Abstract
Perturbing CAX1, an Arabidopsis vacuolar H+/Ca2+ antiporter, and the related vacuolar transporter CAX3, has been previously shown to cause severe growth defects; however, the specific function of CAX3 has remained elusive. Here, we describe plant phenotypes that are shared among cax1 and cax3 including an increased sensitivity to both abscisic acid (ABA) and sugar during germination, and an increased tolerance to ethylene during early seedling development. We have also identified phenotypes unique to cax3, namely salt, lithium and low pH sensitivity. We used biochemical measurements to ascribe these cax3 sensitivities to a reduction in vacuolar H+/Ca2+ transport during salt stress and decreased plasma membrane H+-ATPase activity. These findings catalog an array of CAX phenotypes and assign a specific role for CAX3 in response to salt tolerance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ca2+ homeostasis is the nexus between many facets of plant biology (Allen et al. 1999; Sanders et al. 2002). Ca2+ transporters on various membranes play an important role in orchestrating a myriad of biological responses. Although a growing number of Ca2+ transporters have been identified, it is often difficult to associate functions for particular transporters, possibly due to redundancy among them.
Ca2+ can accumulate to millimolar levels in the vacuole, whereas the concentrations are maintained in the micromolar range in the cytosol (Marty 1999). This concentration gradient is established across the tonoplast in part by high capacity H+/Ca2+ exchange and via Ca2+ pumping directly energized by ATP hydrolysis (Sze et al. 2000). The driving force for cation exchange activity is the pH gradient generated by two electrogenic H+ pumps located on the tonoplast, a vacuolar-type H+-ATPase (V-ATPase) and a H+-pyrophosphatase (Sze et al. 1999; Gaxiola et al. 2007). In addition, plant cells require strict maintenance of pH homeostasis in the cytosol, which is controlled by a metabolic-based regulatory mechanism, referred to as the biochemical pH-stat (Sakano 1998), and also by H+ transport systems incorporating the plasma membrane H+-ATPase (P-ATPase) and the above-mentioned tonoplast pumps (Palmgren 2001; Gaxiola et al. 2007). Some recent work has focused on integrating H+ transport processes with biological functions. For example, studies have clarified how H+ pumps alter cell trafficking and hormone perception (Li et al. 2005; Dettmer et al. 2006). Regulated H+ pumps have also been shown to have a role in preventing sensitivity to high pH (Fuglsang et al. 2007). It is conceivable that these events involve alterations in Ca2+ levels and, in principle, the H+/Ca2+ antiporters could also have an important role in many of these same fundamental processes. Indeed, we have previously provided evidence of a functional link between pH changes and H+/Ca2+ antiport activity, whereby these transporters may regulate cytosolic Ca2+ in response to cytosolic pH (Pittman et al. 2005).
In plants, H+/Ca2+ antiport activity was first measured biochemically in the tonoplast (Schumaker and Sze 1986). Plant H+/Ca2+ antiporters were cloned by the ability of N-terminal truncated versions of the proteins to function in Saccharomyces cerevisiae mutants defective in vacuolar Ca2+ transport (Hirschi et al. 1996; Ueoka-Nakanishi et al. 2000; Pittman and Hirschi 2001). The term CAX (CAtion eXchanger) is now used for the two Arabidopsis transporters, CAX1 and CAX2 (Hirschi et al. 1996). Interestingly, CAX3, which is most similar to CAX1 (77% identical at the amino acid level), is at best, a weak vacuolar Ca2+ transporter when expressed in yeast cells (Shigaki and Hirschi 2000; Cheng et al. 2005). In contrast to CAX1 (Pittman and Hirschi 2001, 2003; Shigaki and Hirschi 2006), little is understood regarding the biological properties of CAX3, although both transporters appear to localize to the plant tonoplast (Cheng et al. 2003, 2005). The biological roles these CAX transporters play in cell growth and in response to environmental stresses is only beginning to emerge.
There is a complex interplay among various ions within plants, particularly between Na+ and Ca2+ (Marschner 1995). For example, supplemental Ca2+ is known to mitigate the adverse effects of salinity on plant growth (Epstein 1972; Zhu 2002). Interestingly, expression of both CAX1 and CAX3 are up-regulated during Ca2+ stress, while only CAX3 expression is significantly enhanced during Na+ stress (Hirschi 1999; Shigaki and Hirschi 2000). Signaling molecules that regulate H+/Na+ transport may also regulate CAX transporters (Cheng et al. 2004). However, functional redundancy and compensatory mechanisms have hindered our ability to directly assess the role of CAX transporters in Na+ tolerance.
The interplay among CAX and other transporters has been shown genetically. Mutants in CAX1 not only exhibit a 50% reduction in vacuolar H+/Ca2+ antiport activity, but also a 40% decrease in V-ATPase activity and a 36% increase in vacuolar Ca2+-ATPase activity (Cheng et al. 2003). The cax1 lines also have compensatory changes in gene expression among a battery of transporters, in particular up-regulation of CAX3 and CAX4. Although CAX2 and CAX3 deletions showed no alteration in H+/Ca2+ antiport when grown under calcium excess conditions, they also demonstrate similar reductions in V-ATPase activity (Pittman et al. 2004; Cheng et al. 2005). While the individual CAX mutants display subtle phenotypes, stunted growth and leaf chlorosis are readily apparent when CAX1 and CAX3 are deleted simultaneously (Cheng et al. 2005). The challenge is to delineate the functional specificity of these endomembrane H+/Ca2+ antiporters and decipher how they are integrated into various biological processes.
In this study, we continue to examine phenotypes associated with perturbed CAX1 and CAX3 expression. We report cax phenotypes related to sugar, salt, ethylene, abscisic acid (ABA) and pH perception. We compare and contrast the effects of cax1 and cax3 on the plasma membrane H+-ATPase and demonstrate the unique biochemical role of CAX3 mediated H+/Ca2+ transport during Na+ stress. Summing up, these findings offer insights into the diversity of CAX functions and the integration of CAX3 activity in salt tolerance.
