Abstract
Pollination is essential for seed reproduction and for exchanges of genetic information between individual plants. In angiosperms, mature pollen grains released from dehisced anthers are transferred to the stigma where they become hydrated and begin to germinate. Pollen grains of wild-type Arabidopsis thaliana do not germinate inside the anther under normal growth conditions. We report two Arabidopsis lines that produced pollen grains able to in situ precociously germinate inside the anther. One of them was a callose synthase 9 (cs9) knockout mutant with a T-DNA insertion in the Callose Synthase 9 gene (CalS9). Male gametophytes carrying a cs9 mutant allele were defective and no homozygous progeny could be produced. Heterozygous mutant plants (cs9/+) produced approximately 50% defective pollen grains with an altered male germ unit (MGU) and aberrant callose deposition in bicellular pollen. Bicellular pollen grains germinated precociously inside the anther. Another line, a transgenic plant expressing callose synthase 5 (CalS5) under the CaMV 35S promoter, also contained abnormal callose deposition during microsporogenesis and displaced MGUs in pollen grains. We also observed that precocious pollen germination could be induced in wild-type plants by incubation with medium containing sucrose and calcium ion and by wounding in the anther. These results demonstrate that precocious pollen germination in Arabidopsis could be triggered by a genetic alteration and a physiological condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reproduction in angiosperms requires the delivery of sperm cells from pollen to the ovary where they fuse with the egg cell and central cell to give rise to the embryo and endosperm, respectively. In most of flowering plants, pollen grains are released from dehisced anthers and transported by wind or animal pollinators to the stigma, where they absorb water and initiate the germination pathway (Taylor and Hepler 1997). The receptive stigmas can be either wet or dry, depending upon plant species. While wet stigmas are covered with a surface secretion containing carbohydrates, lipids, proteins, phenolics and water, which can hydrate pollen indiscriminately, dry stigmas have a papillate surface that discriminately controls pollen adhesion and hydration (Edlund et al. 2004; Hiscock and Allen 2008). Pollen germination leads to the outgrowth of pollen tubes that penetrate the stigma and elongate towards the ovary. The processes of pollen germination and pollen tube guidance are controlled by sequential and complex pollen–pistil interactions (McCormick 2004; Sanchez et al. 2004).
Plants of cleistogamous species can produce closed flowers, in which self-pollination takes place, and open flowers which allow for outcross pollination. In a cleistogamous flower, pollen grains can precociously germinate inside the anther, and pollen tubes grow through the closed anther wall or exit from an open stomium to reach the stigma (Lord 1979, 1981; Culley and Klooster 2007). The molecular mechanism underlying the precocious pollen germination inside the anther of cleistogamous flowers is unknown.
Pollination in Arabidopsis, a chasmogamous plant with dry stigmas, is tightly controlled at the stages of pollen germination and pollen tube growth in the pistil. Although wild-type Arabidopsis pollen grains can germinate and produce pollen tubes on the surfaces of various non-stigmatic floral organs of immature buds (Kandasamy et al. 1994), on the vegetative tissues and non-reproductive floral organs of the fiddlehead (fdh) mutant (Lolle and Cheung 1993), or on the in vitro germination medium (Fan et al. 2001), the establishment of pollen–stigma connection is critical for the initiation of the germination processes under natural conditions. Mature pollen grains within a closed anther are indistinguishable from those of dehisced anthers in their ability to adhere, germinate and produce pollen tubes on pistil (Kandasamy et al. 1994). However, pollen germination inside the anther of wild-type Arabidopsis plants under normal growth conditions has, to our knowledge, not been reported.
In the Arabidopsis raring-to-go (rtg) mutant, callose is deposited on the pollen surface before anther dehiscence and pollen grains can prematurely hydrate, germinate and form pollen tubes within the anther, circumventing the requirement for contact with the female stigma (Johnson and McCormick 2001). The RTG gene has not been cloned and it is not clear how this genetic alteration affects callose synthesis, deposition and dissolution. Overexpression of plantacyanin driven by CaMV 35S promoter in wild-type Arabidopsis plants also shows increased callose deposition on the pollen wall and a small percentage of germinated pollen grains in the closed anthers (Dong et al. 2005a). These data suggest that genetic alterations in Arabidopsis can cause pollen to precociously germinate inside the anther. Our knowledge of how precocious pollen germination in the anther is triggered is still very limited.
Callose, a β-1,3-glucan, is an important cell wall component that plays an essential role in plant development and stress responses (Stone and Clarke 1992; Verma and Hong 2001). During microsporogenesis, callose is first synthesized by pollen mother cells (PMC) and accumulated as a distinct layer between the primary cell wall and the plasma membrane. This unique polysaccharide continues to be massively deposited through meiosis, leading to the formation of a thick callose wall surrounding and separating the four spores within a tetrad. During pollination, adhesion of pollen to the stigma is followed by the massive deposition of callose in the germination holes of pollen grains. As pollen tubes elongate rapidly towards the ovary, callose synthesized by the male gamete is used to form the tube wall and to construct the callose plugs that separate the vacuolated basal portion of the extending pollen tube from the metabolically very active pollen tube tip.
Callose synthase 5 (CalS5; or glucan synthase-like 2, GSL2) has been identified as the key isoform of callose synthases responsible for the formation of the callose wall in pollen mother cells and microspore tetrads. CalS5 is also essential for the accumulation of callose in the tube wall and the callose plug in growing pollen tubes (Dong et al. 2005b; Nishikawa et al. 2005). Mutations in the CalS5 gene result in the formation of sterile pollen grains with an aberrant pollen exine pattern, suggesting that callose wall may provide a structural basis for exine formation. Although pollen grains of cals5 mutants can germinate and produce pollen tubes without callose plugs, their viability and competence are greatly reduced (Dong et al. 2005b; Nishikawa et al. 2005).
Recent studies on Arabidopsis mutants defective in two other callose synthase isoforms, CalS9 (GSL10) and CalS10 (GSL8), have revealed a previously unrecognized role of callose in positioning the male sperm cell inside the mature pollen grains. During microgametogenesis, the haploid microspores undergo two rounds of mitosis, an asymmetric cell division (pollen mitosis I) and a symmetric mitosis. The asymmetric pollen mitosis I results in the formation of two dimorphic cells, the vegetative cell and the generative cell. The smaller generative cell undergoes a round of symmetric pollen mitosis II, giving rise to two sperm cells. The two sperm cells with their own plasma membrane are closely associated with the vegetative nucleus, forming a male germ unit (MGU), which is positioned in the center of mature pollen grains in Arabidopsis. At cytokinesis of pollen mitosis I, callose is transiently present between the vegetative cell nucleus and the generative cell nucleus (Park and Twell 2001). Mutations in either the CalS9 or CalS10 gene in Arabidopsis disrupt pollen mitosis, producing pollen with only one nucleus or two nuclei. In bicellular pollen of the mutants, the generative cell is degenerated, undifferentiated or mislocalized (Töller et al. 2008; Huang et al. 2009).
In this study, we observed precocious pollen germination in two different Arabidopsis lines with genetic alternations in callose synthases. We also examined different growth and medium conditions that might induce precocious pollen germination in wild-type Arabidopsis plants. Our results demonstrate that precocious pollen germination inside the anther in Arabidopsis can be triggered by both genetic and physiological factors.
