Abstract
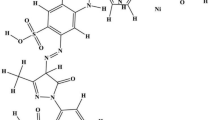
Tagetes patula L. (Marigold) hairy roots were selected among few hairy root cultures from other plants tested for the decolorization of Reactive Red 198. Hairy roots of Tagetes were able to remove dye concentrations up to 110 mg L−l and could be successively used at least for five consecutive decolorization cycles. The hairy roots of Tagetes decolorized six different dyes, viz. Golden Yellow HER, Methyl Orange, Orange M2RL, Navy Blue HE2R, Reactive Red M5B and Reactive Red 198. Significant induction of the activity of biotransformation enzymes indicated their crucial role in the dye metabolism. UV–vis spectroscopy, HPLC and FTIR spectroscopy analyses confirmed the degradation of Reactive Red 198. A possible pathway for the biodegradation of Reactive Red 198 has been proposed with the help of GC–MS and metabolites identified as 2-aminonaphthol, p-aminovinylsulfone ethyl disulfate and 1-aminotriazine, 3-pyridine sulfonic acid. The phytotoxicity study demonstrated the non-toxic nature of the extracted metabolites. The use of such hairy root cultures with a high ability for bioremediation of dyes is discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global industrialization has resulted in the release of large amounts of potentially toxic compounds into the biosphere (Senan and Abraham 2004). Environmental damage by several industrial toxic chemicals and gases is causing serious threats and damaging the natural habitat severely. Cleaning up of the environment by the removal of hazardous contaminants is a crucial and challenging problem needing numerous approaches to reach long-lasting suitable solutions. The textile industries use different chemical dyes and daily discharge millions of liters of untreated effluent containing harmful chemicals into receiving water bodies posing serious health problems. An average textile mill produces 60 × 104 m of fabric and discharges approximately 1.5 million liters of effluent per day in India (COINDS-59/1999-2000). Among these, the reactive group of azo dyes is widely used in the textile dyeing process due to the superior fastness for the fabric, high photolytic stability and resistance to microbial degradation. However, reactive dyes exhibit low levels of fixation with the fiber and about 10–20% of total dye used in the dyeing process remains left in the effluent (Pearce et al. 2003). The discharge of highly colored dye effluents from industries results in serious environmental pollution problems because color is the first contaminant recognized in the textile wastewater. Improper and inadequate chemical disposal of dyes alters the pH, increases the biochemical (BOD) and chemical oxygen demand (COD) (Olukanni et al. 2006) and reduces sunlight penetration (Carias et al. 2007).
Compared to the current available physical and chemical technologies, bioremediation is an alternative effective technique, which is ecofriendly, cost effective, has less sludge producing properties and is used for environmental clean up applications in recent years (Singh et al. 2008). The use of biological sources for decolorization of industrial dangerous chemicals is becoming a promising alternative in which microbes and plants generally replace the present engineering and chemical treatment processes. Among the sources for bioremediation, the use of plants is safe, easy to operate and is a less disruptive technique to the environment (Cunningham and Berti 2000). An extensive research has been focused to develop effective and efficient phytoremediation techniques (Padmavathiamma and Loretta 2007). Plants have a remarkable potential to concentrate and accumulate elements and compounds as well as organic contaminants from the environment and to metabolize these to various molecules in their organs and tissues. The plants also evolved advanced regulatory mechanisms to coordinate effective metabolic activities (Salt et al. 1998).
However, the molecular mechanism of trace element detoxification and hyperaccumulation in plants (Kramer and Chardonnens 2001) is not well understood so far. Many reports have shown that plants successfully transform various environmental xenobiotics including polycyclic aromatic hydrocarbons (Kucerova et al. 2001), nitroaromatic compounds (Goel et al. 1997; Stiborova and Hansikova 1997) and textile dyes (Kagalkar et al. 2009). Recently, phytoremediation studies have been carried out with the help of in vitro cell and tissue cultures techniques and genetic engineering (Mackova et al. 2001; Eapen and D’Souza 2005; Guillon et al. 2008) which offer unique opportunities that complement and extend the existing options. Among these, transgenic hairy roots have proven to be a suitable model system to study xenobiotics detoxification (Nepovim et al. 2004) and were also able to metabolize these compounds through common metabolic pathways (Coniglio et al. 2008). Hairy roots are known for their fast growth on simple nutrient medium, profuse biomass, high metabolic activity, and genetic as well as biochemical stability (Hu and Du 2006; Guillon et al. 2008). Hairy roots of Medicago sativa exhibited higher biotransformation of anthracene compared to the whole plants (Paul and Campanella 2000). Use of hairy root cultures for the biotransformation of various xenobiotic compounds was highly effective (Giri and Narasu 2000). Previous investigations have demonstrated that hairy roots derived from different plant species could be used for the treatment of several contaminants such as PCBs (Mackova et al. 1997), pesticides such as DDT (Suresh et al. 2005a) and nitroaromatic compounds such as 2,4-dinitrotoluene, 2,4,6-trinitrotoluene (TNT) and aminotoluenes (Nepovim et al. 2004).
