Abstract
A steady supply of water is indispensable for leaves to fulfil their photosynthetic function. Understanding water movement in leaves, especially factors that regulate the movement of water flux from xylem to epidermis, requires that the nature of the transport pathway be elucidated. To determine the hydraulic linkage between xylem and epidermis, epidermal cell turgor pressure (P t) in leaves of Tradescantia fluminensis was monitored using a cell pressure probe in response to a 0.2 MPa step change in xylem pressure applied at the leaf petiole. Halftime of P t changes \( {\left( {T^{x}_{{1/2}} } \right)} \) were 10–30 times greater than that of water exchange across an individual cell membrane \( {\left( {T^{m}_{{1/2}} } \right)}, \) suggesting that cell-to-cell water transport constitutes a significant part of the leaf hydraulic path from xylem to epidermis. Furthermore, perfusion of H2O2 resulted in increases of both \( T^{m}_{{1/2}} \) and \( T^{x}_{{1/2}} \) by a factor of 2.5, indicating that aquaporins may play a role in the xylem to epidermis hydraulic link. The halftime for water exchange \( {\left( {T^{m}_{{1/2}} } \right)} \) did not differ significantly between cells located at the leaf base (2.5 s), middle (2.6 s) and tip (2.5 s), indicating that epidermal cell hydraulic properties are similar along the length of the leaf. Following the pressure application to the xylem (0.2 MPa), P t changed by 0.12, 0.06 and 0.04 MPa for epidermal cells at the base, middle and the tip of the leaf, respectively. This suggests that pressure dissipation between xylem and epidermis is significant, and that the pressure drop along the vein may be due to its structural similarities to a porous pipe, an idea which was further supported by measurements of xylem hydraulic resistance using a perfusion technique.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
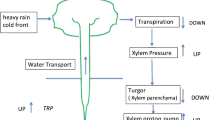
The hydraulic properties of leaves have a major impact on leaf photosynthetic activity, influencing carbon exchange at multiple levels by supplying liquid water for transpiration, protecting chlorophyllous cells from desiccation, and providing water for carbohydrate export (Boyer 1985). Frequently, leaves are treated as uniform evaporative surfaces where the supply of water across the leaf blade is accomplished by the vein network followed by a cell-to-cell path that links hydraulically all of the cells in the leaf. This simplified view, however, cannot explain the presence of multiple water pools in leaves deduced from analysis of rehydration kinetics (Cruiziat et al. 1980; Zwieniecki et al. 2007), or the independent behavior of stomata between different parts or surfaces of a single leaf (Mott and Buckley 2000). Many aspects of the leaf hydraulic system remain poorly understood and leaf hydraulic design and its role in supporting physiological activity remains a topic of active research and discussion (Sack et al. 2003, 2004; Salleo et al. 2003; Cochard et al. 2004; Sack and Holbrook 2006).
Modeling studies suggest that the sites of evaporation occur close to the stomata, indicating an important role for water transport in the liquid, as opposed to the vapor phase in the post-xylary portion of the transpiration stream (Pickard 1981). How water flows through the living tissues of the leaf, however, remains unclear. In particular, discussion of whether water flows predominantly in the apoplast or moves along a cell-to-cell pathway (Kim and Steudle 2007), mirrors those regarding the movement of water across the root cortex (Javot and Maurel 2002). Elucidating the nature of this pathway is critical for understanding what processes might regulate water transport within leaves. Aquaporins provide a potent means for controlling the movement of water across membranes (Chaumont et al. 2005; Maurel 2007), but can only make an important contribution to water flux in leaves if a significant portion of the water flow makes use of the cell-to-cell pathway. Canny (1993) used apoplastic dyes to demonstrate an important role for symplastic transport within leaves of sunflower. However, Westgate and Steudle (1985) argue, based on pressure probe measurements that pressure-driven water transport between xylem and midrib tissue in maize leaves occurs predominantly within the cell walls. Here we examine the hydraulic linkage between epidermis and xylem in Tradescantia fluminensis leaves using a pressure propagation technique (Westgate and Steudle 1985), comparing hydraulic coupling in leaves in which aquaporin activity had been down regulated versus control leaves. We also investigated the ways in which pressure propagates as a function of position.