Materials and methods
Plant materials
Arabidopsis thaliana ecotype Columbia seeds were germinated on the surface of vermiculite in small pots and the plants were grown at 21–23°C in a growth chamber with the normal relative humidity ranged between 50 and 70%. Seeds of cs9-5 (Salk_060111) and cs9-6 (Salk_124294) were obtained from the Arabidopsis Biological Resource Center.
For the observation of early pollen germination inside the anther under the high-humidity condition, 4- to 5-week-old plants were grown for 3 days in a relative humidity maintained at 85–95%. For induction of precocious pollen germination, inflorescences were immersed for 2 days in an in vitro germination buffer containing 18% sucrose, 1.5 mM boric acid, 10 mM CaCl2, 1 mM KCl and 1 mM MgSO4, pH 7.0, or 18% sucrose, 10 mM CaCl2, 1 mM KCl, or 1.5 mM boric acid alone. To remove a stigma or pistil, unopened flowers were hand-opened gently and the stigma or pistil was removed using a small tweezers. For wounding treatments, anthers were squeezed with small tweezers tips, and leaves and stem below the flower were randomly punctured using a small tweezers. Two days later, flowers were fixed and analyzed for pollen germination inside the anther. For temperature treatments, plants were incubated at 4 or 37°C for 12 h. For treatments with bacterial infection, flowers were inoculated with Agrobacterium strains ABI resuspended in 0.05% Silwet L-77 water solution (OD600 = 1.0) or Silwet L-77 alone for 2 days followed by fixation and analysis.
Construction of CalS5 expression vector and transformation
The coding region of CalS5 was amplified by RT-PCR with RNA isolated from Arabidopsis inflorescences using primers 5′-GCA GTC GAC GAG ATG GCA CAG AGT AGT ACA TCT C-3′ and 5′-CGT GTC GAC TTC TTT CTG CTT CTT ACC ACC GGC-3′ (SalI sites underlined). The amplified fragment (5.8 kb) was cloned into pCR2.1 (Invitrogen) and verified by DNA sequencing. The coding region was subcloned into pXCS-HAStrep (Witte et al. 2004) at the XhoI and SalI site, generating pStrep-CS5. Plasmid pStrep-CS5 was introduced into Agrobacterium tumefaciens ABI strain for the transformation to Arabidopsis plants and the transgenic plants were screened for BASTA resistance (50 mg/l) by spraying 5 times at 2–3 weeks after germination. All transgenic lines were confirmed by PCR using genomic DNA.
Pollen staining
To assay pollen viability, anthers were squashed and stained with Alexander’s solution (Alexander 1969). For DNA and callose staining, flowers were fixed in 60% ethanol, 30% chloroform, 10% acetic acid for at least 2 h, and treated with 2N NaOH for 5–10 min. After washing with 0.1M K2HPO4, pH 8.5, the specimens were incubated with 1 μg/ml DAPI (Sigma) for DNA or 0.05% aniline blue (Sigma) for callose. The presence of nuclei (blue fluorescence) or callose (blue-yellow fluorescence) was detected using a fluorescence microscope with a UV filter, whereas the autofluorescence of cell background (red fluorescence) was imaged with a TRITC filter. Superimposed images of the blue and red fluorescence was created using Photoshop software.
RNA extraction and RT-PCR
Total RNA was extracted from Arabidopsis tissues using Trizol reagent (Invitrogen). cDNA was reverse-transcribed from RNA using SuperScript First-Strand Synthesis System (Invitrogen). Relative expression levels of the CalS5 gene were assayed by PCR using primers CS5F2, 5′-AAG ATT TGC AGA GGT CAC TGC AGC-3′ and CS5R2, 5′-CTT CTC GTT CCT CAA CCT CAT C-3′. The expression levels of the CalS5-HA-Strep (S-CalS5) mRNA were assayed using primer CS5F1, 5′-CTG GAA TTC CTT GAC TGG TTA AGA GCT-3′ corresponding to the CalS5 5′-coding region, or primer CS5F3, 5′-TGG CTA GAG GGT ATG AG-3′ corresponding to the CalS5 3′-coding region, and the Strep-specific primer StpR 5′-AAA CAA ATT GAG GAT GAG-3′. The primers used to amplify other CalS mRNAs were described previously (Dong et al. 2008). PCR amplification was carried out in conditions of one initial cycle at 93°C for 3 min, 22 cycles at 93°C for 45 s, 57°C for 45 s, 72°C for 1.5 min, and a final extension at 72°C for 10 min. Actin-2 mRNA, which served as a control, was amplified using primers ActF, 5′-TGG TGT CAT GGT TGG GAT G-3′ and ActR, 5′-CAC CAC TGA GCA CAA TGT TAC-3′.
Genetic analysis
Heterozygous mutant cs9-5/+ plants were pollinated with pollen from homozygous mutant qrt1/qrt1 plants (Preuss et al. 1994; Johnson-Brousseau and McCormick 2004). F2 plants showing both qrt1 and cs9 mutant phenotypes were isolated and their genotype (cs9/+; qrt1/qrt1) confirmed by genomic PCR. Tetrads were stained with Alexander’s dye (Alexander 1969), aniline blue and DAPI solutions.
Scanning electron microscopy
Arabidopsis pollen grains were coated with 9 nm of gold particles using a Technics Hummer V sputter coater, and observed with a Hitachi S-570 scanning electron microscope at 15 kV.
Results
Gametophytic defects of Arabidopsis cs9 mutants
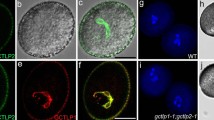
We have previously shown that the CalS9 gene is expressed in several plant tissues, including pollen (Dong et al. 2008). To study its biological function in plants, we characterized two T-DNA insertion mutants, cs9-5 (Salk_060111) and cs9-6 (Salk_124294) (Fig. 1a). Consistent with the recent data obtained by other groups (Töller et al. 2008; Huang et al. 2009), our analysis show that pollen grains carrying cs9 were gametophytic lethal. No cs9 homozygotes could be obtained from the self-pollination of cs9/+ heterozygotes (Fig. 1b; Supplementary Fig. S1). Progenies of self-pollinated cs9/+ plants segregated with a ratio of approximately 1:1:0 for WT:cs9/+:cs9, as confirmed by genomic PCR. There was no observable difference in vegetative growth between the heterozygous mutant (cs9/+) and the wild-type plant. The cs9/+ plants exhibited severe defects in pollen viability. When analyzed by Alexander’s staining, approximately half of pollen grains produced in cs9/+ plants were not viable (Fig. 1c), suggesting that CalS9 is required for male gametophyte development.