Marigold (Tagetes patula) is an annual plant belonging to the Asteraceae family and has been used in traditional herbal medicines. The plant contains bioactive compounds which are widely employed as insecticides, fungicides and nematicides (Vasudevan et al. 1997). Its flowers are attractive and commercially cultivated, harvested and processed in an industrial scale as a source of carotenoid yellow-orange pigments (Hernandez et al. 2006). In addition, non-edible plants are generally preferred for phytoremediation because there is no danger of mixing experimental material in the routine food chain. In this regard, marigold would be an ideal system. Production of secondary metabolites has been reported earlier for Tagetes hairy roots (Suresh et al. 2005b), yet the phytoremediation ability of their hairy roots has not been explored till today. In the present work, we have induced hairy roots of T. patula L. to evaluate (a) the potential for the bioremediation of the textile dye Reactive Red 198 and (b) whether such hairy root cultures possibly might be a useful system to treat wastewater in future.
Materials and methods
Dyes, chemicals and tissue culture media
The textile dyes Reactive Red 198 and other dyes were obtained from local industry of Ichalkaranji, India. Methyl Orange was obtained from Merck (Mumbai, Maharashtra, India). ABTS (2,2′-Azinobis, 3-ethylbenzothiazoline-6-sulfonic acid) was obtained from Sigma (St. Louis, MO, USA). Tartaric acid was obtained from BDH Chemicals (Mumbai, Maharashtra, India). Dichlorophenol indophenol (DCIP) and Murashige and Skoog (MS) medium were obtained from Hi-media (Mumbai). n-Propanol and catechol were purchased from SRL Chemicals (Mumbai).
Plant material
Seeds of Marigold were obtained from a local market and the seeds of tobacco (Havana 425) were given by Dr. T.R. Ganapati (BARC, Mumbai, India). The seeds were removed from the berries and washed thoroughly by immersing them in distilled water with a few drops of Tween 20. After rinsing them well, to remove all the soap traces, seeds were air dried for 2 days. The seeds were then surface sterilized in 0.1% mercuric chloride for 3–5 min and rinsed four times with sterile-distilled water. The seeds were germinated aseptically on half strength MS medium containing 0.2% sucrose and 0.8% agar (Hi-media).
Bacterial strain and culture conditions
Agrobacterium rhizogenes NCIM 5140 (ATCC 5140) was obtained from National Chemical Laboratory (Pune, India) and used for the hairy roots induction. The bacterial culture was revived and maintained on YEB agar medium. A single bacterial colony was inoculated in 25 mL of liquid YEB medium and the culture was placed on a rotary shaker (0.67 g) at 30°C for 16 h till the OD at 600 nm was about 0.5. The bacterial suspension was centrifuged at 4,293g for 10 min and the pellet was resuspended in 5 mL liquid MS medium and used for co-cultivation of the explants.
Preparation of explants
Different parts of Tagetes seedlings including root, hypocotyls, stem and cotyledonary segments were isolated from the in vitro grown seedlings and were precultured for 2 days on MS basal medium (Murashige and Skoog 1962). The precultured explants were taken in the conical flask having bacterial culture with MS liquid medium and kept for 30 min on a rotary shaker in dark. After incubation, the explants were transferred on MS basal medium. After 3 days of incubation, the explants were transferred to MS medium containing 400 mg L−l cefotaxime to kill the residual Agrobacterium. The explants were again subcultured on the same medium after a week. Cefotaxime concentration was then halved in subsequent subcultures every week from 400 to 50 mg L−l and finally cultures free of A. rhizogenes were transferred to B5 medium (Gamborg et al. 1968). Similar experimental procedure was used for the induction of hairy roots in Nicotiana tabacum L., Solanum xanthocarpum Schrad. & Wendl., and Solanum indicum L.
DNA isolation and PCR confirmation
DNA was isolated from the hairy roots of Tagetes grown in the presence of antibiotic using the method described earlier by Dhakulkar et al. (2005). For amplification of coding sequence, following primers were used and amplified 970 bp domain present on the T-DNA region (+) 5′ CGGTCTAAATGAAACCGGCAAACG and (−) 5′ GGCAGATGTCTATCGCTCGCACTCC. And for amplification of ORF13 region, following primers were used and amplified having a 498 bp domain present on the T-DNA region of the Agrobacterium plasmid (+) 5′ CAGCTTCTAAATGTGGAGGCC and (−) 5′ CTTTGCCGATTGCCAGTATGGC. Amplification products were separated by electrophoresis on 1.8% agarose gel in 1× TBE buffer and stained with ethidium bromide and visualized under UV-trans illuminator.
