Abstract
Using apoplastic voltage- and ion selective microprobes, in barley leaves action potentials (APs) have been measured, which propagate acropetally as well as basipetally from leaf to leaf or from root to leaf following the application of mild salt stress (e.g. 30–50 mM KCl or NH4Cl) or amino acids (e.g. 1 mM glutamic acid or 5 mM GABA). Voltage changes were biphasic, followed an ‘all-or-none’ characteristic, and propagated at 20–30 cm min−1 irrespective of the direction. With the salt-induced APs, a strong initial depolarization is the main AP-releasing factor that first causes Ca2+ influx and then anion efflux. Ca2+ influx coincides with an initial slower depolarization, the rapid anion efflux causes the typical voltage ‘break-through’. Subsequently, K+-efflux starts after the depolarizing voltage has passed the K+ equilibrium potential (inversion of the K+ driving force). Glutamic acid and GABA induce APs not through membrane depolarization, but presumably by binding to a putative receptor or to ligand-gated Ca2+-conducting channels, respectively, followed by Ca2+ induced activation of anion efflux. APs are accompanied by transient apoplastic pH increase (about 1 unit), and by cytoplasmic pH decrease (about 0.5 units). The apoplastic pH change is interpreted as an indicator of stress, the cytoplasmic pH change as a prerequisite for defence related gene activation. Since APs are released by agents added in a moderate concentration range, it is suggested that they may serve as first and fast systemic signals following attack from pathogens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The release of action potentials (APs) apparently reflects a basic disposition of all plant cells and thus has been reported in algae (Hope and Walker 1975; Gradmann and Mummert 1980; Steigner et al. 1987; Wayne 1994), bryophytes (Favre et al. 1999) and higher plants (Pickard 1973; Davies 1987; Trebacz et al. 1997a). In higher plants, APs are being discussed as a generic long distance signalling system that may act to potentiate a host response to subsequent signals delivered through alternative long distance information packages. Although propagation of APs along plant organs is well established (Fromm and Bauer 1994; Malone 1996; Rhodes et al. 1996; Mancuso 1999; Dziubinska et al. 2001; Davies 2004), their physiological connection with natural triggering stimuli is still at stake. Whereas for Dionea the causal connection between mechanical stimulus of the trigger-hairs, the release of the AP, and the snapping of the trap are reasonably well described (Forterre et al. 2005), for most plants investigated the causalities are not so evident. It has been demonstrated repeatedly, however, that electrical waves that propagate from organ-to-organ up-regulate proteinase inhibitor genes (Wildon et al. 1992; Stankovic and Davies 1997; Herde et al. 1998), a response obviously following wounding (Graham et al. 1986). Frequently, APs in plants have been brought into contact with defense responses to herbivore attacks which cause mechanical damage of a tissue and thus may bring the cells into contact with a wealth of saliva substances (Walling 2000; Maffei et al. 2004). Whereas these may be transported to distant tissues and cause defence responses there, already at the point of injury chemical messengers may be produced and/or electrical signals are released. Apparently, both xylem and phloem serve to carry electrical signals systemically. Rhodes et al. (1997) carrying out steaming experiments through which potential pathways were selectively interrupted, suggested that signals following severe wounding were distributed within the xylem as hydraulic surge (Malone 1994; Davies 2004), whereas signals released by smaller wounds were carried preferably within or by the phloem. A third way to send messages from organ-to-organ would be through volatile substances, as demonstrated recently by Mithöfer et al. (2005) for Lima bean. Recently, electrical signalling in plants is being discussed in the concept of plant neurobiology (Brenner et al. 2006), a modern view which attempts to understand how plants respond to environmental input in an integrated fashion.
Rapid and systemically propagating APs have been investigated mostly on dicots, whereas monocots, with a few exceptions, have not been taken into consideration much so far, neither with respect to their physiological relevance nor with respect to the ions involved. Malone (1992) reported wound-induced variation potentials in Triticum, and Fromm and Bauer (1994) and Fromm and Fei (1998) demonstrated APs in Zea mays sieve tubes after electric- and cold-shock stimulation or water stress, from which an important role of electrical in root-to-shoot signalling was suggested. In order to demonstrate that in monocots electrical signals may be a genuine mechnism to carry information systemically, i.e. from one leaf to another leaf or from the root to leaves, barley plants were investigated using a non-invasive electrophysiological technique. Propagating APs were characterized by measuring continuously both voltage and apoplastic activities of H+, Ca2+, K+ and Cl– before and after giving releasing stimuli.