Materials and methods
Plant material
Plants of T. fluminensis (Vell. Conc.) (Collections of Biological Laboratories, Greenhouse at Harvard University) were grown in the greenhouse using regular potting mix and 3-l containers. All plants were grown from cuttings. Plants were well-watered and supplied with fertilizer as needed and grown in natural irradiance and photoperiod. Experimental material consisted of fully expanded leaves (approximately 60 mm in length) harvested from 2–4 month-old plants.
Cell pressure probe measurements of cell elasticity (ε) and membrane water permeability (hydraulic conductivity, Lp)
Fully expended leaves of T. fluminensis were detached under water using a razor blade and attached to water supply via a flexible tube. If the petiole was too small to allow this connection to be made securely, a small portion of the stem was also excised. Each leaf was then fixed onto a metal support with an adjustable tilt placed on a hydraulically damped table to avoid vibration induced leaks (sudden pressure drops) during the cell pressure probe measurements (Ye et al. 2004). Using a micromanipulator, leaf epidermal cells were impaled with an oil-filled, freshly pulled micro-capillary, whose tip had been ground to approximately 5 μm in diameter with an angle of approximately 45°. A successful puncture requires that the junction between the cell membrane and the capillary forms a tight seal around its perimeter, such that cell sap enters the capillary to form a visible meniscus between the sap and oil. Cell turgor was restored to almost its original (pre-puncture) level by gently moving the meniscus to a position close to the surface of the leaf. Epidermal cells from three different locations along the leaf were probed: at the base of the leaf (∼10 mm from the stem), middle (∼30 mm) and tip (∼50 mm from the base). Probed cells were located between the leaf midrib and the first parallel vein. Since stomata in T. fluminensis spaced across the leaf surface do not align in any particular directions, i.e., stomata are separated by two to three epidermal cells from each other, we always probed cells that are either in direct contact with subsidiary cells or maximum one epidermal cell from the stomata (Fig. 1). Two types of measurements were conducted: (1) characterization of epidermal cell hydraulic properties (including cell membrane hydraulic conductivity and cell elastic properties), and (2) experimental assays of the hydraulic connection between xylem and epidermal cells.
Cell elastic properties are defined by its elastic modulus (ε) and are quantified as the ratio between induced changes in turgor pressure and the resulting effect on cell volume:
where ΔV/V is the relative change in cell volume. Changes in cell volume, ΔV, were induced by rapidly moving the meniscus forward and backward between two marked positions (with the aid of an eyepiece reticle) along the micro-capillary using a pressure probe, and ΔP the instantaneous changes in cell turgor pressure induced in the movements of the meniscus were simultaneously recorded by a computer (Steudle 1993; Volkov et al. 2007). Induced pressure changes ranged from 0.02 to 0.1 MPa to avoid sudden decreases in cell membrane water permeability or rupture of the capillary seal with the cell (Cosgrove and Steudle 1981; Wan et al. 2004).
To determine membrane hydraulic conductivity (L p), the oil/cell sap meniscus in the tip of the micro-capillary was moved forward or backward and then was held stable in the new position until the pressure relaxation was complete, i.e., pressure returned to a stable value. The halftime \( {\left( {T^{m}_{{1/2}} } \right)} \) for water exchange between the cell interior and the medium was obtained from the pressure relaxation curves and hydraulic conductivity (L p) was calculated as:
where V is cell volume, A cell surface area, π i osmotic pressure of cell sap that was estimated from the initial cell turgor; and ε is the elastic coefficient of the cell (elastic modulus). Both A and V were average values calculated from measurements of cell dimensions (n = 90 cells) which were determined from free-hand sections under a microscope (Tomos et al. 1981).
To determine the hydraulic linkage between xylem and epidermal cells, the leaf petiole was attached to a water-filled plastic tube connected to a pressure tank. This setup allowed for step increases and decreases in the pressure (between 0 and 0.2 MPa) applied to the xylem, while the turgor of an epidermal cell was continuously monitored by holding the meniscus in the micro-capillary tip at a constant position. A typical experimental run is shown in Fig. 2. Initial determination of cell hydraulic properties was followed by a step increase in xylem pressure and then rapid decrease while continuously monitoring cell turgor pressure.