Materials and methods
Plant materials
The cax1-1, cax3-1, cax3-2 and cax1/cax3 lines are in the Arabidopsis thaliana ecotype Col-0 (Cheng et al. 2003, 2005). The deregulated form of CAX1, the N-terminal truncated CAX1 (sCAX1) expressed under the control of the 35S cauliflower mosaic virus promoter in cax1-1 was described previously (Cheng et al. 2003, 2005).
Seed germination
For seed germination measurements, Col-0, cax1-1, cax3-1, cax3-2, and cax1-1/sCAX1 lines were harvested at the same time, dried for 2 days and stored at 4°C for 2 weeks. After being surface-sterilized, these stratified seeds were sown on one-half-strength MS (Murashige and Skoog medium; Murashige and Skoog 1962) agar plates (0.8 % agar, pH 5.6) with or without CaCl2 (50 mM and 100 mM), filter-sterilized ABA (0.1–0.6 μM), or sucrose (0–6%). The plates were placed in the cold room for 48 h and then moved to a growth chamber at 22°C under continuous cool-fluorescent illumination. Germination was monitored daily, and at least three independent experiments (about 60 seeds) were analyzed. For ethylene tolerance, stratified seeds were sown onto one-half-strength MS agar plates (0.8% agar, pH 5.6) containing filter-sterilized 1-aminocyclopropane-1-carboxylic acid (ACC, 10 μM–100 μM). Hypocotyls of at least 50 seedlings from at least three independent trials were scored after 5 days of growth. Seeds with clearly emerged radicals were regarded as germinated; daily germination rate was expressed as a percentage of total sown seeds. Response comparisons were made with similarly stratified seeds of ein2-1 and ein3-1 (provided by Dr. Bonnie Bartel, Rice University, USA).
Root elongation and pH assay
Five-day-old seedlings of cax1, cax3, cax1/cax3 and wild-type controls were grown on one-half-strength MS plates (0.8% agar, pH 5.6) and transferred onto identical media containing 50–100 mM NaCl or 10–30 mM LiCl. Plates were then incubated vertically in a growth chamber at 22°C under continuous cool-fluorescent illumination. The primary root length of seedlings was monitored and measured daily. For pH assays, the pH value of one-half-strength MS medium was adjusted to pH 4.5 with 5 mM MES, or pH 7.5 with 5 mM Tris-MES buffer. These assays were performed on seedlings in plates grown vertically in a growth chamber at 22°C under continuous cool-fluorescent illumination. The primary root length of seedlings was monitored and measured daily.
Preparation of membrane vesicles and transport measurements
Membrane vesicle preparation and Ca2+ uptake were performed as described previously (Shigaki et al. 2001; Cheng et al. 2003). For P-ATPase activity assays, purified plasma membranes were collected from the 32/40% sucrose gradient interface. The hydrolytic activity of the P-ATPase was measured by the release of inorganic phosphate (Pi) according to the method of Ames (1966), as previously described (Vera-Estrella et al. 1999). Plasma membrane vesicles (15 μg of protein) were incubated in 300 μl of solution containing 50 mM KCl, 1 mM sodium molybdate, 3 mM Tris/ATP, 6 mM MgSO4, 30 mM Tris/Mes pH 6.8 in the presence or absence of 200 nM bafilomycin, 10 mM azide and 100 μM vanadate to inhibit the activity of the V-ATPase, the F-type H+-ATPase and the P-ATPase, respectively. The reaction medium was incubated at 37°C for 30 min, and stopped by adding Ames solution (1 volume of 10% ascorbic acid to 6 volumes of 0.42% ammonium molybdate in H2SO4), and the A820 was measured using a Hewlett Packard 8452A Diode-array Spectrophotometer (Hewlett Packard, Mexico, USA). The vanadate-sensitive, bafilomycin- and azide-resistant activity was calculated and is presented as μmol of Pi released min−1 mg−1 of plasma membrane protein. These values of P-ATPase hydrolytic activity are in the range of those previously reported for plants (Vera-Estrella et al. 1999). The time-dependent Ca2+ uptake into plant vacuolar membrane vesicles by Ca2+/H+ exchange was measured using the filtration method (Pittman and Hirschi 2001).
SDS-PAGE and Western blotting
Plasma membrane fractions were precipitated by dilution of the samples 50-fold in 1:1 (v/v) ethanol:acetone and incubated overnight at −30°C. The samples were then centrifuged at 13,000g for 20 min at 4°C using an F2402 rotor in a GS-15R table-top centrifuge (Beckman, Mexico, USA). The pellets were air-dried, re-suspended with sample buffer (2.5% SDS) and heated at 60°C for 2 min before loading (20 μg of protein per lane) onto 10% (w/v) linear acrylamide mini-gels. After electrophoresis, SDS-PAGE-separated proteins were electrophoretically transferred onto nitrocellulose membranes (ECL, Amersham Biosciences) and Western blot analysis was carried out as described above using primary polyclonal antibodies directed against either A. thaliana P-ATPase isoform (AHA3), which also recognizes AHA1 and AHA2 (DeWitt et al. 1996), or HKT1, the plasma membrane Na+-transporter (Su et al. 2003).
Results and discussion
CAX1 or CAX3 deletion impairs germination under sucrose
Arabidopsis has numerous Ca2+ efflux transporters, which include at least 6 H+/Ca2+ antiporters and 14 Ca2+-ATPases (Sze et al. 2000; Shigaki et al. 2006), thus it can be difficult to assign roles to individual transporters, due to redundancy. Function may be identified for a protein that has unique developmental or spatial expression, as is the case for the Ca2+-ATPase ACA9 that is expressed primarily in pollen and is required for pollen tube growth and fertilization (Schiott et al. 2004). There are multiple CAX transporters in Arabidopsis (Shigaki et al. 2006) and clear phenotypes have previously been observed with the cax1 knockout lines (Cheng et al. 2003; Catala et al. 2003; Bradshaw 2005). This may be due in part to the disparate expression pattern of CAX1 compared to the closely related CAX3 in the older plant. It has been shown previously that in older seedlings and mature plants, CAX1 is mainly expressed in leaves and CAX3 is predominantly expressed in roots (Cheng et al. 2005). While the expression of CAX1 and CAX3 do not largely overlap in the mature plant, both genes are strongly expressed during seed germination and early seedling development (Zhao et al. unpublished). Publicly available microarray data confirm these expression profiles (http://www.genevestigator.ethz.ch/at/). In order to more readily compare and contrast the mutants, we thus focused on comparing phenotypes during seedling growth.