We demonstrate that in barley genuine APs are released by a considerable number of substances, which were applied at moderate concentrations to leaves or roots.
Materials and methods
General conditions
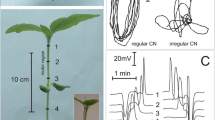
Barley plants (Hordeum vulgare L. cv. Ingrid; Deutsche Saatveredelung, Lippstadt, Germany) were grown at 20–25°C under a 12 h/12 h light/dark regime in a greenhouse. Three- to four-leaved whole barley plants were carefully separated from the soil and the roots submersed in a basal solution which comprised 1–5 mM KCl, 0.1 mM CaCl2, 0.1 mM NaCl, and a 1 mM Mes/Tris buffer solution adjusted to pH 5.5. As described recently (Felle et al. 2005), the entire plant was placed inside a Faraday cage beside the microscope. Part of the first leaf was fixed horizontally to the bottom of a Plexiglass cuvette with double-adhesive tape. A long-distance microscope objective (20×) permitted the positioning of the electrodes at an angle of approx. 45°. As shown in Fig. 1, there were three different approaches. (1) The tip of the leaf, jutting out of the cuvette by approx. 10 cm, was used as test tissue to be submersed in different test solutions and cut therein, if necessary. The monitoring electrodes were placed on the same leaf (Fig. 1a). For recovery from the test solutions, the leaf tip was either put into the basal solution or left in the air after being rinsed with distilled water first, followed by the basal solution. (2) A second leaf was gently pulled away from the cuvette and was fixed with sticky tape to a beaker in which solutions could be exchanged. To trigger the release of APs, leaf tips were submersed in the respective test media (Fig. 1b). (3) As shown in Fig. 1c, the stimulus was given at the roots by exchanging the media without injuring the roots.
Release and systemic propagation of action potentials in barley leaves. a Action potential was monitored on the same leaf the stimulus (e.g. 50 mM KCl) was imposed (first leaf), on a different leaf the stimulus was given (second leaf, b), on a leaf while the stimulus was imposed to the root system (root) through rapid medium exchange (c). Distance from stimulus application to measuring site was 10–12 cm (first leaf) 20–25 cm (second leaf) and 30–40 cm (root). Representative examples of over 20 experiments (first leaf), 14 experiments (second leaf), 6 experiments (root). APs were measured using an apoplastic voltage micro-probe (see text and Materials and methods). Insets indicate the location of stimulus and measuring sites
Electrical measurements
Depending on the type of measurement either the root system or a part of a leaf was submersed in the basal solution and connected to earth (zero voltage). Voltage- or ion-selective microelectrodes were positioned in the sub-stomatal cavity of open stomata, as described recently (Felle et al. 2000, 2004). As soon as the electrode tip had contact with the aqueous phase of the apoplast, the electrical circuit was closed; provided the reading of the signal was stable, the experiments were started by exchanging the media wherever the action potential was to be triggered (first leaf, second leaf, root).