Typical turgor pressure/time curve for a single leaf epidermal cell of T. fluminensis as measured by a cell pressure probe. Halftimes of water exchange across cell membrane \( {\left( {T^{m}_{{1/2}} } \right)} \) calculated from hydrostatic relaxations were around 3.0 s. Application of a pressure of 0.2 MPa in the xylem (by pushing water into the petiole) resulted in an exponential increase of cell turgor pressure of 0.05 MPa with a halftime \( {\left( {T^{x}_{{1/2}} } \right)} \) of 34 s. Within errors, original cell turgor pressure was re-attained upon the withdrawal of pressure in the xylem. A similar result was obtained for the same cell when treatment was repeated
To test the contribution of water channels (aquaporins; AQPs) to water flow across the leaf, hydrogen peroxide (H2O2), an effective and environmentally safe inhibitor for AQPs was used as a gating agent (Aroca et al. 2005; Ye and Steudle 2006). Although the mechanism by which H2O2 gates AQPs is not well understood, it is likely that AQP activity is affected by oxidative stress resulting from the presence of reactive oxygen species (ROS). Specifically, AQPs may be attacked by H2O2 or .OH radicals as produced in the Fenton reaction, which could then result in conformational changes of AQPs and their closure (Ye and Steudle 2006). Leaves were perfused (via the petiole) with H2O2 at a concentration of 40 mM for approximately 1.5 h. Pressure probe measurements as described above were then performed on H2O2 pre-treated leaves.
Measurements of leaf vein hydraulic resistance
To determine the apparent hydraulic resistance of leaf veins (R vein), defined as the axial resistance to water flow lengthwise along a leaf with an open tip, water was perfused through the veins at a pressure of 0.2 MPa (the driving force; P driving), while leaves were submerged in oil. Volume flow rates were measured by collecting the water droplets that formed at the open end of each vein during a given period of time (t = 30 min) with the volume (V water) measured using a dissecting microscope (Fig. 3). This approach was used because leaves of T. fluminensis are extremely fragile, precluding the attachment of a tube to the distal portion of the leaf. Estimation of hydraulic resistance as a function of position along the leaf was determined by making consecutive cuts from tip to base (three cuts were performed at the same distance as measurements of the cell hydraulic properties) and measuring flow through the remaining portion of the leaf. Vein resistance (R vein) was calculated using the following formula:
Measurements of leaf vein hydraulic resistance (R vein). Consecutive cuts in the tip, middle and at the base of the leaf led to significant decreases of R vein as measured by using the perfusion technique. Inset shows that water droplets were visible at the cutting edge of a leaf by applying water pressure (0.2 MPa) at the petiole while the leaf was submerged in oil. Different symbols on the top of each column denote significant differences between results (ANOVA; F = (2, 12) 9.4473; P = 0.0034)
To avoid possible interference of hydraulic resistance with anoxia or irradiance, leaves were illuminated with light levels exceeding 100 μmol m−2 s−1.
Results
Cell wall elasticity (ε) and membrane water permeability (\( L_{p} \sim 1 \mathord{\left/ {\vphantom {1 {T^{m}_{{1/2}} }}} \right. \kern-\nulldelimiterspace} {T^{m}_{{1/2}} },\,T^{m}_{{1/2}} , \)= halftime of water exchange across cell membrane) were not significantly different between epidermal cells from different locations along T. fluminensis leaves. Average values of ε were 3.8, 3.7 and 3.4 MPa for cells at the base, middle and the tip of the leaf, respectively [Fig. 4a; ANOVA; F(2, 13) = 0.1884; P = 0.8305]. Halftimes of water exchange across membranes \( {\left( {T^{m}_{{1/2}} } \right)} \) were 2.5, 2.6 and 2.5 s on average for cells from the three positions [Fig. 4b; ANOVA; F (1, 47) = 0.0005; P = 0.9829]. The cell L p calculated from Eq. 2 was also not statistically significantly different because average cell size and thus membrane area was similar (Fig. 4c; ANOVA; F(1, 47) = 0.0095; P = 0.9227). In the present study, cells tested had turgor pressures between 0.2 and 0.4 MPa. Within ± 0.04 MPa, cell turgor pressure remained constant during experiments that usually lasted for about 1 h.