All lines displayed similar germination rates in normal media (Cheng et al. 2003, 2005; Fig. 1a). Preliminary findings suggested that CAX1 and CAX3 are up-regulated in seedlings and plants treated with various concentrations of sugars (data not shown), implicating the involvement of these transporters in the plant’s response to altered sugar levels. In order to determine if cax1 and cax3 showed altered responses to sugar stress during seed germination, both lines were germinated on media containing various concentrations of sucrose. Both cax1 and cax3 seeds were sensitive to high levels of sucrose in a dose-dependent manner, but the sensitivity of cax3 seeds to high sucrose was much greater than that of cax1 seeds, suggesting that CAX3 may play a significant role in the sucrose response (Fig. 1b). There was no difference between the cax3-1 and cax3-2 lines. The cax1 lines expressing 35S:sCAX1 germinated in a manner indistinguishable from the wild type, suggesting that sCAX1 expression ameliorated the germination sensitivity of the cax1 lines to high sucrose.
Germination phenotypes of cax1 and cax3 mutant seedlings. The matched seed lots for Col-0 (wild type), cax1–1, cax3-1, cax3-2 and cax1-1/sCAX1 were sown onto one-half-strength Murashige and Skoog (MS) medium agar plates (a) and the identical medium supplemented with increasing concentrations of sucrose (b) or ABA (c). A time course of germination over 6 days is shown in (a). Germination rate in the presence of sucrose or ABA after 6 days incubation is shown in (b) and (c). Plates were incubated at 22°C under continuous fluorescence light. All data are means ± SE from three independent experiments (each experiment included 60 seeds per line)
cax1 and cax3 knockout seedlings display similar phytohormone phenotypes
Recent work has suggested that sugar sensitivity during germination is related to hormone signaling (Rolland et al. 2006). Indeed it has been shown that different hormones regulate similar processes through various non-overlapping transcriptional responses (Nemhauser et al. 2006). Previous studies suggest that cax mutants may have altered responses to a variety of phytohormones (Catala et al. 2003; Cheng et al. 2003). To directly address hormone phenotypes, cax mutants were germinated on various hormone-containing media. Compared to controls, cax1 and cax3 seeds displayed impaired germination on media supplemented with ABA, and the rate of inhibition of germination was identical for both mutants (Fig. 1c). In contrast, both cax1 and cax3 seeds displayed robust germination on media containing various concentrations of ACC (Fig. 2a; data not shown). ACC is the immediate precursor of ethylene in the biosynthesis pathway and can be used in growth assays to investigate ethylene responses. All three lines, cax1, cax3 and cax1/cax3, displayed tolerance to ACC, as visualized by their elongated hypocotyls when grown in the dark, compared to Col-0, which displayed the characteristic ethylene-dependent inhibition of hypocotyl elongation of etiolated seedlings (Fig. 2). The length of hypocotyls from cax mutant seedlings was significantly longer than those from Col-0 seedlings, although still shorter than seedlings grown in the absence of ACC, indicating that tolerance to ACC in the absence of CAX1 or CAX3 was not complete (Fig. 2b). The cax1/cax3 double mutant grown on ACC had a very slight increase in hypocotyl length compared to single mutants, but this was not significantly different; there was no significant difference in mean hypocotyl length between all the cax mutant lines (Fig. 2b). The cax1 line expressing 35S:sCAX1 was indistinguishable from Col-0.
Ethylene sensitivity of cax1 and cax3 mutant seedlings. a Seeds of Col-0 (wild type) and various cax mutant lines, as indicated, were germinated on one-half-strength Murashige and Skoog (MS) medium agar plates in the dark, in the absence (upper panel) or presence (lower panel) of 40 μM ACC. Pictures were taken after 7 days. b Length of hypocotyls of 6-day-old, Col-0 and cax mutant seedlings grown in the dark on one-half-strength MS medium containing 40 μM ACC. Hypocotyl measurements of Col-0 seedlings grown on medium without ACC is shown for comparison (1/2 MS). Data are means ±SE of hypocotyl length from at least three independent experiments (each experiment included 30 seeds per line)
Our working hypothesis is that genes thought to be involved in Ca2+ homeostasis and hormone responses are deregulated in the cax mutants. We postulate that Ca2+ homeostasis has been perturbed in the cax mutants; thus, it is not surprising that cax1 and cax3 lines were sensitive to sugar, ABA and ethylene. It is well known that Ca2+ signaling plays important roles in seed germination and seedling development (Raz and Fluhr 1992; Finkelstein et al. 2002). Vacuolar Ca2+ homeostasis appears to be important in ABA signaling. ABA induces cytosolic Ca2+ elevation, which is in part due to Ca2+ release from the vacuole. Recently, it has been observed that a tpc1 mutant is insensitive to ABA-induced repression of germination (Peiter et al. 2005). TPC1 encodes a vacuolar SV-type Ca2+ channel required for Ca2+ release, thus it is plausible that vacuolar H+/Ca2+ antiporter mutants that have reduced vacuolar Ca2+ uptake displayed the opposite phenotype.