The preparation of the microelectrodes was carried out as described before (Felle et al. 1998, 2004). Briefly: microelectrodes for apoplastic tests were fabricated from 1.5 mm (O.D.) glass tubing (Hilgenberg, Malsfeld, Germany). Capillaries were pulled on a two-stage puller (List, Darmstadt, Germany) to tips of 2–5 μm in diameter, which were heat-polished over a hot platinum wire. These capillaries were filled with 0.5 M KCl to be used as apoplastic voltage electrodes. To prepare ion-selective electrodes, capillaries were heated to 200°C in an oven for 1 h and were then silanized by dipping the rear end of the hot capillaries into a 0.2% tributylsilane/chloroform solution. The capillaries were kept at 200°C for approx.1 h before the silanization procedure was repeated. Silanized capillaries (cooled to room temperature) were backfilled with the respective sensor mixture and topped up with the reference solution: K+ and Cl– electrodes were filled with 100 mM KCl, the Ca2+-electrode with 1 mM CaCl2. All electrodes were stored dry and cool for several days before use. Double-barrelled electrodes were fabricated from double-barrelled tubing (Hilgenberg). The capillaries were pulled to 5 μm tips, were silanized, and then each barrel was filled with the respective sensor, as described above. Ready-to-use electrodes were connected with a high-impedance (1015 Ω) amplifier (FD223, World Precision Instruments, Sarasota, FL, USA). Kinetics were recorded on a chart recorder (L 2200, Linseis, Selb,Germany). Signals picked up by ion-selective electrodes consist of both apoplastic voltage and ion-specific voltage. To obtain the ion-specific net signal, traces were subtracted from each other by a differential amplifier (WPI, Sarasota). pH microelectrodes for intracellular use were fabricated from double-barrelled ‘piggy-back’ glass tubing (Hilgenberg) which consisted of two barrels with different diameters. After pulling the capillaries to 0.5–0.6 μm tips, the barrel with the larger diameter (O.D. 1.5 mm) as the future pH-sensitive electrode was treated as described above; the smaller barrel (O.D. 0.75 mm) as reference electrode was filled with 0.5 M KCl. To prevent cell turgor to push the sensor into the electrode, the pH-sensitive barrel was connected to a home-built pressure controller to hold the sensor in place. Membrane potential measurements in mesophyll cells were carried out by inserting the electrodes through the cut end of a barley leaf which was kept constantly in solution. Agents were tested in a flow through regime.
Results
Basic responses
Application of 50 mM KCl to the open end of a barley leaf triggers an AP, which is picked up by blunt microelectrodes that were non-invasively placed in sub-stomatal cavities. Action potentials are released regardless whether the stimulus is imposed to the leaf the electrode measures on (first leaf; Fig. 1a) or to a neighbouring leaf (second leaf; Fig. 1b). They are also observed, when the salt is added to the root (Fig. 1c). This indicates that such electrical signals propagate systemically through the plant in both directions, i.e. acropetally (Fig. 1c), basipetally (Fig. 1a) or in both directions (Fig. 1b). Since the surface of barley leaves is water repellent, and to guarantee better access of the test agents to the plasma membrane, the leaf tips were cut open under water, i.e. within the respective test solution. This procedure was not always necessary, but it increased the probability of an AP being released. Cutting the leaves in air or in the basal solution with a salt content in the lower millimolar range (comparable to the apoplastic fluid; Felle et al. 2000) did not trigger APs (data not shown). The responses of barley leaves to a selection of different agents are shown in Fig. 2; more are listed in Table 1, where also the responses to different concentrations of the tested substances is taken into consideration. Exchange of the respective anion (Cl– vs. NO –3 , data not shown) did not change the response. 100 mM sorbitol did not trigger an AP, thus ruling out osmotic effects in this concentration range. After taking the cut end of the treated leaf out of the solution, followed by rinsing with the basal solution, an AP can be released again after due time of recovery (approx. 20 min), when brought into contact with the respective substance again; this procedure could be repeated several times. Spontaneous APs were not observed. Irrespective of the treatment, the APs had always the same voltage amplitude of 70–80 mV, propagated with a velocity of 20–30 cm min–1 and as such qualify for a genuine action potential. The APs are biphasic: they start with a minor depolarization after which the depolarization picks up speed and “breaks through”. Since the APs sometimes run very fast, this biphasic characteristic cannot be recognized clearly in all of the shown kinetics. It is stressed that the experiments with KCl presented here are not to be compared with those carried out by Favre et al. (2001) on Arabidopsis leaves, where repetitive APs were triggered through the addition of 250 mM or higher concentrations of KCl following pricking.