Decolorization experiments
Initially, experiments were performed with T. patula L. hairy roots (120 mg dry weight) to check their ability to decolorize various dyes, mainly Reactive Red 198, Golden Yellow HER, Methyl Orange, Orange M2RL, Navy Blue HE2R and Reactive Red M5B. All the further decolorization experiments were carried out with Reactive Red 198 under static condition, at 20°C. The decolorization experiments were performed in sterile MS medium containing Reactive Red 198 (30 mg L−1). All decolorization experiments were performed in three sets. Aliquots (3 mL) were withdrawn after decolorization and the residual dye content (%) in the supernatant was measured at 510 nm. Decolorization was expressed in terms of percentage and was calculated as follows:
In order to study the effect of initial dye concentrations on the decolorization of Reactive Red 198 by hairy roots of T. patula L., the decolorization performance was assessed by initial addition of different concentrations of dye (30, 50, 70, 90 and 110 mg L−l) to MS medium and measured as percent decolorization. Repetitive decolorization capacity of the hairy roots was studied by repeated transfer of hairy root cultures in Reactive Red 198 (30 mg L−1) containing medium.
Enzymatic status of hairy roots
Tagetes patula L. hairy roots were mashed in mortal pestle and harvested in 50 mM phosphate buffer (pH 7.4, 1 mg mL−1) and were chilled properly (+4°C), homogenized and centrifuged (2,415g at 4°C for 20 min) and the supernatant was used for an intracellular enzyme assays. After removal of hairy roots, the medium was used for measuring the extracellular enzyme activities before and after decolorization. Activities of biotransformation enzymes, viz. lignin peroxidase, laccase, tyrosinase, Mn peroxidase, NADH-DCIP reductase and azo reductase were assayed spectrophotometrically at room temperature. All enzyme assays were run in triplicates and average rates were calculated.
Lignin peroxidase, laccase and tyrosinase enzyme activity were determined using a procedure reported earlier (Kalyani et al. 2008). Lignin peroxidase was determined by monitoring the formation of propanaldehyde at 300 nm in a reaction mixture of 2.5 mL (pH 3.5) containing 100 mM n-propanol, 250 mM tartaric acid, 10 mM H2O2. Laccase was determined in a reaction mixture of 2 mL containing 10% ABTS in 0.1 M acetate buffer (pH 4.9) and optical density was measured at 420 nm. Catechol (0.01%) in 0.1 M phosphate buffer (pH 7.4) constituted the reaction mixture for tyrosinase activity that was measured at 495 nm. NADH-DCIP reductase was measured as per the earlier report (Salokhe and Govindwar 1999). The assay mixture contained 50 μM DCIP, 50 μM NADH in 50 mM potassium phosphate buffer (pH 7.4) and 0.1 mL of enzyme solution in a total volume of 5.0 mL. The DCIP reduction was monitored at 595 nm. Azoreductase assay was performed in a reaction mixture (2 mL) containing 4.45 μM methyl red, 100 μM NADH in 50 mM potassium phosphate buffer at pH 7.4. The initial rate was determined by measuring the decrease in absorbance at 430 nm (Dhanve et al. 2008). Mn peroxidase was determined by the modified method of Hatvani and Mecs (2001). The 2.5 mL assay mixture contained 0.05 M sodium tartarate buffer (pH 4.5), 1 mM MnSO4 and the reaction was started by the addition of 10 mM H2O2 and monitored at 238 nm.
Decolorization and biodegradation analysis
UV–vis spectral analysis was carried out using Hitachi UV-Vis spectrophotometer (UV 2800) and changes in its absorption spectrum (400–800 nm) were recorded. The supernatant samples obtained at 0 h and after decolorization were subjected to spectral analysis. Metabolites produced in the biodegradation of the Reactive Red 198 were extracted with an equal volume of ethyl acetate. The extract was dried over anhydrous Na2SO4 and evaporated solvent on a rotary evaporator. The residues obtained after evaporation were dissolved in small volume of high-performance liquid chromatography (HPLC) grade methanol and used for analytical studies. HPLC analysis was performed in an isocratic Waters 2690 system equipped with dual absorbance detector, using C18 column (4.6 × 250 mm) and HPLC grade methanol as a mobile phase. The FTIR analysis was done in the mid-IR region of 400–4,000 cm−1 with 16 scan speed using Perkin Elmer 783 spectrophotometer and compared with control dye. The samples were mixed with spectroscopically pure KBr in the ratio of 5:95. Pellets were fixed in sample holders for the analyses. GC–MS analysis for the identification of metabolites formed after degradation was carried out using a QP2010 gas chromatography coupled with mass spectroscopy (Shimadzu). The ionization voltage was 70 eV. Gas chromatography was conducted in the temperature programming mode with a Restek column (0.25 mm, 60 m; XTI-5). The initial column temperature was 80°C for 2 min, which was increased linearly at 10°C min−1 to 280°C, and held for 7 min. The temperature of the injection port was 280°C and the GC/MS interface was maintained at 290°C. The helium carrier gas flow rate was 1.0 mL min−1. NIST spectral library stored in the computer software (version 1.10 beta, Shimadzu) of the GC–MS was used for comparison of retention times and mass spectra of degradation metabolites based on their fragmentation pattern.