Many genetic and physiological studies have documented that overall sensitivity of plant seed germination to a hormone is at least partially established by the interplay of several hormones (Beaudoin et al. 2000; Ghassemian et al. 2000). ABA and ethylene are known to control seed dormancy and germination, most often through transiently stimulating an elevation in cytosolic Ca2+ (Raz and Fluhr 1992; Finkelstein et al. 2002). Our hypothesis is that CAX1 and CAX3 play a role in ABA-dependent seed germination by regulating ABA-induced Ca2+ elevation patterns. In contrast to the effect of ABA, ethylene has a positive role in promoting seed germination. It has been shown that ABA and ethylene counteract each other during seed germination (Beaudoin et al. 2000; Ghassemian et al. 2000). As expected, cax1 and cax3 mutants were less sensitive to ethylene, as indicated by longer hypocotyl elongation of these mutants in the presence of the ethylene precursor ACC (Fig. 2). Furthermore, because sugar signaling involves both ABA and ethylene signal transduction, it is consistent that cax1 and cax3 mutants also showed sensitivity to high sucrose during germination (Fig. 1b).
The responses of cax1 and cax3 mutants to ABA and ethylene were very similar to those of mutants directly involved in ABA or ethylene signaling pathways, such as ein1 and ein2 (Beaudoin et al. 2000; Ghassemian et al. 2000; Leon and Sheen 2003). These characterized mutants show differential growth responses to ethylene and ABA. Compared with ein2 mutants, cax1 and cax3 were much more sensitive to ACC, although they were significantly less sensitive to ACC as compared to wild-type seedlings (Fig. 2). Isolation and identification of an array of sugar mutants suggest that some sugar-insensitive mutants are actually new loci of ethylene or ABA signaling component genes such as ctr1 (gin4) and aba2 (gin1), whereas sugar hypersensitive mutants include abi1 or ein2 (Rolland et al. 2002, 2006). Both cax1 and cax3 mutants were not as sensitive to sucrose stress (Fig. 1b) as compared with ein2 mutants (data not shown).
cax1 and cax3 seedlings have dissimilar growth rates in response to NaCl, LiCl and low pH
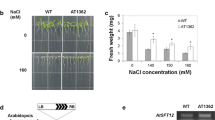
While many of the sugar and phytohormone-dependent germination and growth phenotypes were similar in the cax1 and cax3 lines, we were able to delineate important differences that suggest unique roles for the individual transporters. Previous studies have shown that CAX3 expression is strongly induced by salt stress (Shigaki and Hirschi 2000; Cheng et al. 2003) and this has been confirmed in microarray experiments (http://www.genevestigator.ethz.ch/at/). At the same time, CAX1 is only weakly induced by this stress (Hirschi 1999), suggesting that CAX3 may have a distinct function in response to NaCl. To follow up on this observation, we examined the effect of salt stress on the cax1 and cax3 single and double mutants. The two cax3 lines, as well as cax1/cax3, showed significant sensitivity when grown on media containing 50 and 100 mM NaCl, whereas the cax1 and control lines displayed equivalently less sensitivity to this stress (Fig. 3a). In a similar fashion, the cax3 and cax1/cax3 lines showed similar sensitivity to 10 mM LiCl that was not observed in the control or cax1 lines (Fig. 3b). The cax3 seedlings also showed hypersensitivity to acidic media (pH 4.5), which was not observed in the Col-0 or cax1 lines (Fig. 3c). Under these conditions, the cax1/cax3 double mutants were only slightly sensitive to these conditions (Fig. 3c), exhibiting slower growth and shorter roots as compared with cax1 and Col-0 lines, but less sensitive than the cax3 lines. In all, the absence of a cax1 phenotype in response to these three environmental stresses, yet the similarity of the phenotypes for the cax3 and cax1/cax3 mutants, indicates that they are due specifically to loss of CAX3 function.
Salt, lithium and pH sensitivity of cax3 mutant seedlings. Five-day-old Col-0 (wild type) and various cax mutant seedlings grown on one-half-strength Murashige and Skoog (MS) medium agar plates (pH 5.6) were transferred onto (a) identical plates or plates supplemented with 50 mM or 100 mM NaCl and grown for 5 days, (b) identical plates or plates supplemented with 10 mM LiCl and grown for 7 days, and (c) identical plates at pH 4.5, 5.6 or 7.5 and grown for 7 days. For all treatments, mean root length measurements are shown. All data are means ±SE from at least three independent experiments
To summarize the new phenotypes we have observed in this study, we have found that cax1-1, cax3-1 and cax3-2 have inhibited seed germination in response to ABA and sucrose, although the sensitivity to sucrose is greater in both cax3 lines. Secondly, cax1-1, cax3-1 and cax3-2 have increased tolerance to the ethylene precursor ACC. The degree of tolerance is identical for cax1 and cax3, and, intriguingly, the tolerance to ACC was no greater for the cax1/cax3 double mutant, although the reason for this is unclear. Finally, we have shown that the cax3-1, cax3-2 and cax1/cax3 lines had increased sensitivity to NaCl, LiCl and acidic pH, but cax1-1 was equivalent to Col-0 in response to all of these environmental stresses.
Transport differences between cax1 and cax3 mutant plants
The specific NaCl, LiCl and pH sensitivity of the cax3 lines may be explained in part by the fact that CAX3 is expressed predominantly in root tissue (Cheng et al. 2005; http://www.genevestigator.ethz.ch/at/). This may be of significance, as toxic concentrations of ions such as Na+ in the growth medium will initially accumulate in the roots, and the root is the primary sensor of stresses, such as salt, and the initial site for cytosolic Ca2+ elevations in response to these stresses (Kiegle et al. 2000).