T-DNA insertional mutants of cs9 gene. a Genomic DNA structure of the CalS9 gene. Black boxes represent exons and lines represent introns. Triangles indicate the T-DNA insertion sites. ATG translation start site. TAA translation stop site. b Genomic PCR of cs9-5 and cs9-6 mutant lines. Genomic DNA isolated from cs9-5 and cs9-6 plants were used for PCR amplification using three primers, LP 5′-ATA TAC CCT TGC ACC ACC ATG-3′, RP 5′-AAT ACT TAG CAG TTA GCA GGC G-3′ and LBb1 5′-GCG TGG ACC GCT TGC TGC AAC T-3′. The amplification products were a 1.05-kb genomic fragment in wild type (WT), a 1.05-kb genomic fragment and two T-DNA specific fragments (0.8 and 0.5 kb, arrowheads) in cs9-5/+, and 1.05 kb genomic fragment, a 0.2 kb T-DNA specific fragment (arrow) in cs9-6/+. A 0.45-kb nonspecific fragment was present in all reactions. See supplemental Fig. S1 for Genomic PCR using two primers. c Viability of pollen grains of wild-type control and cs9 mutants as stained with the Alexander solution (Alexander 1969). Arrows indicate the defective mutant pollen grains. d Precocious pollen germination inside the anther in cs9-5/+ and cs9-6/+ plants. Fluorescence images taken using a UV filter for the presence of callose (light blue) or a TRITC filter for autofluorescence background (red) were overlaid with Photoshop. Bars 10 μm in c, 40 μm in d
We used callose staining and a nucleus DNA binding dye to examine how the pollen development in cs9/+ plants was affected by the mutation. The callose wall of pollen mother cells and tetrads of cs9–5/+ and cs9–6/+ plants contained a uniform layer of callose as in the wild-type control (yellow arrows, Fig. 8a, b, d, e), indicating that CalS9 is not responsible for callose wall biosynthesis, and is not required for microsporogenesis. However, when stained with DAPI, approximately half of the mature cs9/+ pollen grains were tricellular, containing one disperse vegetative nucleus and two compact sperm cells associated closely in the center of a pollen grain, as shown in the wild-type control (Fig. 4a). The remaining 50% pollen grains of cs9/+ plants were deformed or lacked one or both sperm cells. Most bicellular pollen grains contained a vegetative nucleus in the center of pollen grain and one sperm cell displaced to a bulge formed by extrusion of the pollen wall. (type III in Fig. 4a), forming a morphologically distinguishable from the normal round-shaped tricellular pollen. Consistent with the data described in two recent reports (Töller et al. 2008; Huang et al. 2009), our results demonstrating that the CalS9 gene is required for pollen mitosis and plays a pivotal role in positioning the MGU inside the pollen.
We also examined if there were any other alterations in callose deposition and flower development. To our surprise, germinated pollen grains with growing pollen tubes were found inside the anthers of cs9–5/+ and cs9–6/+ mutant lines, but not in the control plants (Figs. 1d, 3). Further characterization of the cs9/+ mutant was then focused on this pollen germination phenotype.
Transgenic expression of 35S::S-CS5 in Arabidopsis
We also observed similar precocious pollen germination phenotypes in transgenic Arabidopsis plants expressing S-CalS5 under the CaMV 35S promoter (35S::S-CS5, Fig. 2). Previous studies have indicated that CalS5 is the main callose synthase responsible for the formation of the callose wall during microsporogenesis, and the pollen tube wall and callose plug during pollen germination (Dong et al. 2005b; Nishikawa et al. 2005). Callose synthases are believed to exist as enzyme complexes. To investigate how elevated expression levels and ectopic expression of CalS5 might affect callose synthesis during plant growth and development, we generated transgenic plants expressing 35S::S-CS5 (for Strep-tagged CalS5) (Fig. 2a). Sixteen (16) independent transgenic lines (T1) selected on Basta resistance were maintained and verified by genomic PCR (data not shown). T2 plants that showed no segregation for Basta resistance in the T3 generation were identified as homozygous transgenic lines. Growth and development phenotypes of transgenic plants were observed in T3 and T4 homozygous plants.
We randomly selected five transgenic lines and analyzed the expression levels of the native CalS5 gene and the S-CS5 transgene in flowers (Fig. 2b). To detect the expression of the S-CS5 transgene, RT-PCR was performed using a pair of primers, one being Strep specific (StpR, Fig. 2a, b) and the other corresponding either to the 5′-end or the 3′-sequence of the CalS5 coding region (CS5F1 and CS5F3, Fig. 2a, b). Expression of the S-CS5 transgene was readily detected in these lines. Using a different pair of primers corresponding to the middle CalS5 coding region, which would amplify both the native CalS5 and the S-CS5 transgene, we could detect higher levels of the transcripts in the transgenic lines than in the wild-type plants (Fig. 2b), suggesting that the native CalS5 expression was not silenced by the S-CS5 transcripts. The vegetative parts of homozygous S-CS5 transgenic plants were morphologically indistinguishable from those of the wild-type control. However, the transgenic plants exhibited phenotypes in the reproductive parts similar to those observed in cs5-1 mutant plants. The defective phenotypes included shorter siliques, aborted embryos, shrunken anthers and deformed pollen grains with rougher exine structures (Supplementary Figs. S2, S3). Compared with wild type and cs5-1 mutant, S-CS5/WT plants developed an uneven peripheral callose wall surrounding pollen mother cells and tetrads (red arrowheads, Fig. 8), while maintained a regular interstitial callose wall that separated the spores within a tetrad. Under a fluorescence microscope, the interstitial callose wall appeared as bright lines after staining with aniline blue (yellow arrows, Fig. 8h). The irregular callose patches observed in the peripheral callose wall of the pollen mother cells and the tetrads in transgenic S-CS5/WT plants (red arrowheads, Fig. 8g, h) were persistent in the newly released microspores (Fig. 8i). In contrast, callose was degraded completely in the newly released microspores in wild type, cs9/+ and cs5-1 plants (Fig. 8c, f, l). This abnormal and uneven tetrad callose wall, and persistent callose deposition on the microspore surface might have disrupted exine formation, resulting in the development of deformed and sterile pollen in S-CS5/WT transgenic plants. In this work, we focused on the phenotypes of pollen germination inside the anther (Figs. 2c, 3), which was not observed in wild type, cs5-1 mutant (Figs. 2c, 3b, c, q, r) or transgenic plants expressing the empty vector (pXCS-HAStrep; Witte et al. 2004; data not shown).