Summary of wall elasticity (ε), halftime of water exchange \( {\left( {T^{m}_{{1/2}} } \right)}, \) and membrane water permeability (Lp) of leaf epidermal cells of T. fluminensis. There were no significant differences between cells in the three parameters (ε, \( T^{m}_{{1/2}} \)and Lp) measured from three different positions of the leaf (base, middle, and tip), as indicated by a same symbol “a” on the top of each column
Application of a positive pressure to the xylem (0.2 MPa) induced an exponential increase of cell turgor pressure in all three locations along the leaf. The magnitude of the increase differed significantly as a function of position, i.e., decreasing from ∼0.12 MPa in the cell located at the leaf base to ∼0.04 MPa at the leaf tip (Fig. 5; ANOVA; F(2, 17) = 19.6453; P = 0.00004). However, the halftimes of the pressure increase \( {\left( {T^{x}_{{1/2}} } \right)} \) measured in epidermal cells along the leaf length varied from 30 to 150 s with no significant effect of location (Fig 6; ANOVA; F (2, 16) = 0.0805; P = 0.9231). These halftimes for pressure increase in epidermal cells were 10–30 times greater than for water exchange across single cell membrane \( {\left( {T^{m}_{{1/2}} \, \approx \,3.0\,s} \right)}. \) In each case, a return to the initial xylem pressure resulted in a drop of cell pressure, while a subsequent step increase resulted in a similar pressure increase and halftime of pressure change (Fig. 2).
Magnitude of epidermal cell turgor increase (ΔP) in response to the application of pressure (0.2 MPa) in the xylem was largest for cells from the base part of the leaf, and smallest for cells from the tip. Different letters on the top of each column denote significant differences between results (ANOVA; F = (2, 17) 19.6453; P = 0.00004)
The above analysis indicates that cell membrane properties, cell integrity and its hydraulic connection to the xylem were not affected during the experiment. However, the significantly lower increase in turgor pressure measured at the more distal positions in response to a step increase in xylem pressure might be associated with pressure dissipation along the vein, due to either vein resistance, water loss from veins, or both. Measurements along the veins showed a non-linear relation between pressure and flow rate, consistent with the idea that a significant portion of the pressure drop is due to leakiness of the pathway rather than hydraulic resistance. As an example shown in Fig. 1, the xylem structure had no significant changes as a function of leaf length, i.e., size of conduits were not obviously changed (ANOVA; F (2, 6) = 4.6431; P = 0.0605).
Perfusion of leaves with H2O2 resulted in increases of both \( T^{m}_{{1/2}} \) and \( T^{x}_{{1/2}} \) by a factor of 2.5 (Fig. 7a, b; t-test, P = 0.05; n = 10 cells). This means that both the water permeability of the epidermal cells and the hydraulic conductivity of the tissue, i.e., the connection between xylem and epidermis, were reduced by the same factor. Treatment with H2O2 did not affect epidermal cell turgor pressure (Fig. 7c; t-test, P = 0.05; n = 10 cells) indicating that cell membranes remained intact, at least for the duration of experiments. The magnitude of epidermal cell turgor pressure change (ΔP) in response to the step increase of pressure in the xylem did not differ between control leaves and those pre-treated with H2O2 (Fig. 7d; t-test, P = 0.05; n = 10 cells).