In addition, there may be a biochemical basis for these phenotypes, which we were interested in investigating. In an earlier study, cax1, cax3 and cax1/cax3 lines were characterized for their nearly uniform reduction in V-ATPase activity at the tonoplast (Cheng et al. 2003, 2005). To summarize, previously we showed that V-ATPase activity is reduced by 40% in cax1 lines, by 22% in cax3 lines and by 47% in cax1/cax3 double mutant lines. Conversely, cax1 lines that overexpressed sCAX1 showed a 26% increase in V-ATPase activity (Cheng et al. 2003). Here, we extended our analysis to examine changes in P-ATPase activity among these lines. Plasma membrane vesicles isolated from cax1 plants showed a 32% increase in P-ATPase hydrolytic activity over activity measured in the wild-type plants (Fig. 4a). In contrast, P-ATPase activity in cax3 plants was reduced compared to the wild type (42% reduction; Fig. 4a). The cax1/cax3 double mutant displayed a 20% reduction in P-ATPase activity. In an attempt to correlate these changes in P-ATPase activity to changes at the level of protein in the different mutants, Western blot analysis was carried out on purified plasma membrane fractions using a peptide antibody specific for the three major AHA isoforms: AHA1, AHA2 and AHA3 (DeWitt et al. 1996; Fig. 4b). No significant changes in AHA protein expression were observed suggesting that these specific P-ATPase isoforms are not affected in cax1 or cax3. This may imply that differences in post-translational modification between the mutants are the explanation for the observed P-ATPase changes. However, we cannot rule out that the changes in activity are a result of changes in protein levels of other AHA isoforms from this large multi-gene family (Baxter et al. 2005). While inconclusive, these results confirm that there were no related changes in the distribution of the plasma membranes in the gradients, which could account for the changes in activity measured in the mutants, as no significant change in protein abundance of AHA or HKT1 protein, the plasma membrane Na+ transporter, were observed during multiple membrane preparations (Fig. 4b).
Plasma membrane H+-ATPase and vacuolar H+/Ca2+ antiport activities in cax1 and cax3 mutants. a P-ATPase hydrolytic activity in cax mutants. Rates of vanadate-sensitive, bafilomycin- and azide-resistant ATP hydrolysis were calculated and reported as μmol of Pi released min−1 mg−1 protein in plasma membrane vesicles isolated from Col-0, cax1, cax3 and cax1/cax3 Arabidopsis lines. Results are means ± SE of four independent experiments. b Time course of Mg2+-ATP-energized pH-dependent 10 μM Ca2+ uptake into vacuolar membrane vesicles isolated from wild type, cax1 and cax3 Arabidopsis plants determined in the presence of 0.5 mM orthovanadate (a Ca2+-ATPase inhibitor). At 18 h prior to tissue harvest, the plants were pretreated with 80 mM NaCl. H+/Ca2+ antiport activity was determined as the difference between Ca2+ uptake in the absence and presence of 5 μM carbonyl cyanide p-(trifluoromethoxy)phenylhydrazone (FCCP; protonophore) and net H+/Ca2+ antiport activity is shown after the subtraction of the FCCP background values. The Ca2+ ionophore A23187 (5 μM) was added at the 12 min time point and significantly dissipated Ca2+ accumulation mediated by H+/Ca2+ antiport when measured at the 22 min time point. Data are means ± SE from at least three independent experiments. c Western blot analysis of plasma membranes from wild-type and cax mutants. Plasma membrane fractions, containing 20 μg of protein, were separated on 10% SDS-PAGE gels and transferred to nitrocellulose membranes. Primary antibodies used to probe protein fractions are: anti-AHA3, which was designed to recognize the Arabidopsis plasma membrane H+-ATPase AHA3, but also recognizes isoforms AHA1 and AHA2 (DeWitt et al. 1996); and anti-HKT1, which recognizes the Arabidopsis plasma membrane Na+ transporter (Su et al. 2003). Blots are representative of three independent experiments
Previously, we were unable to detect any change in H+/Ca2+ antiport activity in cax3 lines, while cax1/cax3 displayed cax1-like antiport activity (Cheng et al. 2005). However, these assays were performed following pre-treatment with exogenous calcium application as a means of heightening H+/Ca2+ activity, because this has been shown to induce CAX1 activity (Hirschi 1999). It was conceivable that these conditions do not induce CAX3-mediated Ca2+ transport, hence there was no difference in the total H+/Ca2+ antiport activity of cax3 and Col-0 (Cheng et al. 2005). Given the before-mentioned salt and lithium stress phenotype of the cax3 lines, we measured H+/Ca2+ antiport activity when plants had been pretreated with a sodium stress. When the cax3 lines were grown in the presence of exogenous sodium (80 mM NaCl), there was an approximately 50% reduction in vacuolar H+/Ca2+ transport activity compared to the wild type, while there was no change under these conditions in vacuolar H+/Ca2+ transport activity in the cax1 mutant (Fig. 4c). This suggests that CAX3 is the predominant Ca2+/H+ antiporter in the plant under salt stress conditions, while CAX1 is essentially inactive. This correlates with expression data, as CAX3 is up-regulated by salt but CAX1 is not (Shigaki and Hirschi 2000).
The sensitivity of the cax3 lines to salt stress may be related to the observed decrease in P-ATPase activity observed in this mutant (Fig. 4a). Physiological and genetic evidence suggests that alterations in P-ATPase activity cause salt sensitivity (Yan et al. 1998; Young et al. 1998; Vitart et al. 2001). It is anticipated that reduced P-ATPase activity at the plasma membrane would down-regulate plasma membrane H+-coupled Na+ efflux transporters, such as the SOS1 H+/Na+ antiporter (Zhu 2002), and thus reduce the plant’s ability to tolerate salt stress. This, along with the previously reported decrease in V-ATPase activity (Cheng et al. 2005), which would lead to reduced activity of a tonoplast H+/Na+ antiporter such as AtNHX1 (Zhang and Blumwald 2001), would result in a plant with a much higher sensitivity to salt. In a similar manner, the LiCl sensitivity could be due to impaired activity of H+-coupled Li+ transporters at both membranes following down-regulation of the H+ pumps. AtNHX1 can transport Li+ in addition to Na+ and K+ (Venema et al. 2002), while a plasma membrane-localized homolog, AtNHX8, is important for providing Li+ tolerance (An et al. 2007). The possibility also remains that CAX3 may directly transport Na+ or Li+ in addition to Ca2+, although we have been unable to demonstrate this. The putative transport of such monovalent metals by a CAX-type transporter is not without precedent. Overexpression of soybean GmCAX1 in Arabidopsis gave LiCl and NaCl tolerance, leading the authors of this study to suggest that GmCAX1 may be a Li+ and Na+ transporter (Luo et al. 2005).
