblotting in isolated plastids and total leaf extracts. Planta 169:162–171
Desveaux D, Després C, Joyeux A, Subramaniam R, Brisson N (2000) PBF-2 is a novel single-stranded DNA binding factor implicated in the PR-10a gene activation in potato. Plant Cell 12:1477–1489
Desveaux D, Allard J, Brisson N, Sygusch J (2002) A new family of plant transcription factors displays a novel ssDNA-binding surface. Nat Struct Biol 9:512–517
Desveaux D, Maréchal A, Brisson N (2005) Whirly transcription factors: defense gene regulation and beyond. Trends Plant Sci 10:95–102
Fling SP, Gregerson DS (1986) Peptide and protein molecular weight determination by electrophoresis using high-molarity tris buffer without urea. Anal Biochem 155:83–88
Grabowski E, Miao Y, Mulisch M, Krupinska K (2008) Single-stranded DNA binding protein Whirly1 in barley leaves is located in chloroplasts and nuclei of the same cell. Plant Physiol 147:1800–1804
Gruissem W, Greenberg BM, Zurawski G, Hallick RB (1986) Chloroplast gene expression and promoter identification in chloroplast extracts. Methods Enzymol 118:253–270
Hallick RB, Lipper C, Richards OC, Rutter WJ (1976) Isolation of a transcriptionally active chromosome from chloroplasts of Euglena gracilis. Biochemistry 15:3039–3045
Hensel G, Valkov V, Middlefell-Williams J, Kumlehn J (2008) Efficient generation of transgenic barley: the way forward to modulate plant-microbe interactions. J Plant Physiol 165:71–82
Humbeck K, Quast S, Krupinska K (1996) Functional and molecular changes in the photosynthetic apparatus during senescence of flag leaves from field-grown barley plants. Plant Cell Environ 19:337–344
Igloi GL, Kössel H (1992) The transcriptional apparatus of chloroplasts. Crit Rev Plant Sci 10:525–558
Jeong SY, Rose A, Meier I (2003) MFP1 is a thylakoid-associated, nucleoid-binding protein with a coiled-coil structure. Nucleic Acids Res 31:5175–5185
Krause K, Krupinska K (2000) Molecular and functional properties of highly purified transcriptionally active chromosomes from spinach chloroplasts. Physiol Plant 109:188–195
Krause K, Kilbienski I, Mulisch M, Rödiger A, Schäfer A, Krupinska K (2005) DNA-binding proteins of the Whirly family in Arabidopsis thaliana are targeted to the organelles. FEBS Lett 579:3707–3712
Krause K, Herrmann U, Fuß J, Miao Y, Krupinska K (2009) Whirly proteins as communicators between plant organelles and the nucleus? Endocytobiosis Cell Res 19:51–62
Krupinska K (1992) Transcriptional control of plastid gene expression during development of primary foliage leaves of barley grown under a daily light-dark regime. Planta 186:294–303
Krupinska K, Apel K (1989) Light-induced transformation of etioplasts to chloroplasts of barley without transcriptional control of plastid gene expression. Mol Gen Genet 219:467–473
Krupinska K, Falk J (1994) Changes in RNA-polymerase activity during biogenesis, maturation and senescence of barley chloroplasts. Comparative analysis of transcripts synthesized either in run-on assays or by transcriptionally active chromosomes. J Plant Physiol 143:298–305
Lakhani S, Khanna NC, Tewari KK (1993) Nascent transcript-binding protein of the pea chloroplast transcriptionally active chromosome. Plant Mol Biol 23:963–979
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Maréchal A, Parent J-S, Véronneau-Lafortune F, Joyeux A, Lang F, Brisson N (2009) Whirly proteins maintain plastid genome stability in Arabidopsis. Proc Natl Acad Sci USA 106:14693–14698
Meier I, Phelan T, Gruissem W, Spiker S, Schneider D (1996) MFP1, a novel plant filament-like protein with affinity for matrix attachment region DNA. Plant Cell 8:2105–2115
Mullet JE (1988) Chloroplast development and gene expression. Ann Rev Plant Physiol Plant Mol Biol 39:475–502
Pfalz J, Liere K, Kandlbinder A, Dietz K-J, Oelmüller R (2006) pTAC2, -6, and -12 are components of the transcriptionally active plastid chromosome that are required for plastid gene expression. Plant Cell 18:176–197
Poulsen C (1983) The barley chloroplast genome: physical structure and transcriptional activity in vivo. Carlsberg Res Commun 48:57–80
Prikryl J, Watkins KP, Friso G, van Wijk KJ, Barkan A (2008) A member of the Whirly family is a multifunctional RNA- and DNA-binding protein that is essential for chloroplast biogenesis. Nucleic Acids Res 36:5152–5165
Saski C, Lee SB, Fjellheim S, Guda C, Jansen RK, Luo H, Tomkins J, Rognli OA, Daniell H, Clarke JL (2007) Complete chloroplast genome sequences of Hordeum vulgare, Sorghum bicolor and Agrostis stolonifera, and comparative analyses with other grass genomes. Theor Appl Genet 115:571–590
Schmitz-Linneweber C, Williams-Carrier R, Barkan A (2005) RNA immunoprecipitation and microarray analysis show a chloroplast Pentatricopeptide repeat protein to be associated with the 5′ region of mRNAs whose translation it activates. Plant Cell 17:2791–2804
Suck R, Zeltz P, Falk J, Acker A, Kössel H, Krupinska K (1996) Transcriptionally active chromosomes (TACs) of barley chloroplasts contain the α-subunit of plastome-encoded RNA polymerase. Curr Genet 30:515–521
Terasawa K, Sato N (2005) Visualization of plastid nucleoids in situ using the PEND-GFP fusion protein. Plant Cell Physiol 46:649–660
Weber P, Fulgosi H, Piven I, Müller L, Krupinska K, Duong VH, Herrmann RG, Sokolenko A (2006) TCP34, a nuclear-encoded response regulator-like TPR protein of higher plant chloroplasts. J Mol Biol 357:535–549
Wessel D, Flügge UI (1984) A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem 138:141–143
Xiong JY, Lai CX, Qu Z, Yang XY, Qin XH, Liu GQ (2009) Recruitment of AtWHY1 and AtWHY3 by a distal element upstream of the kinesin gene AtKP1 to mediate transcriptional repression. Plant Mol Biol 71:437–449
Yoo HH, Kwon C, Lee MM, Chung IK (2007) Single-stranded DNA binding factor AtWHY1 modulates telomere length homeostasis in Arabidopsis. Plant J 49:442–451
Acknowledgments
We thank Susanne Braun, Marita Beese, Cornelia Marthe and Reik Modrozynski for technical assistance. Christine Desel (CAU Kiel) is thanked for expert guidance in confocal laser scanning microscopy. Anke Schäfer is thanked for helpful ideas and Ying Miao (CAU Kiel) for critical reading of the manuscript and for help in preparation of the figures. Iris Meier (The Ohio State University, USA) is thanked for providing the antibody towards MFP1. We thank the Center of Biochemistry and Molecular Biology (CAU Kiel) for providing the phosphorimaging equipment. This work was supported by grants of the Deutsche Forschungsgemeinschaft (Kr1350/8, Kr1350/9) and an Emmy-Noether stipend to CSL.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Melonek, J., Mulisch, M., Schmitz-Linneweber, C. et al. Whirly1 in chloroplasts associates with intron containing RNAs and rarely co-localizes with nucleoids. Planta 232, 471–481 (2010). https://doi.org/10.1007/s00425-010-1183-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-010-1183-0