Effects of H2O2 on halftimes of cell membrane water permeability \( {\left( {T^{m}_{{1/2}} } \right)} \) and epidermal cell turgor pressure change \( {\left( {T^{x}_{{1/2}} } \right)}. \) Perfusion of 40 mM H2O2 to the leaf resulted in increases of both \( T^{m}_{{1/2}} \) (a) and \( T^{x}_{{1/2}} \) (b) by a factor of 2.5, while cell turgor pressure P (c) and the magnitude of pressure change (ΔP) was not significant different (d). Cells tested were from the middle part of the leaf blade. Different letters on the top of each column denote significant differences between results (t-test; P = 0.05; n = 10 cells)
Discussion
A recent analysis of the rehydration kinetics of leaves (Zwieniecki et al. 2007) suggests that many leaves do not function as a well-mixed pool, raising the possibility that differences in the hydraulic linkage between leaf tissues may make a substantial contribution to leaf function. Spatially explicit measurements of hydraulic linkages, however, are needed to determine the degree of hydraulic compartmentalization in leaves. The results of the present study demonstrate a tight hydraulic linkage between veins and epidermis within T. fluminensis leaves. Positive pressure, applied via the xylem, increased epidermal cell turgor with a halftime of approximately 70 s (Fig. 6), which is in the range of halftimes measured for the first phase of leaf rehydration kinetics observed in former studies in angiosperm species (Zwieniecki et al. 2007).
Strong hydraulic coupling of xylem and epidermis provides a simple mode for transduction of information about leaf water status. For example, changes in evaporative demand due to light or heat flux (Sharkey and Yeh 2001) results in a drop in epidermal turgor pressure creating water potential gradient between epidermis and xylem. This increases water supply to the sites of evaporation while potentially bypassing mesophyll cells, thus protecting them from sudden changes in water potential. Alternatively, when xylem water delivery is interrupted by cavitation, pressure signals will spread first to the epidermis forcing stomata to close while again protecting mesophyll cells from excess water loss (Zwieniecki et al. 2007). A large number of studies suggest that hydraulic signals within a plant can be transmitted quickly from shoot to root, and vice versa. For example, Wegner and Zimmermann (1998) demonstrate that light can induce pressure changes in both leaf vessels and root xylem; Comstock and Mencuccini (1998) showed a rise in water potential throughout the shoot upon soil pressurization, which subsequently influenced stomatal conductance; and more recently, Grams et al. (2007) reported that in drought-stressed maize plants, turgor pressure of leaf epidermal cells increased in response to re-irrigation. Such studies underscore the importance of understanding the nature of the hydraulic connection between xylem and epidermal layers, as well as how this pathway relates to the structure and function of the leaf as a whole.
Given that the xylem and epidermis are hydraulically connected, it might be viewed as trivial that the application of pressure at one end results in an increased delivery at the other end. However, upon closer inspection, it is not obvious that the application of positive pressure to the xylem should lead to a turgor pressure change in epidermal cells. An equally plausible scenario is that water will “leak” into intercellular spaces, such as routinely occurs in studies which measure leaf hydraulic properties via pressure perfusion (Tyree et al. 2005). However, if the epidermal cells, prior to xylem pressurization, experience some degree of water deficit due to transpirational water loss from the illuminated leaf surface, the increase in epidermal cell turgor pressure may reflect their rehydration as the water supply via the xylem is increased. Alternatively, any impediment to water movement through the apoplast will enhance pressure transmission through the symplast.
Water movement across plant tissues is often characterized as consisting of three potential pathways. Water may move apoplastically, i.e., in the cell walls (Boyer 1974, 1977); transcellularly, i.e., crossing cell membranes (Tyree et al. 1999; Martre et al. 2002; Sack et al. 2004); or symplastically, i.e., via plasmodesmata (Tyree et al. 1981; Oparka and Prior 1992; Murphy and Smith 1998; Fricke 2000). Most likely all three pathways are used to some degree, although their relative allocation is likely to vary between species and depends on environmental conditions (Sack and Holbrook 2006). Under experimental conditions, the preferential pathway of water transport within leaf tissues might also depend on the physical nature of the driving force used to induce flow, e.g., hydrostatic versus osmotic (Steudle and Jeschke 1983; Westgate and Steudle 1985). For example, Westgate and Steudle (1985) determined water relation parameters (\( T_{{1/2}} , \) L p and ε) of individual parenchyma cells located in different zones (abaxial and adaxial) of the midrib tissue of the maize leaf. Based on data obtained from the sorption kinetics of tissue rehydration and pressure propagation (as performed in the present study), they conclude that under osmotically driven flow (leaf rehydration) water moves primarily using the cell-to-cell pathway, whereas under pressure driven flow the apoplastic path dominates. However, Kramer and Boyer (1995) interpret the same results as indicative of cell-to-cell water movement even in the case of pressure driven flow. Our results are consistent with the latter interpretation. In T. fluminensis leaves, the halftime of epidermal cells turgor pressure change \( {\left( {T^{x}_{{1/2}} } \right)} \) in response to pressure applied to the xylem was 10–30 times greater than that of water exchange across an individual cell membrane \( {\left( {T^{m}_{{1/2}} } \right)}. \) This difference in time constants is consistent with the anatomical structure of T. fluminensis leaves (the number of cells between xylem and measured epidermal cell varied from 10 to 15 on average), thus water molecules on its way from xylem to epidermis had to cross 20–30 cell membranes. Assuming that all cells have similar water exchange characteristics, we believe that requirement for membrane crossing explains the difference in halftimes reported in this study.