Transgenic Arabidopsis plants expressing S-CS5. a Plasmid pStrep-CS5 expressing CalS5 tagged with hemagglutinin (HA) and Strep (Stp) at the C-terminus was used to transform wild-type Arabidopsis and cs5-1 mutant plants. PAT phosphinothricin acetyl transferase used for selection of transgenic plants resistant to herbicide Basta. RB and LB, right and left borders of the T-DNA. Primers CS5F2 and CS5R2 were used to detect the mRNA of the native CalS5 and the S-CS5 transgene. The expression of the S-CS5 transgene was amplified by a Strep-specific primer (StpR) and an oligonucleotide corresponding to the 5′-end (CS5F1) or the 3′-sequence (CS5F2) of the CalS5 coding region. b RT-PCR results showing the expression of the native CalS5 gene and the S-CS5 transgene in the flowers of 5 randomly selected transgenic plants. Actin2 mRNA was used as an internal control. Three replications were conducted and one representative result is shown. c Precocious pollen germination inside the anther in S-CS5/WT plant. Only the representative lines, line 3, 5 and 11, were shown. Fluorescent images taken using a UV filter for the presence of callose (light blue) or a TRITC filter for autofluorescent background (red) were overlaid with Photoshop. Bar 20 μm
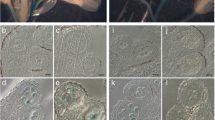
Precocious pollen germination inside the anther and pollen germination on the stigma. Anthers at the early tricellular (a, f, k, p), late tricellular (b, g, l, q) and dehiscence stages (c, h, m, r), or treated with high humidity (d, i, n, s) and non-treated stigmas in open flowers (e, j, o, t) were fixed and stained with aniline blue. Fluorescent images taken using a UV filter for the presence of callose (light blue) or a TRITC filter for autofluorescent background (red) were overlaid with Photoshop. Callose accumulation was observed in cs9-5/+ mutant and S-CS5/WT transgenic plants (line 3) at the late tricellular stage. Treatment with the high-humidity condition (85–95% relative humidity) induced callose deposition in all genotypes tested, while pollen germination in the anther was observed only cs9-5/+ mutant and S-CS5/WT transgenic plants. Pollen tubes of cs5-1 mutant (t) contained little callose (Dong et al. 2005b; Nishikawa et al. 2005). Bar 20 μm
Precocious pollen germination
As shown in Fig. 3 and Table 1, under normal growth conditions, no callose could be detected on pollen grains of the tested lines at the early tricellular stage (Fig. 3a, f, k, p). However, significant callose deposition was detected on pollen grains of S-CS5/WT (18–35%) and cs9/+ lines (24–28%) at the late tricelluar stage (Figs. 3g, l, 4b; Table 1). At anther dehiscence, germinated pollen grains with growing pollen tubes were observed inside the anthers of S-CS5/WT (4–8%) and cs9/+ lines (about 8%) (Fig. 3h, m), but not in those of wild-type control or cs5-1 mutant (Fig. 3c, r; Table 1). The pollen tubes grew inside the anther or extended from the open stomium, but did not penetrate the anther cell wall. As the anthers would inevitably have some physical contact with the stigma during flower opening, we asked whether the precocious pollen germination was induced by a signal from the stigma. We removed the stigma and whole pistil before flower opening, and found no significant change in germination rates inside the anthers in the two lines (data not shown), suggesting that precocious pollen germination was not induced by a stigma signal, and was not affected by wounding in the pistil.
As high humidity is known to promote early pollen germination in Arabidopsis rtg mutant plants (Johnson and McCormick 2001), we tested how high humidity affected pollen germination in cs9/+ and S-CS5/WT plants. When incubated in a high-humidity condition (>85%), the percentages of pollen grains with abnormal callose accumulation and germinated pollen in the two lines increased significantly (14–19% in S-CS5/WT and 16% in cs9/+) (Fig. 3; Table 1). The high-humidity condition also induced accumulation of callose in pollen of the wild-type control and in cs5-1 mutant plants (Fig. 3), suggesting that callose deposition itself was not sufficient to cause early pollen germination inside the anther, and that this humidity-induced callose was not synthesized by CalS5.
We also examined pollen germination on stigma. In cs9/+ plants, a 1:1 ratio of wild type:cs9 pollen grains were produced. Only pollen grains with the normal shape (wild type) germinated on stigma and produced visible callose plugs, whereas the deformed pollen grains that carried the mutated cs9 gene did not germinate on stigma (Fig. 3j). Therefore, the mutated cs9 gene could not be transmitted to the next generation, and no homozygote of cs9 plants could be obtained. Pollen grains of S-CS5/WT plants germinated normally and produced normal callose plugs (Fig. 3o) as observed in the wild-type plants (Fig. 3e), while the pollen tubes of cs5-1 mutant plants contained nearly undetectable callose (Fig. 3t).
Callose deposition and male germ unit (MGU) position in mature pollen grains
As described above, an abnormal callose deposition in pollen grains of cs9-5/+ plants was detected during the late tricellular stage (Fig. 3g). We stained the mature pollen grains of cs9-5/+ plants before anther dehiscence with aniline blue and DAPI (Fig. 4b; Table 2). We also examined callose deposition and MGU position in wild-type pollen grains. Mature wild-type pollen grains contained two sperm cells in the center of the grain in close vicinity of the vegetative nucleus and had no detectable callose (Fig. 4aI). During germination on stigma, more than 50% of wild-type pollen grains produced callose in and around the germination pore and the MGU migrated from the pollen center to a diffusion pattern. Incubation of flowers with water could also induce callose accumulation in the pollen wall and initiate MGU migration from the center of pollen to a cortical position, although no pollen germination could be detected under this condition (Fig. 4b; Table 2).
Male germ unit (MGU) positions and callose deposition in pollen grains. a DAPI-stained cs9 pollen. Yellow arrows indicate the vegetative nucleus (VN) and sperm cells (SC). MGU position I normal position with one vegetative nucleus and two sperm cells associated closely in the center of pollen grain, II abnormal position with the vegetative nucleus and two sperm cells mislocalized closely to the pollen wall, III bicellular pollen with the vegetative nucleus in the pollen center and one sperm cell mislocalized to a bulge formed by the pollen wall, IV pollen with two nucleus in the grain, V mononuclear pollen, VI collapsed pollen with no detectable nucleus. b Localization of callose (red arrowheads) and mislocalized MGU. Pollen grains stained with aniline blue and DAPI were photographed with a fluorescence microscope using a UV filter. WT on stigma means the hand-pollinated wild-type pollens on the stigma after 30 min. Arrows indicate the vegetative nucleus and sperm cells of cs9 pollen. Bar 5 μm in a and b
In cs9/+ plants, almost all of the callose-containing pollen grains were bicellular, having only one sperm cell mislocated to vicinity of the pollen wall, and callose was deposited specifically on the pollen wall just around the sperm cell (Fig. 4a, type III; Fig. 4b; Table 2). As described below, these bicellular pollen grains were the ones that germinated precociously inside the anther. It seemed that the abnormal callose deposition and MGU mislocalization in cs9/+ pollen grains were directly related to the precocious pollen germination.
At the late tricellular stage, most pollen grains of S-CS5/WT plants contained one vegetative cell and two sperm cells (Fig. 4a; Table 2). However, the sperm cells of 20–27% of S-CS5/WT pollen grains were placed closely to the pollen wall. Irregular callose patches were found on the surface of many S-CS5/WT pollen grains (Figs. 3, 4; Table 2). Consistently, S-CS5/WT also produced about 7–10% of pollen grains contained both irregular callose patches on the wall and mislocalized sperm cells (Fig. 4b; Table 2).
Migration of MGU in precociously germinated pollen tubes
Similar to the wild-type pollen tubes germinated in vitro or in vivo, precociously germinated S-CS5/WT pollen tubes contained two sperm cells and one vegetative nucleus, migrating from the grain towards the growing tip (Fig. 5a–e, l, m). In contrast, precociously germinated pollen tubes in cs9/+ plants only contained one vegetative nucleus and one sperm cell, indicating that they were developed from the defective bicellular pollen grains carrying the cs9 gene. In addition, the vegetative nucleus and sperm cell could not migrate towards the growing tip (Fig. 5f–i). Pollen tubes of cs9 could emerge from either the bulgy site containing the sperm cell or a pore in other site (Fig. 5h–k). It is important to note that while heterozygous cs9/+ plants produced an equal amount of WT and cs9 pollen grains, precocious germination was found only in the irregularly developed cs9 mutant pollen grains, but not in the normal WT pollen. This indicated that precocious pollen germination in cs9/+ plants was a gametophytic defect that resulted from the lack of the CalS9 gene.