The sensitivity of cax3 to acidic pH may also be related to decreased activity of the P-ATPase (Fig. 4a) and V-ATPase (Cheng et al. 2005). Following down-regulation of H+-pumps at both tonoplast and plasma membrane, pH homeostasis would also be severely challenged as is observed in the cax3 mutant (Fig. 3c). In our experiments, the P-ATPase activity was also slightly reduced in the cax1/cax3 knockout, but not as greatly as cax3 (Fig. 4a). These P-ATPase activity measurements correlated with the pH phenotypes, as the cax3 lines were more pH sensitive than the cax1/cax3 lines.
To explain the increase in P-ATPase in cax1, our hypothesis is that a decrease in H+ leak into the cytosol due to reduction in H+ antiport activity (in cax1 lines) at the tonoplast simultaneously dampens V-ATPase, causing feedback activation of the P-ATPase. Other works suggest a relationship between P-ATPase activity and Ca2+ fluctuations, mediated by ABA (Allen et al. 2000; Brault et al. 2004). Our model fits our observation that when sCAX1 is expressed in transgenic lines, V-ATPase activity increases (Cheng et al. 2003). While V-ATPase activity is similarly reduced in both cax1 and cax3, this H+ feedback mechanism does not explain why only cax1 lines, and not cax3, displayed induced P-ATPase activity. We reason that CAX1 is active under Ca2+-induced conditions, while CAX3 is induced by factors such as Na+. Possibly, the P-ATPase activity also changes in the cax mutants depending on the environmental conditions.
An alternative model is that alterations in cytosolic Ca2+ elevation patterns following perturbation of CAX1-dependent or CAX3-dependent H+/Ca2+ antiport activity differentially regulates P-ATPase activity in a Ca2+-dependent manner. A recent study has demonstrated that the P-ATPase isoform AHA2 is negatively regulated via the activity of a calcineurin B-like interacting protein kinase (CIPK) CIPK11 (also named PKS5), which is a homolog of the salt tolerance regulator SOS2 (Fuglsang et al. 2007). These CIPK-type kinases are activated through interaction with calcineurin B-like (CBL) Ca2+-binding proteins, which sense cytosolic Ca2+ levels (Batistic and Kudla 2004). The difference in P-ATPase regulation in the cax mutant backgrounds may be explained by differences in cytosolic Ca2+ modulation by CAX1 and CAX3, which differentially regulate CIPK–CBL activity. In addition, variation in CAX1 and CAX3 tissue expression will mean that they are co-expressed with different CIPK/CBL isoforms, which may have varied downstream targets.
CAX transporters appear to play important roles in nutrient acquisition and signal transduction (Hirschi 2004; Shigaki and Hirschi 2006). This may be intimately associated with the effect these transporters have on the H+ pumps at both the tonoplast and plasma membrane (Cheng et al. 2003, 2005; Fig. 4a). These indirect, and difficult-to-interpret, alterations in H+ pumps on different membranes serve as a reminder that transgenic plants altered in the expression of a single transporter will be difficult to predictably engineer due to complex interactions with other proteins (Zhang and Blumwald 2001; Hirschi 2004; Park et al. 2005).
Previous work has presented models positing that CAX1 negatively controls specific regulators during adaptation (Catala et al. 2003). The accumulating phenotypes associated with the cax1 and cax3 lines suggest that many regulons will be impacted by the activity of these transporters. An illuminating example of the breadth of functions of antiporters is seen with yeast NHX1, an endosomal member of the NHE family of Na+/H+ exchangers (Brett et al. 2005). While it transports both K+ and Na+, it also regulates luminal and cytoplasmic pH to control vesicle trafficking out of the endosome. In flowers of morning glory (Ipomoea tricolor), the vacuolar lumen pH is tightly regulated by an NHX-type H+/Na+ antiporter and by H+ pumps as a means to control flower color. In these flowers, increased vacuolar pH is due to increased NHX1 activity, which correlates with increased V-ATPase and V-PPase activity, in addition to a twofold increase in P-ATPase activity (Yoshida et al. 2005). Similarly, CAX-type H+/Ca2+ transporters may directly regulate Ca2+ levels and also impact on the secretory pathway and pH regulation.
Conclusions
Using standard phenotypic analysis of cax1 and cax3, our data further demonstrate the importance of these transporters in an array of biological responses. Previously, we catalogued the striking phenotypes of the cax1/cax3 double mutants in order to demonstrate the importance of CAX function within the plant (Cheng et al. 2005); however, those studies failed to distinguish the biological differences between CAX1 and CAX3. Here, we have further evaluated hormone and stress phenotypes and biochemical properties of the cax1 and cax3 lines, strengthening the concept that these transporters function in diverse cellular events. The most compelling observation from these studies is the distinct role of CAX3 during salt stress and the opposing roles CAX1 and CAX3 play on P-ATPase activity. In summary, we suggest that functional differences between CAX1 and CAX3 explain the responses of these genes to environmental stress. Firstly, CAX1 and CAX3 appear to differentially regulate plasma membrane and tonoplast H+ pumps (Fig. 4a; Cheng et al. 2005), which will in turn affect various cellular functions including H+-coupled ion transporters and pH regulation. Secondly, we have shown further evidence that the CAX1 and CAX3 Ca2+ transport activities are differentially regulated by environmental stress: CAX1 by Ca2+ stress and CAX3 by Na+ stress (Fig. 4c; Cheng et al. 2005). Finally, we suggest that the root-specific localization of CAX3, but not CAX1, in older plants is important in the root response to some environmental stresses. Future work will be needed to address the specific interactions among the CAX transporters.