Phytotoxicity studies
Lethal effect of dye and its metabolites was tested on the seeds of Phaseolus mungo L. and Triticum aestivum L. Reactive Red 198 degraded product extracted in ethyl acetate was dried and dissolved in water to the final concentration of 700 ppm for phytotoxicity studies. The phytotoxicity study was carried out at room temperature (33 ± 2°C). Ten seeds of P. mungo and T. aestivum were taken and watered separately with 5 mL Reactive Red 198 dye solution at 700 ppm concentration per day for control. Same concentration of its degradation products was used for the test. Control set was done using plain water at the same time. Length of plumule (shoot), radical (root) and germination (%) was recorded after 7 days.
Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA) with Tukey–Kramer multiple comparisons test. Values are mean of three experiments. Readings were considered significant when P was ≤0.05.
Results
Hairy roots induction
For the induction of hairy roots in T. patula L. using A. rhizogenes (ATCC15064) (Fig. 1), different explants such as cotyledonous leaves, hypocotyl and stem portion of in vitro grown seedlings were used. Total of 24 explants from each type were infected along with N. tabacum leaf as a control. The percent observations were taken after 3 weeks. All three explants showed 100% response in tobacco, whereas Tagetes showed a 76% response with an average of 8 ± 0.32 roots per explant. The time period for the root induction varied from explant to explant. The initiation of hairy roots was observed within a week after the infection of the explants. The explants continued to increase in size with more and more explants showing roots over a period of time. Fast growing roots were separated from slow growing ones for further studies.
Induction of hairy roots in Tagetes patula L. (a, b) and PCR confirmation of hairy roots (c). a Initiation of hairy roots and their growth after 1 week on the semi-solid medium. b Flask culture of hairy roots after 3 weeks in the liquid medium. c PCR confirmation of transformed nature of hairy roots after 3 weeks of culture. Lane M marker ladder, lanes 1 and 2 transformed hairy roots
The growth performance of the hairy roots was evaluated using MS, 1/2 MS and B5 media and 500 mg of fresh hairy roots (Table 1). The growth of the hairy roots was exponentially increased within a week, while maximum growth was seen on B5 media compared to that of MS and 1/2 MS.
Dye decolorization by various plant hairy root cultures
Besides Tagetes hairy roots, hairy roots of N. tabacum, S. xanthocarpum and S. indicum were also tested in preliminary experiments to assess their potentiality for the decolorization of Reactive Red 198 dye. All these hairy roots were exposed to 30 mg L−l dye. The hairy root culture of N. tabacum decolorized 95% Reactive Red 198 within 12 days, and S. xanthocarpum and S. indicum decolorized 96 and 86%, respectively, within 30 days. Among the tested four cultures, Tagetes hairy roots showed the most promising results and were selected for further studies.
Screening of different textile dyes for the decolorization
Dyes of different chemical structures are often used in the textile processing industry, and the effluents from the industry are markedly variable in composition. As shown in Table 2, the Tagetes hairy roots decolorized all the six different reactive textile dyes tested after 10 days. The maximum decolorization was observed for Reactive Red 198, while the minimum decolorization was observed for the dye Reactive Red M5B.
Repeated use of Tagetes hairy roots
One of the objectives of this study was to check the ability of Tagetes hairy roots for the repeated dye decolorization. Hence, hairy roots (120 mg dry weight) were repeatedly transferred in the media (20 mL) with the dye (30 mg L−l). The Tagetes hairy roots successively decolorized Reactive Red 198 up to five cycles of subcultures and produced a complete decolorization. In the first cycle, complete decolorization of Reactive Red 198 was observed within 8 days, and for the second cycle time of decolorization was 7 days which remained constant up to the last cycle.
Effect of different dye concentrations
Tagetes hairy roots efficiently decolorized increasing concentrations of dyes with a decolorization efficiency varying from 54 to 99%. The rate of decolorization was affected by addition of increasing concentrations of the dye ranging from 30 up to 110 mg L−l. Dye concentration 50 mg L−1 was decolorized up to 99% within 10 days, whereas 110 mg L−l dye gets decolorized up to 54%.