Migration of the MGU during pollen tube growth. The MGU consisting of the vegetative nucleus (yellow arrows) and the sperm cells (red arrowheads) migrated from the pollen grain to the growing pollen tube (stars) in wild type and S-CS5/WT plants. a–c Transmission image (a) and fluorescent image (b) of a DAPI-stained wild-type pollen grain germinated in vitro. Close-up view of the MGU in b is shown in c. d–e Transmission image (d) and fluorescent image (e) of precociously germinated wild-type pollen induced by sucrose. f–k Transmission images (f, h, j) and fluorescent images (g, i, k) of precociously germinated pollen of cs9-5/+ plants. Note that the MGU remained in the pollen grain. l–m Transmission image (l) and fluorescent image (m) of precociously germinated pollen of S-CS5/WT plants. Bars 80 μm in a, b; 5 μm in c; 10 μm in d–m
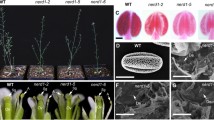
To verify the 1:1 segregation ratio of cs9:WT spores in a tetrad produced in the cs9/+ plants, we crossed cs9-5/+ plants with quartet1 (qrt1) mutant, which keeps all four microspores of a tetrad attached together during pollen development and allows for tetrad analysis of microspore segregation (Preuss et al. 1994; Johnson-Brousseau and McCormick 2004). Plants from the F2 generation with the genotype of cs9-5/+; qrt1/qrt1 were selected for tetrad analysis. Stained with Alexander’s dye (Alexander 1969), the tetrad of qrt1 mutant plants (+/+; qrt1/qrt1) contained all four purple-stained viable pollen grains (Fig. 6a, left panel). In contrast, the tetrad of cs9 qrt1 double-mutant plant (cs9-5/+; qrt1/qrt1) contained exactly two purple-stained viable pollen grains and two green-stained mutant grains (Fig. 6a, right panel). When stained with aniline blue and DAPI, about 95% of the tetrads produced in the qrt1 mutant plants (+/+; qrt1/qrt1) contained all four normal pollen grains, having the centralized MGU with no detectable callose patches (Fig. 6bI). In cs9 qrt1 double-mutant plants (cs9-5/+; qrt1/qrt1), however, no tetrads with all four normal pollen grains were detected. Instead, most tetrads contained two irregular pollen grains and two normal pollen grains. These defective pollen grains contained only one or two nuclei, as opposed to the three nuclei in WT pollen grains. The defective bicellular pollen grains of the cs9 qrt1 double-mutant plants had the characteristic callose patches on the pollen wall surrounding the mislocalized sperm cell, which was also detected in the cs9/+ single mutant plants (Fig. 6b, c). As expected, precocious pollen germination was detected in the tetrads in cs9 qrt1 double-mutant plants (about 20%), and only the defective bicellular pollen grains could germinate precociously in the anther. As in the cs9/+ single mutant, the MGU of the cs9 qrt1 double mutant did not migrate to the pollen tube during pollen tube growth (Fig. 6b). Therefore, these tetrad analysis results confirmed that precocious pollen germination in cs9/+ plants was resulted from the gametophytic defect.
Tetrad analysis of cs9-5/+ pollen grains. a Alexander solution stained tetrads of the qrt1 control (+/+; qrt1/qrt1) and cs9 qrt1 double mutant (cs9-5/+; qrt1/qrt1) plants. b Aniline blue and DAPI-stained tetrads. Tetrad type I a tetrad consisting of four normal pollen grains, each containing one vegetative nucleus and two sperm cells associated closely in the center of pollen grain, and undetectable callose deposits on pollen surface; II a tetrad consisting of two normal pollen grains, a mononuclear pollen grain, and a MGU-mislocalized bicellular pollen grain with visible callose deposition (indicated by red arrowhead); III a tetrad consisting of two normal pollen grains, a defective pollen grain with no visible nucleus, and a MGU-mislocalized bicellular pollen with callose deposition on the germinating pollen tube (indicated by *); IV a tetrad consisting of two normal pollen grains, a defective pollen grain with no visible nucleus, and a mononuclear pollen grain; V a tetrad consisting of two normal pollen grains, and two defective pollen grains with no nucleus. Yellow arrows in II and III indicate the vegetative nucleus and sperm cells in bicellular pollen grains. c Quantification of tetrad analysis using aniline blue and DAPI staining. The MGU types were indicated in b. Columns were the means ± standard deviations from three replicate assays. At least 50 tetrads in newly dehisced anthers were analyzed in each assay. Bars 10 μm in a, b
Induction of precocious pollen germination in WT Arabidopsis anthers
Under normal growth conditions, wild-type Arabidopsis pollen does not germinate in the anther. We asked whether there existed external and internal cues that might trigger pollen to germinate in the anther of wild-type plants. We treated Arabidopsis flowers with different ions, wounding, temperature stress and bacterial infection. As shown in Fig. 7 and Table 3, precocious pollen germination could be easily observed by treating flowers with in vitro germination buffer or sucrose alone. Ca2+ (10 mM) or K+ (1.5 mM) alone had a weak effect in inducing precocious germination, despite their importance in pollen germination and pollen tube growth. Pollen germination in the anther was not detected in treatments with water or 1.5 mM boric acid, suggesting that humidity and ions were not sufficient to induce precocious germination, although callose accumulation in the pollen wall and MGU migration could be induced (Fig. 4b). Sucrose (18%) alone or in vitro germination buffer, which also contained sucrose together with Ca2+ and K+ ions, might act both as a carbon source and as a germination signal, and was sufficient to trigger precocious pollen germination and sustain pollen tube growth in the anther.
Induction of precocious pollen germination inside the anther in wild-type plants. Flowers treated with water (a), sucrose solution (b), wounding (c), temperature stress (d) and bacterial infection (e) were stained with aniline blue. Deposition of callose in pollen grains was induced by the treatments. However, precocious pollen germination (arrowheads) was induced only treatments with sucrose (18%; b), in vitro germination medium and wounding (c). Images were taken with a fluorescence microscope using a UV filter to detect callose (light blue) and a TRITC filter to record the autofluorescent background. Images were overlaid using Photoshop software. Bar 20 μm. f RT-PCR analysis of CalS gene expression in wild-type Arabidopsis flowers treated with different germination conditions. RT-PCR products of Actin2 mRNA were used as an internal control. Three replications were conducted and one representative result is shown
Other treatments, including temperatures, wounding and bacterial infection could only induce callose accumulation in pollen grains, except that very low percentages of pollen germination were detected by wounding in the anthers and stigmas, being 0.7 and 1.1%, respectively (Table 3; Fig. 7). It was not clear if this was caused by the nutrition of wound exudates or an unidentified wound signal(s). All these results clearly indicated that certain environmental factors could mimic the function of stigma signals or genetic alterations such as cs9 and rtg mutations in promoting early pollen germination inside the anther.