Abbreviations
- 35S:
-
35S Cauliflower mosaic virus promoter
- ABA:
-
Abscisic acid
- ACC:
-
1-Aminocyclopropane-1-carboxylic acid
- AHA:
-
Arabidopsis H+-ATPase
- CAX:
-
Cation exchanger
- MS:
-
Murashige and Skoog medium
- P-ATPase:
-
Plasma membrane H+-ATPase
- sCAX:
-
N-terminal truncated cation exchanger
- V-ATPase:
-
Vacuolar-type H+-ATPase
References
Allen GJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JF, Chory J, Schroeder JI (2000) Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289:2338–2342
Ames BN (1966) Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol 8:115–118
An R, Chen QJ, Chai MF, Lu PL, Su Z, Qin ZX, Chen J, Wang XC (2007) AtNHX8, a member of the monovalent cation:proton antiporter-1 family in Arabidopsis thaliana, encodes a putative Li+/H+ antiporter. Plant J 49:718–728
Batistic O, Kudla J (2004) Integration and channeling of calcium signaling through the CBL calcium sensor/CIPK protein kinase network. Planta 219:915–924
Baxter IR, Young JC, Armstrong G, Foster N, Bogenschutz N, Cordova T, Peer WA, Hazen SP, Murphy AS, Harper JF (2005) A plasma membrane H+-ATPase is required for the formation of proanthocyanidins in the seed coat endothelium of Arabidopsis thaliana. Proc Natl Acad Sci USA 102:2649–2654
Beaudoin N, Serizet C, Gosti F, Giraudat J (2000) Interactions between abscisic acid and ethylene signaling cascades. Plant Cell 12:1103–1115
Bradshaw HD Jr (2005) Mutations in CAX1 produce phenotypes characteristic of plants tolerant to serpentine soils. New Phytol 167:81–88
Brault M, Amiar Z, Pennarun AM, Monestiez M, Zhang Z, Cornel D, Dellis O, Knight H, Bouteau F, Rona JP (2004) Plasma membrane depolarization induced by abscisic acid in Arabidopsis suspension cells involves reduction of proton pumping in addition to anion channel activation, which are both Ca2+ dependent. Plant Physiol 135:231–243
Brett CL, Tukaye DN, Mukherjee S, Rao R (2005) The yeast endosomal Na+K+/H+ exchanger Nhx1 regulates cellular pH to control vesicle trafficking. Mol Biol Cell 16:1396–1405
Catala R, Santos E, Alonso JM, Ecker JR, Martinez-Zapater JM Salinas J (2003) Mutations in the Ca2+/H+ transporter CAX1 increase CBF/DREB1 expression and the cold-acclimation response in Arabidopsis. Plant Cell 15:2940–2951
Cheng N-H, Pittman JK, Barkla BJ, Shigaki T, Hirschi KD (2003) The Arabidopsis cax1 mutant exhibits impaired ion homeostasis, development, and hormonal responses, and reveals interplay among vacuolar transporters. Plant Cell 15:347–364
Cheng N-H, Pittman JK, Shigaki T, Lachmansingh J, LeClere S, Lahner B, Salt DE, Hirschi KD (2005) Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion homeostasis. Plant Physiol 138:2048–2060
Cheng N-H, Pittman JK, Zhu JK, Hirschi KD (2004) The protein kinase SOS2 activates the Arabidopsis H+/Ca2+ antiporter CAX1 to integrate calcium transport and salt tolerance. J Biol Chem 279:2922–2926
Dettmer J, Hong-Hermesdorf A, Stierhof YD, Schumacher K (2006) Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 18:715–730
DeWitt ND, Hong B, Sussman MR, Harper JF (1996) Targeting of two Arabidopsis H+-ATPase isoforms to the plasma membrane. Plant Physiol 112: 833–844
Epstein E (1972) Mineral nutrition in plants: principles and perspectives. Wiley, New York
Finkelstein RR, Gampala SSL, Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell 14(suppl):15–45
Fuglsang AT, Guo Y, Cuin TA, Qiu Q, Song C, Kristiansen KA, Bych K, Schulz A, Shabala S, Schumaker KS, Palmgren MG, Zhu JK (2007) Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+-ATPase by preventing interaction with 14-3-3 protein. Plant Cell 19:1617–1634
Gaxiola RA, Palmgren MG, Schumacher K (2007) Plant proton pumps. FEBS Lett 581:2204–2214
Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P (2000) Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell 12:1117–1126
Hirschi KD (1999) Expression of Arabidopsis CAX1 in tobacco: altered calcium homeostasis and increased stress sensitivity. Plant Cell 11:2113–2122
Hirschi KD (2004) The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiol 136:2438–2442
Hirschi KD, Zhen R, Cunningham KW, Rea PA, Fink GR (1996) CAX1, an H+/Ca2+ antiporter from Arabidopsis. Proc Natl Acad Sci USA 93:8782–8786
Kiegle E, Moore CA, Haselhoff J, Tester MA, Knight MR (2000) Cell-type-specific calcium responses to drought, salt and cold in the Arabidopsis root. Plant J 23:267–278
Leon P, Sheen J (2003) Sugar and hormone connections. Trends Plant Sci 8:110–116
Li J, Yang H, Peer WA, Richter G, Blakeslee J, Bandyopadhyay A, Titapiwantakun B, Undurraga S, Khodakovskaya M, Richards EL, Krizek B, Murphy AS, Gilroy S, Gaxiola R (2005) Arabidopsis H+-PPase AVP1 regulates auxin-mediated organ development. Science 310:121-125
Luo GZ, Wang HW, Huang J, Tian AG, Wang YJ, Zhang JS, Chen SY (2005) A putative plasma membrane cation/proton antiporter from soybean confers salt tolerance in Arabidopsis. Plant Mol Biol 59:809–820
Marschner H (1995) Mineral nutrition of higher plants. Academic, New York
Marty F (1999) Plant vacuoles. Plant Cell 11:587–599
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Nemhauser JL, Hong F, Chory J (2006) Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell 126:467–475
Palmgren MG (2001) Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake. Annu Rev Plant Physiol Plant Mol Biol 52:817–845