We used water channel analysis in search of further proof that we are indeed dealing here with a cell-to-cell path in which the flow crosses multiple membranes. Water channels (aquaporins; AQPs) play an important role in water relations as 75–95% of the trans-membrane movement of water is mediated by AQPs (Steudle and Henzler 1995; Kjellbom et al. 1999; Tyerman et al. 1999). Among numerous internal or external factors that could affect AQP activities, hydrogen peroxide (H2O2) has been shown an effective and environmentally safe inhibitor for root AQPs (Aroca et al. 2005; Ye and Steudle 2006). In the present study, perfusion of H2O2 into leaves resulted in increases of \( T^{m}_{{1/2}} \) by a factor of 2.5 (Fig. 7), suggesting that H2O2 also inhibits AQP activity in T. fluminensis leaves, potentially due to the same mechanism as in the roots (Ye and Steudle 2006). Interestingly, the halftime of leaf epidermal cells turgor pressure change \( {\left( {T^{x}_{{1/2}} } \right)} \) in response to pressure applied to the xylem also increased by a factor of 2.5, further supporting the idea that the cell-to-cell pathway dominates the xylem–epidermis hydraulic connection in T. fluminensis leaves. A number of studies report that plasmodesmata may also contribute to water movement between cells (Oparka and Prior 1992; Fricke 2000). While the results of present study cannot rule out this possibility, we provide evidence that majority of flux is mediated by channels affected by oxidative stress. To date, we are not aware of any studies suggesting that plasmodesmata are sensitive to H2O2 treatment.
Leaf veins function both to distribute water across the leaf and to supply water to surrounding tissues and sites of evaporation (Zwieniecki et al. 2006). Decreases in xylem pressure thus result from either the frictional losses due to Poiseuille flow or the lateral movement of water out of the vein, resulting either from flow into minor veins or leakage into surrounding tissues. Using video recordings of the dye Phloxine B diffusion applied under positive pressure, Salleo et al. (2003) provide visual evidence that major veins of leaf are leaky in the radial directions, as described for roots (Landsberg and Fowkes 1978; Zwieniecki et al. 2002). In the present study, we were unable to measure directly the pressure in the vein, but with the aid of a cell pressure probe we measured the maximum turgor change in epidermal cells at three different locations along the main vein of the leaf blade (base, middle and tip) in response to pressure applied at the petiole (0.2 MPa). Epidermal cell turgor pressure (P t) changed by 0.12, 0.06 and 0.04 MPa for cells at the base, middle and the tip of the leaf, respectively. As the cellular properties of the epidermal cells did not change along the leaf, the observed distribution and magnitude of turgor changes denotes a decrease in xylem pressure as a function of distance along the leaf which we believe are due largely to the porous nature of the vein (Zwieniecki et al. 2006).
In conclusion, the results of present study demonstrate a close hydraulic linkage between xylem and epidermal cells in T. fluminensis leaves, consistent with the hypothesis that leaves may exhibit a significant degree of hydraulic compartmentalization (Zwieniecki et al. 2007). Further, the hydraulic link between xylem and epidermis relies on the cell-to-cell path that is strongly mediated and regulated by activity of membrane aquaporins. Significant pressure drops observed along the vein and pressure dissipation between xylem and epidermis indicates that porous nature of the vein is an important hydraulic component of the leaf vasculature.