Enzymatic analysis
The biotransformation enzymes, viz. lignin peroxidase, laccase, tyrosinase, Mn peroxidase, NADH-DCIP reductase and azo reductase were analyzed during Reactive Red 198 degradation in hairy roots. It highlighted the combined action of studied oxidative and reductive enzymes during the dye degradation. Table 3 shows the differences in enzyme activities in Tagetes hairy roots that were cultured without (control) or with Reactive Red 198. The activity of biotransformation enzymes was demonstrated extracellular as well as intracellular in control hairy roots and in hairy roots collected after decolorization. After decolorization of the dye, extracellular as well as intracellular lignin peroxidase activity was induced. Mn peroxidase and tyrosinase were induced only intracellular and extracellular, respectively, while laccase activity was absent in the control sets and induced intracellularly during the dye decolorization. The intracellular enzyme DCIP reductase and azoreductase were induced during decolorization of Reactive Red 198.
Decolorization and biodegradation analysis
UV–vis spectral analysis (Fig. 2) of Reactive Red 198 showed a maximum absorbance at 530 nm. Absorbance was reduced in samples withdrawn after decolorization by Tagetes hairy roots.
The FTIR spectrum of control Reactive Red 198 (Fig. 3a) displayed a peak at 3,570 cm−1 indicating an OH stretching of asymmetric intramolecular hydrogen bonded single bridge alcoholic or phenolic compound. Peaks at 2,947, 2,119 and 1,575 cm−1 showed CH stretching of alkanes, CC stretching of alkynes and NN stretching of azo compound, respectively. Chloride containing compound as well as sulfonic acid (SO stretch) indicated peaks displayed at 1,186 and 1,028 cm−1, respectively. The FTIR spectrum of the products formed after decolorization (Fig. 3b) displayed a peak at 2,926 cm−1 demonstrating CH stretching of asymmetric alkane, and a peak at 2,850 cm−1 demonstrating CH stretching of aldehyde. Peaks at 2,290 and 1,469 cm−1 showed CN stretching of saturated alkyl and CH deformation alkane, respectively. Peaks at 1,349 cm−1 for SO resulted from stretching of sulfonyl compound, 1,255 cm−1 showed aliphatic ester compound, 1,069 cm−1 COH stretching of primary alcohol, and 808 cm−1 CH deformation of trisubstituted alkanes.
HPLC chromatogram (Fig. 4a) of Reactive Red 198 produced major and minor peaks at 1.904 and 2.343 retention times, respectively. Analysis of the metabolites obtained after degradation of the dye by Tagetes hairy roots (Fig. 4b) resulted in five additional peaks at retention times 1.984, 2.597, 2.837, 3.269 and 3.502 min.
Gas chromatography and mass spectra (GC–MS) analysis was carried out to investigate the metabolites formed during the biodegradation process. GC–MS analysis showed three metabolites, viz. 1-aminotriazine, 3-pyridine sulfonic acid (molecular weight 254, m/z 252, retention time 24.492), p-aminovinylsulfone ethyl sulfate (molecular weight 289, m/z 291, retention time 27.042) and 2-aminonaphthol (molecular weight 159, m/z 157, retention time 24.192) as final products.
Phytotoxicity studies
Germination of P. mungo and T. aestivum seeds was 100% in water and in 770 ppm degradation metabolites, yet only 70 and 60%, respectively, with Reactive Red 198 treatment (Table 4). In distilled water as control, the mean length of plumule and radicle of Phaseolus was 5.5 ± 0.4 and 3.16 ± 0.27 cm, respectively, and in case of T. aestivum 3.5 ± 0.43 and 4.7 ± 0.28 cm, respectively. Both the length of plumule and radicle was significantly affected by Reactive Red 198 (Table 4). In contrast, plumule and radicle length of Phaseolus and Triticum was scarcely and not significantly affected when treated with 700 ppm degradation metabolites (Table 4).
Discussion
The present study confirmed the ability of T. patula L. hairy roots to decolorize six structurally different textile dyes with decolorization efficiency of more than 62%. The difference in decolorization of dyes was due to structural differences (Paszcezynski et al. 1992), higher molecular weight and the presence of inhibitory groups such as –NO2 and –SO3Na in the dyes (Mohandass et al. 2007). The time required for the decolorization was proportional to the dye concentration. The higher concentrations of dye reduced the color removal rate; it might be due to toxicity of the dyes towards hairy roots metabolic activities, decreased growth rate and inadequate mass culture for the uptake of higher extent of dyes. The efficiency of Tagetes hairy roots with the ability of repeated decolorization cycles indicates an appropriate system for commercial application.