Park S, Li J, Pittman JK, Berkowitz GA, Yang H, Undurraga S, Morris J, Hirschi KD, Gaxiola RA (2005) Up-regulation of a H+-pyrophosphatase (H+-PPase) as a strategy to engineer drought-resistant crop plants. Proc Natl Acad Sci USA 102:18830–18835
Peiter E, Maathuis FJM, Mills LN, Knight H, Pelloux J, Hetherington A, Sanders D (2005) The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature 434:404–408
Pittman JK, Hirschi KD (2001) Regulation of CAX1, an Arabidopsis Ca2+/H+ antiporter: identification of an N-terminal autoinhibitory domain. Plant Physiol 127:1020–1029
Pittman JK, Hirschi KD (2003) Don’t shoot the (second) messenger: endomembrane transporters and binding proteins modulate cytosolic Ca2+ levels. Curr Opin Plant Biol 6:257–262
Pittman JK, Shigaki T, Hirschi KD (2005) Evidence of differential pH regulation of the Arabidopsis vacuolar Ca2+/H+ antiporters CAX1 and CAX2. FEBS Lett 579: 2648–265
Pittman JK, Shigaki T, Marshall JL, Morris JL, Cheng NH, Hirschi KD (2004) Functional and regulatory analysis of the Arabidopsis thaliana CAX2 cation transporter. Plant Mol Biol 56: 959–971
Raz V, Fluhr R (1992) Calcium requirement for ethylene-dependent responses. Plant Cell 4:1123–1130
Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57:675–709
Rolland F, Moore B, Sheen J (2002) Sugar sensing and signaling in plants. Plant Cell 14(suppl):185–205
Sakano K (1998) Revision of biochemical pH-stat: involvement of alternative pathway metabolisms. Plant Cell Physiol 39:467–473
Sanders D, Pelloux J, Brownlee C, Harper JF (2002) Calcium at the crossroads of signaling. Plant Cell 14(suppl):401–417
Schiott M, Romanowsky SM, Baekgaard L, Jakobsen MK, Palmgren MG, Harper JF (2004) A plant plasma membrane Ca2+ pump is required for normal pollen tube growth and fertilization. Proc Natl Acad Sci USA 101:9502–9507
Schumaker KS, Sze H (1986) Calcium transport into the vacuole of oat roots. Characterization of H+/Ca2+ exchange activity. J Biol Chem 261:12172–12178
Shigaki T, Hirschi KD (2000) Characterization of CAX-like genes in plants: implications for functional diversity. Gene 257:291–298
Shigaki T, Hirschi KD (2006) Diverse functions and molecular properties emerging for CAX cation/H+ exchangers in plants. Plant Biol 8:419–429
Shigaki T, Cheng N-H, Pittman JK, Hirschi KD (2001) Structural determinant of Ca2+ transport in the Arabidopsis H+/Ca2+ antiporter CAX1. J Biol Chem 276:43152–43159
Shigaki T, Rees I, Nakhleh L, Hirschi KD (2006) Identification of three distinct phylogenetic groups of CAX cation/proton antiporters. J Mol Evol 63:815–825
Su H, Balderas E, Vera-Estrella R, Golldack D, Quigley F, Zhao C, Pantoja O, Bohnert HJ (2003) Expression of the cation transporter McHKT1 in a halophyte. Plant Mol Biol 52:967–980
Sze H, Li X, Palmgren MG (1999) Energization of plant cell membranes by H+-pumping ATPases: regulation and biosynthesis. Plant Cell 11:677–689
Sze H, Liang F, Hwang I, Curran AC, Harper JF (2000) Diversity and regulation of Ca2+ pumps: insights from expression in yeast. Annu Rev Plant Physiol Plant Mol Biol 51:433–462
Ueoka-Nakanishi H, Tsuchiya T, Sasaki M, Nakanishi Y, Cunningham KW, Maeshima M (2000) Functional expression of mung bean Ca2+/H+ antiporter in yeast and its intracellular localization in the hypocotyl and tobacco cells. Eur J Biochem 267:3090–3098
Venema K, Quintero FJ, Pardo JM, Donaire JP (2002) The Arabidopsis Na+/H+ exchanger AtNHX1 catalyzes low affinity Na+ and K+ transport in reconstituted liposomes. J Biol Chem 277:2413–2418
Vera-Estrella R, Barkla BJ, Bohnert HJ, Pantoja O (1999) Salt stress in Mesembryanthemum crystallinum L. cell suspensions activates adaptive mechanisms similar to those observed in the whole plant. Planta 207:426–435
Vitart V, Baxter I, Doerner P, Harper JF (2001) Evidence for a role in growth and salt resistance of a plasma membrane H+-ATPase in the root endodermis. Plant J 27:191–201
Yan F, Feuerle R, Schäffer S, Fortmeier H, Schubert S (1998) Adaptation of active proton pumping and plasmalemma ATPase activity of corn roots to low root medium pH. Plant Physiol 117:311–319
Young JC, DeWitt ND, Sussman MR (1998) A transgene encoding a plasma membrane H+-ATPase that confers acid resistance in Arabidopsis thaliana seedlings. Genetics 149:501–507
Yoshida K, Kawachi M, Mori M, Maeshima M, Kondo M, Nishimura M, Kondo T (2005) The involvement of tonoplast proton pumps and Na+(K+)/H+ exchangers in the change of petal color during flower opening of morning glory, Ipomoea tricolor cv. heavenly blue. Plant Cell Physiol 46:407–415
Zhang HX, Blumwald E (2001) Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat Biotechnol 19:765–768
Zhu J-K (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273
Acknowledgments
This work was supported by the United States Department of Agriculture/Agricultural Research Service under Cooperative Agreement 58-6250-6001, National Science Foundation Grant #90344350 and USDA-CSREES #2005-34402-17121, Designing Foods for Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, J., Barkla, B.J., Marshall, J. et al. The Arabidopsis cax3 mutants display altered salt tolerance, pH sensitivity and reduced plasma membrane H+-ATPase activity. Planta 227, 659–669 (2008). https://doi.org/10.1007/s00425-007-0648-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-007-0648-2