We also used RT-PCR to examine the transcription levels of the 12 CalS genes in the flower tissue treated with different germination conditions. As shown in Fig. 7f, the mRNA level of CalS12, a CalS isoform involved in flower development and wounding- or pathogen responses (Jacobs et al. 2003; Nishimura et al. 2003; Enns et al. 2005), was increased by the treatments. The expression of CalS1, 5, 9 and 11 was up-regulated by the 37°C treatment, whereas CalS2, 8, 10 were down-regulated by the wounding treatment. Treatment with sucrose repressed the expression of CalS9, suggesting that a reduced level of CalS9 may be important in inducing precocious pollen germination. This data is consistent with the conclusion drawn from the observation in the cs9/+ mutant plants. Expression of CalS2, 8, 10 and 11 were also down-regulated by sucrose. However, CalS5 expression was not changed significantly under the same condition.
Discussion
Pollen germination and pollen tube growth are strictly controlled in flowering plants. In Arabidopsis, pollen germination is triggered by a complex interaction with the stigma under natural conditions. In this report, we described two novel Arabidopsis lines that exhibited precocious pollen germination inside the anther. One of these lines was a T-DNA insertional mutant at CalS9, a gene essential for microgametogenesis. The mutant could be maintained as a heterozygote (cs9–5/+ and cs9–6/+). The other line exhibiting precocious pollen germination was a transgenic plant expressing a callose synthase 5 isoform under the CaMV 35S promoter (S-CS5/WT). These two lines, cs9/+ and S-CS5/WT, are a new addition to the genetic resources useful for the study of pollen germination and reproduction in plants. Previously, the Arabidopsis rtg mutant (Johnson and McCormick 2001) and the transgenic plant with increased plantacyanin accumulation (Dong et al. 2005a) have been described to exhibit precocious pollen germination. Flowers formed in these precocious pollen germination lines of Arabidopsis resemble a cleistogamy with the respect that the pollen can germinate before leaving the anther. We also presented evidence for the induction of precocious pollen germination in the wild-type Arabidopsis plants by treatments with sugar solutions and wounding. Taken together, these findings demonstrate that precocious pollen germination in Arabidopsis could be triggered by genetic alternations and nutrition/wounding treatments.
The two Arabidopsis lines, cs9/+ and S-CS5/WT, were related to each other with respect to the genetic alterations of callose synthases, suggesting that callose synthesis and deposition are important for pollen development and germination. Our results and the recent data obtained by other groups (Töller et al. 2008; Huang et al. 2009) have indicated a critical role of CalS9 in regulating the progression of pollen mitosis I and II. Two other callose synthases, CalS1 and CalS10, have been identified as the main isoforms involved in cell-plate formation in the meristems and during pollen development (Hong et al. 2001; Thiele et al. 2008; Chen et al. 2009). Whether CalS9 is targeted to the cell plate during pollen mitosis remains to be determined. The molecular mechanism underlying the regulation of MGU position inside the pollen by CalS9 also warrants further examination. The data described in this work have suggested a new function of CalS9 in temporal control of pollen germination. In cs9/+ plants, only the irregularly bicellular pollen grains with a mislocalized MGU position would germinate in the anther (Figs. 4, 5, 6), whereas the remaining wild-type pollen grains remained intact, suggesting that the environment of the anther locule, a sporophytic tissue, was not the factor that induced early pollen germination. It is also interesting to note that in precociously germinated cs9 mutant pollen, the MGU consisting of one vegetative nucleus and one sperm cell could not migrate from the pollen grain to the extending tube. These findings suggested a new role of CalS9 in regulating MGU movement during pollen tube extension.
In addition to stress conditions, abnormal development of plant tissues can also induce ectopic callose accumulation. Defects in cell elongation and shape, alterations in pollen cell fate, or severe morphological deformation in mutant pollen often couple with ectopic callose deposition in cell walls (Park and Twell 2001; Ko et al. 2006; Van Damme et al. 2006). The abnormal pollen division and shape in cs9 mutant pollen and the deformed pollen shape and surface patterning in S-CS5 transgenic plants might contribute to the ectopic callose accumulation in the two Arabidopsis lines described in this report. However, in spite of the different genetic backgrounds, callose deposition and MGU mislocalization could be observed in the pollen grains of cs9/+ and S-CS5/WT plants. These events, occurring while pollen grains were inside the anther with no contact with stigma, might play a role in the observed precocious germination. The high callose deposition might increase the water-withholding ability (Vithanage et al. 1980) and allow absorption of nutrition from the anther locule. The displaced MGU might be helpful in initiating pollen tube growth and pollen cell wall remodeling. However, callose deposition and MGU mislocalization were not sufficient to promote the entire pollen germination processes, because in wild-type plants, water treatment could also induce these events but failed to trigger pollen tube growth. Thus, it is possible that other genes involved in pollen germination might be up- or down-regulated in cs9/+ and S-CS5/WT plants. This hypothesis is consistent with a recent report on the phenotype of a knockout mutant of the CalS12 gene (GSL5), in which a group of host defense genes are up-regulated (Nishimura et al. 2003).
Previous studies have suggested that CaMV 35S promoter is constitutive in most plant tissues, but not uniform and may be variable in the flower (Wilkinson et al. 1997; Jenik and Irish 2000). Although the activity of CaMV 35S promoter has long been thought to be very low in Arabidopsis pollen grains, transgenic plants expressing genes under this promoter have also been shown to induce phenotypes in pollen, such as male sterility (Eyüboglu et al. 2007), pollen tube growth retardation (Singh et al. 2002) and precocious pollen germination (Dong et al. 2005a). In this study, precocious pollen germination in S-CS5/WT plants could be just one of the pleiotropic effects brought about by the ectopic expression of CalS5 under the CaMV 35S promoter, which also resulted in synthesis of a defective callose wall during microsporogenesis, formation of rough exine structures, production of shrunken anthers and partial male sterility. These results also highlight the requirement of a normal expression level and pattern of CalS5 for reproduction in Arabidopsis.
As described before, pollen exine pattern formation is closely associated with callose deposition and dissolution in microspore development (Paxson-Sowders et al. 1997). In cs5 mutant, the callose wall is defective and the microspores are enclosed in a thinner cell wall that is depleted of callose. Primexine is not formed on microspores, leading to the failure of establishing the bacula and tectum structure (Dong et al. 2005b). Pollen exine pattern is also alternated in other plants with abnormal callose deposition during microgametogenesis. Transgenic tobacco plants expressing a β-1,3-glucanase that removes the callose wall prematurely (Worrall et al. 1992), and the Arabidopsis mutants with reduced expression levels of β-1,3-glucanases and delayed callose dissolution (Zhang et al. 2007; Zhu et al. 2008), produce defective pollen grains with irregular pollen surface patterns. The irregular exine pattern of S-CS5 pollen grains could be resulted from the abnormal callose deposition during pollen development, including the disrupted peripheral callose wall of tetrads and the elevated callose deposition in the late pollen development stages (Fig. 8). The exact reason for the disruption of the peripheral callose wall by the expression of the S-CS5 transgene remains to be determined.