The induction of extracellular and intracellular enzymes was correlated with their involvement in the dye degradation. Biotransformation enzymes were induced in Tagetes hairy roots during the dye decolorization, suggesting that the presence of dye in the culture media was a prerequisite for the increased production of specific enzymes that were involved in the biotransformation. Most of the above-studied enzymes have been well known for their involvement in microbial biotransformation processes (Jadhav et al. 2007). Interestingly, the presence of these enzymes has also been noticed in Tagetes hairy roots. The enhancement of the total peroxidase activity after cultivation with a mixture of polychlorinated biphenyls in Solanum nigrum hairy roots has been reported (Mackova et al. 1997). Similarly, peroxidase from plant sources such as Ipomoea palmata and Sacharum spontaneum has been proven effective for the degradation of textile dyes (Shaffiqu et al. 2002). Crude extract precipitates from the leaves of the plant Phragmites australis have also been reported as successful in the decolorization of the dye Acid Orange 7 (Carias et al. 2006). Significantly, high amount of the NADH-DCIP reductase and laccase was observed in B. juncea roots and shoots during the degradation of the textile effluent (Ghodake et al. 2009). The azoreductase is a key enzyme expressed in azo dye degrading bacteria that cleaves azo bonds reductively (Dhanve et al. 2008). After decolorization, induced azoreductase activity in Tagetes hairy roots indicated and confirmed its role for the reduction of azo bonds in the dye degradation.
The major visible light absorbance peak completely disappeared or a new peak appeared, when the dye was removed due to biodegradation. Disappearance of peak at 510 nm indicates removal of color. Difference in FTIR spectrum of Reactive Red 198 and metabolites indicated that the dye molecule degraded into different metabolites in Tagetes hairy roots. HPLC analysis confirmed the biodegradation of Reactive Red 198 in different metabolites. A possible degradation pathway for Reactive Red 198 based on GC–MS analysis was proposed as shown in Fig. 5, in which the azo dye underwent an asymmetric cleavage by peroxidase to form 1-aminotriazine, 3-pyridine sulfonic acid (molecular weight 254, m/z 252, retention time 24.492) and intermediate I. Further, the action of azoreductases leading to the breaking azo bond of the intermediate I to form p-aminovinylsulfone ethyl sulfate (molecular weight 289, m/z 291, retention time 27.042) and 2-aminonaphthol (molecular weight 159, m/z 157, retention time 24.192) as a final product (Table 5). Degradation of dye Reactive Red 198 using microbial consortium PMB11 (Proteus sp. SUK7, Morganella morganii SUK5 and Bacillus odyssey SUK3) produced different metabolites, viz. triazine with pyridine molecule, ethyl 2-amiobenzenesulfonate and 2,4-diaminonaphthol (unpublished data). This indicates that metabolites produced by microbial biodegradation were different from the metabolites formed by hairy roots.
The non-toxic nature of degradation metabolites of Reactive Red 198 with respect to germination and growth of P. mungo and T. aestivum indicates detoxification of the dye. Similar results were shown by degraded metabolites of Reactive Red 198 using consortium PMB11 (data not shown).
From the present work, it has become apparent that hairy roots may be interesting candidates for phytoremediation applications to understand the key enzyme pathways involved in the detoxification of hazardous pollutants. However, the underlying mechanisms of phytoremediation have still remained unanswered and need further experimentation and opening up a new era of bioremediation. Although being transgenic products, hairy roots do not pose environmental threat problems thus avoiding stringent regulations. Cultivation of hairy roots in bioreactors under precise controllable conditions has been demonstrated (Choi et al. 2006; Mehrotra et al. 2008). Such a system could be extended further for phytoremediation applications on a larger scale.
Use of microbes for dye degradation always had a potential threat of escaping of mutant microbes into the environment. Such a threat is completely eliminated using the hairy roots. Several novel genes have been identified recently responsible for hyperaccumulation of hazardous substances in plants (Hanikenne et al. 2008). A recombinant Escherichia coli strain (E. coli NO3) containing genomic DNA fragments from azo-reducing wild-type Pseudomonas luteola strain showed enhanced decolorization of reactive azo dye (Chang et al. 2000). Isolation and incorporation of these genes into the hairy root gene construct would be an attractive proposition (Bulgakov 2008; Wood 2008) and would allow analysis of functional as well as discovery of new metabolic genes (Guillon et al. 2008). The present work opens an additional avenue of hairy roots for dye degradation and would be a base for planning further experiments. To our knowledge, this work is the first report regarding the textile dye degradation using hairy roots of T. patula L., showing efficient decolorization of Reactive Red 198, tolerance to higher dye concentration and the presence of biotransformation enzymes in dye degradation. The use of hairy root constructs with enhanced ability to treat wastewater will be a useful set up in future.