References
Aroca R, Amodeo G, Fernandez-Illescas S, Herman EM, Chaumont F, Chrispeels MJ (2005) The role of aquaporins and membrane damage in chilling and hydrogen peroxide induced changes in the hydraulic conductance of maize roots. Plant Physiol 137:341–353
Boyer JS (1974) Water transport in plants: mechanism of apparent changes in resistance during absorption. Planta 117:187–207
Boyer JS (1977) Regulation of water movement in whole plants. In: Jennings DH (ed) Integration of activity in the higher plant. Cambridge University Press, Cambridge, pp 455–470
Boyer JS (1985) Water transport. Annu Rev Plant Physiol 36:473–516
Canny MJ (1993) The transpiration stream in the leaf apoplast: water and solutes. Philos Trans R Soc Lond B 341:87–100
Chaumont F, Moshelion M, Daniels MJ (2005) Regulation of plant aquaporin activity. Biol Cell 97:749–764
Cochard H, Nardini A, Coll L (2004) Hydraulic architecture of leaf blades: where is the main resistance? Plant Cell Environ 27:1257–1267
Comstock JP, Mencuccini M (1998) Control of stomatal conductance by leaf water potential in Hymenoclea salsola (T&G.), a desert subshrub. Plant Cell Environ 21:1029–1038
Cosgrove D, Steudle E (1981) Water relations in growing pea epicotyl segments. Planta 153:343–350
Cruiziat P, Tyree M, Bodet C, Logullo M (1980) Kinetics of rehydration of detached sunflower leaves following substantial water loss. New Phytol 84:293–306
Fricke W (2000) Water movement between epidermal cells of barley leaves—a symplastic connection? Plant Cell Environ 23:991–997
Grams TEE, Koziolek C, Lautner S, Matyssek R, Fromm J (2007) Distinct roles of electric and hydraulic signals on the reaction of leaf gas exchange upon re-irrigation in Zea mays L. Plant Cell Environ 30:79–84
Javot H, Maurel C (2002) The role of aquaporins in root water uptake. Ann Bot 90:301–313
Kim YMX, Steudle E (2007) Light and turgor affect the water permeability (aquaporins) of parenchyma cells in the midrib of leaves of Zea mays. J Exp Bot 58:4119–4129
Kjellbom P, Larsson C, Johannson I, Karlsson M, Johansson U (1999) Aquaporins and water homeostasis in plants. Trends Plant Sci 4:308–314
Kramer PJ, Boyer JS (1995) Water relations of plants and soils. Academic Press, San Diego
Landsberg JJ, Fowkes ND (1978) Water movement through plant roots. Ann Bot 42:493–508
Martre P, Morillon R, Barrieu F, North GB, Nobel PS, Chrispeels MJ (2002) Plasma membrane aquaporins play a significant role during recovery from water deficit. Plant Physiol 130:2101–2110
Maurel C (2007) Plant aquaporins: novel functions and regulation properties. FEBS Lett 581:2227–2236
Mott KA, Buckley TN (2000) Patchy stomatal conductance: emergent collective behaviour of stomata. Trends Plant Sci 5:258–262
Murphy R, Smith A (1998) Determination of cell water-relation parameters using the pressure probe: extended theory and practice of the pressure-clamp technique. Plant Cell Environ 21:637–657
Oparka K, Prior D (1992) Direct evidence for pressure-generated closure of plasmodesmata. Plant J 2:741–750
Pickard WF (1981) How does the shape of the substomatal chamber affect transpirational water loss? Math Biosci 56:111–127
Sack L, Holbrook NM (2006) Leaf hydraulics. Annu Rev Plant Biol 57:361–381
Sack L, Cowan P, Jaikumar N, Holbrook N (2003) The ‘hydrology’ of leaves: coordination of structure and function in temperate woody species. Plant Cell Environ 26:1343–1356
Sack L, Streeter CM, Holbrook NM (2004) Hydraulic analysis of water flow through leaves of sugar maple and red oak. Plant Physiol 134:1824–1833
Salleo S, Raimondo F, Trifilo P, Nardini A (2003) Axial-to-radial water permeability of leaf major veins: a possible determinant of the impact of vein embolism on leaf hydraulics? Plant Cell Environ 26:1749–1758