Callose deposition during microsporogenesis. Pollen mother cells (a, d, g, j), tetrads (b, e, h, k), and microspores (c, f, i, l) were stained with aniline blue for the presence of callose. Fluorescence images were taken under a UV filter. Yellow arrows indicate the normal callose wall of pollen mother cells (a, d), the normal peripheral callose wall surrounding the tetrad (b, e), and the normal interstitial callose wall separating microspores in a tetrad (b, e, h, k). Red arrowheads indicate the defective callose wall of pollen mother cells in S-CS5/WT transgenic plants (line 3) and cs5-1 mutant (g, j), the defective peripheral callose wall of tetrads in S-CS5/WT and cs5-1 (h, k), and the aberrant callose patches in S-CS5/WT microspores (i). Bar 10 μm
Precocious pollen germination inside the anther is rare in Arabidopsis plants under normal growth conditions, despite that the mature pollen grains, before anther dehiscence, already have the ability to adhere, germinate and produce pollen tubes on pistil (Kandasamy et al. 1994). Certain repression mechanism might exist in the anther, which prevents premature germination of pollen grains before they leave the anther. To search for a clue of such a repression mechanism, we treated anthers with different nutrition solutions and physiological factors (Table 3). In vitro germination medium and sucrose solution could strongly induce callose deposition on the pollen grains and pollen germination inside the anther. Wounding and Ca2+ in the anther and stigma had a tiny effect on the precocious pollen germination (Table 3). These data suggest that sugar nutrition and other signals may circumvent the inhibitory mechanism in the anther locule and promote pollen germination and pollen tube growth. How mature pollen grains are kept ungerminated in anthers of chasmogamous plants is a poorly understood topic in reproduction biology. Development of genetic resources and application of biochemical and molecular approaches to this field of research would be very important for the advancement of our understanding on pollen viability and fertility.
Abbreviations
- CalS:
-
Callose synthase
- GSL:
-
Glucan synthase-like
- MGU:
-
Male germ unit
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- DAPI:
-
4′6-Diamidino-2-phenylindole
References
Alexander MP (1969) Differential staining of aborted and nonaborted pollen. Stain Technol 44:117–122
Chen XY, Liu L, Lee E, Han X, Rim Y, Chu H, Kim SW, Sack F, Kim JY (2009) The Arabidopsis callose synthase gene GSL8 is required for cytokinesis and cell patterning. Plant Physiol 150:105–113
Culley TM, Klooster MR (2007) The cleistogamous breeding system: a review of its frequency, evolution, and ecology in angiosperms. Bot Rev 73:1–30
Dong J, Kim ST, Lord EM (2005a) Plantacyanins plays a role in reproduction in Arabidopsis. Plant Physiol 138:778–789
Dong X, Hong Z, Sivaramakrishnan M, Mahfouz M, Verma DPS (2005b) Callose synthase (CalS5) is required for exine formation during microgametogenesis and for pollen viability in Arabidopsis. Plant J 42:315–328
Dong X, Hong Z, Chatterjee J, Kim S, Verma DPS (2008) Expression of callose synthase genes and its connection with Npr1 signaling pathway during pathogen infection. Planta 229:87–98
Edlund AF, Swanson R, Preuss D (2004) Pollen and stigma structure and function: the role of diversity in pollination. Plant Cell 16:S84–S97
Enns LC, Kanaoka MM, Torii KU, Comai L, Okada K, Cleland RE (2005) Two callose synthases, GSL1 and GSL5, play an essential and redundant role in plant and pollen development and in fertility. Plant Mol Biol 58:333–349
Eyüboglu B, Pfister K, Haberer G, Chevalier D, Fuchs A, Mayer KF, Schneitz K (2007) Molecular characterization of the STRUBBELIG-RECEPTOR FAMILY of genes encoding putative leucine-rich repeat receptor-like kinases in Arabidopsis thaliana. BMC Plant Biol 7:16
Fan LM, Wang YF, Wang H, Wu WH (2001) In vitro Arabidopsis pollen germination and characterization of the inward potassium currents in Arabidopsis pollen grain protoplasts. J Exp Bot 52:1603–1614
Hiscock SJ, Allen AM (2008) Diverse cell signalling pathways regulate pollen–stigma interactions: the search for consensus. New Phytol 179:286–317
Hong Z, Delauney AJ, Verma DPS (2001) A cell-plate specific callose synthase and its interaction with phragmoplastin. Plant Cell 13:755–768
Huang L, Chen XY, Rim Y, Han X, Cho WY, Kim SW, Kim JY (2009) Arabidopsis glucan synthase-like 10 functions in male gametogenesis. J Plant Physiol 166:344–352
Jacobs AK, Lipka V, Burton RA, Panstruga R, Strizhov N, Schulze-Lefert P, Fincher GBJ (2003) An Arabidopsis callose synthase, GSL5, is required for wound and papillary callose formation. Plant Cell 15:2503–2513
Jenik PD, Irish VF (2000) Regulation of cell proliferation patterns by homeotic genes during Arabidopsis floral development. Development 127:1267–1276
Johnson SA, McCormick S (2001) Pollen germinates precociously in the anthers of raring-to-go, an Arabidopsis gametophytic mutant. Plant Physiol 126:685–695
Johnson-Brousseau SA, McCormick S (2004) A compendium of methods useful for characterizing Arabidopsis pollen mutants and gametophytically expressed genes. Plant J 39:761–775
Kandasamy MK, Nasrallah JB, Nasrallah ME (1994) Pollen–pistil interactions and developmental regulation of pollen tube growth in Arabidopsis. Development 12:3405–3418
Ko JH, Kim JH, Jayanty SS, Howe GA, Han KH (2006) Loss of function of COBRA, a determinant of oriented cell expansion, invokes cellular defense responses in Arabidopsis thaliana. J Exp Bot 57:2923–2936
Lolle SJ, Cheung AY (1993) Promiscuous germination and growth of wild type pollen from Arabidopsis and related species on the shoot of the Arabidopsis mutant, fiddlehead. Dev Biol 155:250–258
Lord EM (1979) The development of cleistogamous and chasmogamous flowers in Lamium amplexicaule (Labiatae): an example of heteroblastic inflorescence development. Bot Gaz 140:39–50
Lord EM (1981) Cleistogamy: a tool for the study of floral morphogenesis, function and evolution. Bot Rev 47:421–449
McCormick S (2004) Control of male gametophyte development. Plant Cell 16:S142–S153
Nishikawa S, Zinkl GM, Swanson RJ, Maruyama D, Preuss D (2005) Callose (beta-1,3 glucan) is essential for Arabidopsis pollen wall patterning, but not tube growth. BMC Plant Biol 5:22
Nishimura MT, Stein M, Hou BH, Vogel JP, Edwards H, Somerville SC (2003) Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science 301:969–972
Park SK, Twell D (2001) Novel patterns of ectopic cell plate growth and lipid body distribution in the Arabidopsis Gemini pollen1 mutant. Plant Physiol 126:899–909