Abbreviations
- ABTS:
-
2,2′-Azinobis, 3-ethylbenzothiazoline-6-sulfonic acid
- B5:
-
Gamborg et al. medium
- MS medium:
-
Murashige and Skoog medium
- NADH-DCIP:
-
Dichlorophenol indophenol
- SE:
-
Standard error
- YEB:
-
Yeast extract broth
References
Bulgakov VP (2008) Functions of rol genes in plant secondary metabolism. Biotechnol Adv 26:318–324
Carias CC, Novais JM, Martins-Dias S (2006) Are Phragmites australis enzymes involved in the degradation of the textile azo dye acid orange 7. Bioresour Technol 99:243–251
Carias CC, Novais JM, Martins-Dias S (2007) Phragmites australis peroxidases role in the degradation of an azo dye. Water Sci Technol 56:263–269
Chang JS, Kuo TS, Chao YP, Ho JY, Lin PJ (2000) Azo dye decolorization with a mutant Escherichia coli strain. Biotechnol Lett 22:807–812
Choi YE, Yoon SK, Kee YP (2006) Design of bioreactors for hairy root culture. In: Gupta SD, Ibaraki Y (eds) Focus on biotechnology: plant tissue culture engineering. Springer, The Netherlands, pp 161–172
Comprehensive Industry Documents Series on Textile Industry. COINDS-59/1999-2000 Central pollution Control Board, India
Coniglio MS, Busto VD, Gonzalez PS, Medina MI, Milrad S, Agostini E (2008) Application of Brassica napus hairy roots cultures for phenol removal from aqueous solutions. Chemosphere 72:1035–1042
Cunningham SD, Berti WR (2000) Phytoextraction and phytostabilization: technical, economic, and regulatory considerations of the soil-lead issue. In: Terry N, Banuelos G (eds) Phytoremediation of contaminated soil and water. CRC Press, Boca Raton, FL, pp 359–376
Dhakulkar S, Ganapathi TR, Bhargava S, Bapat VA (2005) Induction of hairy roots in Gmelina arborea Roxb. and production of verbascoside in hairy roots. Plant Sci 169:812–818
Dhanve RS, Kalyani DC, Phugare SS, Jadhav JP (2008) Coordinate action of exiguobacterial oxidoreductive enzymes in biodegradation of Reactive Yellow 84A dye. Biodegradation 13:1–8
Eapen S, D’Souza SF (2005) Prospects of genetic engineering of plants for phytoremediation of toxic metals. Biotechnol Adv 23:97–114
Gamborg OK, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soyabean cells. Exp Cell Res 50:151–158
Ghodake GS, Telke AA, Jadhav JP, Govindwar SP (2009) Potential of Brassica juncea in order to treat textile effluent contaminated sites. Int J Phytorem 11:297–312
Giri A, Narasu ML (2000) Transgenic hairy roots recent trends and applications. Biotechnol Adv 18:1–22
Goel A, Kumar G, Payne GF, Dube S (1997) Plant cell biodegradation of a xenobiotic nitrate ester nitroglycerin. Nat Biotechnol 15:174–177
Guillon S, Tremouillaux-Guiller J, Pati PK, Gantet P (2008) Hairy roots: a powerful tool for plant biotechnological advances. In: Ramawat KH, Merillon LM (eds) Bioactive molecules and medicinal plants. Springer, Berlin, pp 271–283
Hanikenne M, Talke IN, Haydon MJ, Lanz C, Nolte A, Motte P, Kroymann J, Weijel D, Kramer U (2008) Evolution of metal hyper accumulation require cis-regulatory changes and triplication of HMA4. Nature 453:391–396
Hatvani N, Mecs I (2001) Production of laccase and manganese peroxidase by Lentinus edodes on malt containing by product of the brewing process. Process Biochem 37:491–496
Hernandez GG, Berzunza EA, Concha LC, Miranda-Ham ML (2006) Agrobacterium mediated transient transformation of Marigold (Tagetes erecta). Plant Cell Tiss Org Cult 84:365–368
Hu ZB, Du M (2006) Hairy roots and its application in plant genetic engineering. J Integr Plant Biol 48:121–127
Jadhav JP, Parshetti GK, Kalme SD, Govindwar SP (2007) Decolorization of azo dye Methyl Red by Saccharomyces cerevisiae MTCC 463. Chemosphere 68:394–400
Kagalkar AN, Jagatap UB, Jadhav JP, Bapat VA, Govindwar SP (2009) Biotechnological strategies for phytoremediation of the sulphonated azo dye Direct Red 5B using Blumia Malcolmii Hook. Bioresour Technol 100:4104–4110
Kalyani DC, Patil PS, Jadhav JP, Govindwar SP (2008) Biodegradation of reactive textile dye Red RBL by an isolated bacterium Pseudomonas sp. SUK1. Bioresour Technol 99:4635–4641
Kramer U, Chardonnens AN (2001) The use of transgenic plants in the bioremediation of soils contaminated with trace elements. Appl Microbiol Biotechnol 55:661–672