Sharkey T, Yeh S (2001) Isoprene emission from plants. Annu Rev Plant Physiol Plant Mol Biol 52:407–436
Steudle E (1993) Pressure probe techniques: basic principles and application to studies of water and solute relations at the cell, tissue, and organ level. In: Smith J, Griffith H (eds) Water deficits: plant responses from cell to community. BIOS Sicentific Publishers, Oxford, pp 5–36
Steudle E, Henzler T (1995) Water channels in plants: do basic concepts of water transport change. J Exp Bot 46:1067–1076
Steudle E, Jeschke WD (1983) Water transport in barley roots. Planta 158:237–248
Tomos AD, Steudle E, Zimmermann U, Schulze ED (1981) Water relations of leaf empidermal cells of Tradescantia virginiana. Plant Physiol 68:1135–1143
Tyerman SD, Bohnert HJ, Maurel C, Steudle E, Smith JA (1999) Plant aquaporins: their molecular biology, biophysics and significance for plant water relations. J Exp Bot 25:1055–1071
Tyree M, Cruiziat P, Benis M, Logullo M, Salleo S (1981) The kinetics of rehydration of detached sunflower leaves from different initial water deficits. Plant Cell Environ 4:309–317
Tyree MT, Sobrado MA, Stratton LJ, Becker P (1999) Diversity of hydraulic conductance in leaves of temperate and tropical species: possible causes and consequences. J Trop For Sci 11:47–60
Tyree MT, Nardini A, Salleo S, Sack L, El Omari B (2005) The dependence of leaf hydraulic conductance on irradiance: any role for stomatal response? J Exp Bot 56:737–744
Volkov V, C Hachez, Moshelion M, Draye X, Chaumont F, Fricke W (2007) Water permeability differs between growing and non-growing barley leaf tissues. J Exp Bot 58:377–390
Wan X, Steudle E, Hartung W (2004) Gating of water channels (aquaporins) in cortical cells of young corn roots by mechanical stimuli (pressure pulses): effects of ABA and HgCl2. J Exp Bot 55:411–422
Wegner LH, Zimmermann U (1998) Simultaneous recording of xylem pressure and trans-root potential in roots of intact glycophytes by using a novel xylem pressure probe technique. Plant Cell Environ 21:849–865
Westgate M, Steudle E (1985) Water transport in the midrib tissue of maize leaves. Direct measurements of the propagation of changes in cell turgor across a plant tissue. Plant Physiol 78:183–191
Ye Q, Steudle E (2006) Oxidative gating of water channels (aquaporins) in corn roots. Plant Cell Environ 29:459–470
Ye Q, Wiera B, Steudle E (2004) A cohesion/tension mechanism explains the gating of water channels (aquaporins) in Chara internodes by high concentration. J Exp Bot 55:449–461
Zwieniecki M, Thompson M, Holbrook N (2002) Understanding the hydraulics of porous pipes: tradeoffs between water uptake and root length utilization. J Plant Growth Regul 21:315–323
Zwieniecki MA, Stone H, Leigh A, Boyce CK, Holbrook NM (2006) Hydraulic design of pine needles: one-dimensional optimization for single-veined leaves. Plant Cell Environ 29:803–809
Zwieniecki M, Brodribb T, Holbrook NM (2007) Hydraulic design of leaves: insights from rehydration kinetics. Plant Cell Environ 30:910–921
Acknowledgments
The authors thank Whitney Kress for her valuable assistance in the experiments. We are also grateful to Fulton Rockwell for reading and discussing the manuscript. This work was supported by the National Science Foundation (Grant no.10B-0517071).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ye, Q., Holbrook, N.M. & Zwieniecki, M.A. Cell-to-cell pathway dominates xylem-epidermis hydraulic connection in Tradescantia fluminensis (Vell. Conc.) leaves. Planta 227, 1311–1319 (2008). https://doi.org/10.1007/s00425-008-0703-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-008-0703-7