Paxson-Sowders DM, Owen HA, Makaroff CA (1997) A comparative ultrastructural analysis of exine pattern development in wild-type Arabidopsis and a mutant defective in pattern formation. Protoplasma 198:53–65
Preuss D, Rhee SY, Davis RW (1994) Tetrad analysis possible in Arabidopsis with mutation of the Quartet (Qrt) genes. Science 264:1458–1460
Sanchez AM, Bosch M, Bots M, Nieuwland J, Feron R, Mariani C (2004) Pistil factors controlling pollination. Plant Cell 16:S98–S106
Shedletzky E, Unger C, Delmer DP (1997) A microtiter-based fluorescence assay for (1,3)-beta-glucan synthases. Anal Biochem 249:88–93
Singh DP, Jermakow AM, Swain SM (2002) Gibberellins are required for seed development and pollen tube growth in Arabidopsis. Plant Cell 14:3133–3147
Stone BA, Clarke AE (1992) Chemistry and physiology of higher plant 1,3-β-glucans (callose). In: Stone BA, Clarke AE (eds) Chemistry and biology of (1, 3)-β-glucans. La Trobe University Press, Melbourne, pp 365–429
Taylor LP, Hepler PK (1997) Pollen germination and tube growth. Annu Rev Plant Physiol Plant Mol Biol 48:461–491
Thiele K, Wanner G, Kindzierski V, Jürgens G, Mayer U, Pachl F, Assaad FF (2008) The timely deposition of callose is essential for cytokinesis in Arabidopsis. Plant J 58:13–26
Töller A, Brownfield L, Neu C, Twell D, Schulze-Lefert P (2008) Dual function of Arabidopsis glucan synthase-like genes GSL8 and GSL10 in male gametophyte development and plant growth. Plant J 54:911–923
Van Damme D, Coutuer S, De Rycke R, Bouget FY, Inze D, Geelen D (2006) Somatic cytokinesis and pollen maturation in Arabidopsis depend on TPLATE, which has domains similar to coat proteins. Plant Cell 18:3502–3518
Verma DPS, Hong Z (2001) Plant callose synthase complexes. Plant Mol Biol 47:693–701
Vithanage HI, Gleeson PA, Clarke AE (1980) The nature of callose produced during self-pollination in Secale cereale. Planta 148:498–509
Wilkinson JE, Twell D, Lindsey K (1997) Activities of CaMV 35S and NOS promoters in pollen: implications for field release of transgenic plants. J Exp Bot 48:265–275
Witte CP, Noel LD, Gielbert J, Parker JE, Romeis T (2004) Rapid one-step protein purification from plant material using the eight-amino acid StrepII epitope. Plant Mol Biol 55:135–147
Worrall D, Hird DL, Hodge R, Paul W, Draper J, Scott R (1992) Premature dissolution of the microsporocyte callose wall causes male sterility in transgenic tobacco. Plant Cell 4:759–771
Zhang ZB, Zhu J, Gao JF, Wang C, Li H, Zhang HQ, Zhang S, Wang DM, Wang QX, Huang H, Xia HJ, Yang ZN (2007) Transcription factor AtMYB103 is required for anther development by regulating tapetum development, callose dissolution and exine formation in Arabidopsis. Plant J 52:528–538
Zhu J, Chen H, Li H, Gao JF, Jiang H, Wang C, Guan YF, Yang ZN (2008) Defective in Tapetal Development and Function 1 is essential for anther development and tapetal function for microspore maturation in Arabidopsis. Plant J 55:266–277
Acknowledgments
We thank the Arabidopsis Biological Resources Center (ABRC) for providing Arabidopsis seeds. This work was supported by grants from NSF (MCB 0548525 and IOB 0543923).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
425_2009_1091_MOESM1_ESM.tif
Supplemental Fig. S1 Genomic PCR of cs9-5 and cs9-6 T-DNA insertion mutants. a Map of T-DNA insertion sites and primers used for genomic PCR reactions. There are two T-DNA inserts in cs9-5 (Salk_060111) and one in cs9-6 (Salk_124294). The directions of primers and T-DNA inserts are indicated by arrows. Black boxes represent exons and open boxes represent T-DNA. Primers used for genomic PCR reactions are LP 5′-ATA TAC CCT TGC ACC ACC ATG-3′, RP 5′-AAT ACT TAG CAG TTA GCA GGC G-3′ and LBb1 (Lb) 5′-GCG TGG ACC GCT TGC TGC AAC T-3′. b Genomic PCR products using two primers as indicated. In wild type, the amplified PCR products were a 1.05 kb genomic fragment from wild-type plants, two T-DNA specific fragments (0.8 and 0.5 kb) for cs9-5 and one T-DNA specific product (0.2 kb) for cs9-6. A nonspecific amplification product of 0.45 kb, indicated by star (*), was present in reactions with the LBb1 primer(TIFF 220 kb)
425_2009_1091_MOESM2_ESM.tif
Supplemental Fig. S2 Defects in reproduction in Arabidopsis plants expressing CalS5. a Phenotypes of newly opened anthers of wild type (WT), transgenic plants (S-CS5/WT, line 3) and cs5-1 mutant (cs5-1). Note that anthers of the transgenic plants and cs5-1 mutant were shrunken and contained fewer pollen grains. Bar, 0.5 mm b Silique phenotypes of wild type, S-CS5/WT transgenic plants (line 3) and cs5-1 mutant. Bar, 10 mm. c Embryos in the siliques of wild type, S-CS5/WT transgenic plants and cs5-1 mutant. Bar, 2.5 mm. d Differential interference contrast (DIC) microscope images of pollen grains of wild type (WT), S-CS5/WT plants (line 3) and cs5-1 mutant. Bar, 5 μm. e Scanning electron microscopy (SEM) images of pollen grains of wild type, and 3S-CS5 /WT plants (line 3). Bar, 5 μm (TIFF 3100 kb)
425_2009_1091_MOESM3_ESM.tif
Supplemental Fig. S3 Expression of CalS genes, callose contents and aborted embryos in transgenic plants expressing S-CS5. a-b RT-PCR analysis of the expression of CalS5 gene and the transgene S-CS5 in the leaf (a) and root (b) of 5 randomly selected S-CS5/WT transgenic plants. Primer CS5F2 and CS5R2 were used to detect the mRNA of CalS5. Primer CS5F1 corresponding to the CalS5 N-terminal region and the Strep specific primer StpR were used to detect the mRNA of S-CS5, Actin2 mRNA was used as an internal control. Three replications were conducted and one representative result is shown. c Callose contents of the tissues of wild type, cs5-1 mutant and S-CS5/WT (left-right: line 3, 5, 9, 11 and 14) plants. Quantitative measurements of callose content were performed as described previously (Shedletzky et al. 1997). Error bars were the means ± standard deviations from three replicate assays. In each plant tissues, Columns marked with different letters were significantly different according to Duncan multirange test, p≤ 0.05. d-e Percentages of short siliques (less than 5 mm), and aborted embryos in wild type, cs5-1 mutant and S-CS5/WT (left-right: line 3, 5, 9, 11 and 14) plants. Error bars were the means ± standard deviations from three replicate assays. Columns marked with different letters were significantly different according to Duncan multirange test, p≤ 0.05(TIFF 716 kb)
Rights and permissions
About this article
Cite this article
Xie, B., Wang, X. & Hong, Z. Precocious pollen germination in Arabidopsis plants with altered callose deposition during microsporogenesis. Planta 231, 809–823 (2010). https://doi.org/10.1007/s00425-009-1091-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-009-1091-3