Kucerova P, in der Wiesche C, Wolter M, Macek T, Zadrazil F, Mackova M (2001) The ability of different plant species to remove polycyclic aromatic hydrocarbons and polychlorinated biphenyls from incubation media. Biotechnol Lett 23:1355–1359
Mackova M, Macek T, Ocenaskova J, Burkhard J, Demnerova K, Pazlarova J (1997) Biodegradation of polychlorinated biphenyls by plant cells. Int Biodeter Biodegrad 39:317–325
Mackova M, Chroma L, Kucerova P, Burkhard J, Demnerova K, Macek T (2001) Some aspects of PCB metabolism by horseradish cells. Int J Phytoremediat 3:401–414
Mehrotra S, Kukreja AK, Khanya SPS, Mishra BN (2008) Genetic transformation studies and scale up of hairy root culture of Glycyrrhiza glabra in bioreactor. Electron J Biotechnol. doi:10.2225/vol11-issue-2fulltext-6
Mohandass R, Bhaskar A, Kalavathy S, Devilaksmi S (2007) Biodecolorization and biodegradation of Reactive Blue by Aspergillus sp. Afr J Biotechnol 6:1441–1445
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Plant Physiol 15:473–479
Nepovim A, Podlipna R, Soudek P, Schroder P, Vanek T (2004) Effects of heavy metals and nitroaromatic compounds on horseradish glutathione S-transferase and peroxidase. Chemosphere 57:1007–1015
Olukanni OD, Osuntoki AA, Gbenle GO (2006) Textile effluent biodegradation potentials of textile effluent-adapted and non-adapted bacteria. Afr J Biotechnol 5:1980–1984
Padmavathiamma PK, Loretta YL (2007) Phytoremediation technology: hyper accumulation metals in plant. Water Air Soil Pollut 184:105–126
Paszcezynski A, Pasti-Grigsby M, Goszceynski S, Crawford R, Crawford DL (1992) Mineralization of sulfonated azo dyes and sulfanilic acid by Phanerochaete chrysosporium and Streptomyces chromofuscus. Appl Environ Microbiol 58:3598–3604
Paul R, Campanella B (2000) Use of Alfalfa (Medicago sativa L.) to stimulate biodegradation of anthracene in dredging sludges. Inter-COST workshop on bioremediation, COST Action 831, Sorrento, Italy
Pearce CI, Lloyd JR, Guthrie JT (2003) The removal of color from textile wastewater using whole bacterial cells: a review. Dyes Pigment 58:179–196
Salokhe MD, Govindwar SP (1999) Effect of carbon source on the biotransformation enzyme in Serratia marcescens. World J Microbiol Biotechnol 15:229–232
Salt DE, Smith RD, Raskin I (1998) Phytoremediation. Annu Rev Plant Physiol Plant Mol Biol 49:643–668
Senan RC, Abraham TE (2004) Bioremediation of textile azo dyes by aerobic bacterial consortium. Aerobic degradation of selected azo dyes by bacterial consortium. Biodegradation 15:275–280
Shaffiqu TS, Roy JJ, Nair RA, Abraham TE (2002) Degradation of textile dyes mediated by plant peroxidases. Appl Biochem Biotechnol 102–103:315–326
Singh S, Kang SH, Mulchandani A, Chen W (2008) Bioremediation: environmental clean-up through pathway engineering. Curr Opin Biotechnol 19:437–444
Stiborova M, Hansikova H (1997) Peroxidases from tulip bulbs (Tulipa fosteriana L.) oxidize xenobiotics N-nitrosodimethylamine and N-nitroso-N-methylaniline in vitro. Collect Czech Chem Commun 62:1804–1814
Suresh B, Bais HP, Raghavarao SMS, Ravishankar GA, Ghildyal NP (2005a) Comparative evaluation of bioreactor design using Tagetes patula L. hairy roots as a model system. Process Biochem 40:1509–1515
Suresh B, Sherkhane PD, Kale S, Eapen S, Ravishankar GA (2005b) Uptake and degradation of DDT by hairy root cultures of Cichorium intybus and Brassica juncea. Chemosphere 61:1288–1292
Vasudevan P, Kashyap S, Sharma S (1997) Tagetes: a multipurpose plant. Bioresour Technol 62:29–35
Wood TK (2008) Molecular approaches in bioremediation. Curr Opin Biotechnol 19:572–578
Acknowledgments
VB expresses gratitude to Council of Scientific and Industrial Research (CSIR), New Delhi, India, for Emeritus Scientist Fellowship. PP is thankful to Shivaji University, Kolhapur, India, for awarding Departmental Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patil, P., Desai, N., Govindwar, S. et al. Degradation analysis of Reactive Red 198 by hairy roots of Tagetes patula L. (Marigold). Planta 230, 725–735 (2009). https://doi.org/10.1007/s00425-009-0980-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-009-0980-9