Responses of barley leaves to a selection of substances, added at the indicated concentrations, according to condition shown in Fig. 1b (50 mM and flame, propagating distance 35–45 cm), and according to Fig. 1a (1 mM Glu, 10 mM Glu and 5 mM GABA, propagating distance 10–15 cm). For experimental details, see Materials and methods. Table 1 gives a more comprehensive view of the compounds and concentrations the experiments were carried out with. Representative examples of at least five experiments, each
Action potentials are triggered by different factors
All agents tested depolarize the cells at the site of application. This is illustrated in Fig. 3, where the respective voltage responses of mesophyll cells to the application of different agents are shown in a comparative manner. For some of the substances (e.g. KCl, NH4Cl) the extent of this depolarization apparently correlates with their ability to trigger an AP, with others clearly not. As shown in Fig. 2, APs are already triggered by 1 mM Glu and 5 mM GABA, concentrations that do not depolarize mesophyll cells at all (Fig. 3). Ca2+ seems to be a special case: although the depolarization at the mesophyll cells does not exceed that of l-serine (Fig. 3), Ca2+ is very potent to trigger an AP, whereas l-serine is not (Fig. 4). For the sake of reference, the effect of heat (flame) was tested; it also caused the release of an AP, regardless whether imposed at the measuring leaf or neighbouring leaf.
Depolarizations, measured in mesophyll cells of barley leaves, following the addition of different agents at the indicated concentrations. Only the first 5–10 min of the responses are shown. Representative kinetics from at least three experiments, each. Average membrane potential was −164.7 ± 4 mV (n = 35). To get a better impression of the depolarization ratios, initial membrane voltages have been aligned
Propagating voltage transients
The release of an AP also depends on the used concentration of the respective agent. Concentrations around 30 mM KCl or NH4Cl seemingly mark a threshold at which an AP may be triggered or not (Table 1). But even if not, transients are released that propagate at least along the measuring leaf. Voltage transients that propagate from leaf to leaf are currently under investigation in our laboratory. Such transients are also observed with other substances (sugars, amino acids, xylanase), some kinetics of which are shown in Fig. 4, more responses are documented in Table 1.
Ion fluxes during an AP
Both depolarization and repolarization of an AP are caused by ion fluxes, their kinetics of which may or may not overlap. The typical two-phased kinetics of an AP may be caused by rapid transmembrane ion fluxes that move down their steep electrochemical gradient after the respective channels have been activated by either ligand binding or voltage changes. Although the ionic background of APs has already been identified to some extent in some plant cells, e.g. in Characeae (Kikujama and Tazawa 1982; Williamson and Ashley 1982), in the liverwort Conocephalum conicum (Favre et al. 1999), in Mimosa pudica (Samejima and Sibaoka 1980; Abe 1981), just to name a few, for genuine APs in monocots, the problem seems largely unresolved. The non-invasive approach with ion-selective microelectrodes, which are inserted into the leaf apoplast, provides valuable hints there, because these probes measure continuous ion movements in a selective and time-dependent manner as soon as ions are extruded from cells or taken up by cells during an AP. From the potentially effective ions (K+, Na+, Ca2+, Cl–, H+) only Ca2+ and Cl– (anions) may have to be seriously considered as relevant depolarizing charge carriers. This is because the transmembrane electrochemical gradients of Ca2+ and Cl– are steep, whereas those of K+ and Na+ are not; H+, on the other hand, does not passively pass through channels in plasma membranes and hence does not have to be considered. H+ pump deactivation is a possibility, but is not regarded very likely.
Figure 5a shows the temporal sequence of ion fluxes in case the AP was released through membrane depolarization. Obviously, Ca2+ is the first to move: its activity within the apoplast transiently decreases during a KCl-triggered AP, the kinetics of which starts with an initial, comparatively slow depolarization. With some delay, apoplastic Cl– rapidly increases, apparently coinciding with the second phase of the AP, the accelerated depolarization (“break-through”). It is noteworthy, that even during repolarization Cl– still increases before a very slow recovery sets in. Shortly after Cl– efflux has started, but before the AP peaks, apoplastic K+ increases and rapidly decreases again during repolarization. These ion movements occur regardless whether the AP was triggered on the first or second leaf.
Sequence of ion movements during an AP, measured with ion-selective microelectrodes in the apoplast of barley leaves. Changes in apoplastic K+ (pK), Cl– (pCl), and Ca2+ (pCa) accompanying an action potential E(apo), which was triggered by the addition of 50 mM KCl (a) or 1 mM glutamic acid (Glu, b) on another leaf (condition Fig. 1b). Dashed lines in a help to follow up the sequence of events. E(apo), pCa and pCl were always measured simultaneously. Addition of 1 mM LaCl3 (La3+) abolished the pCa response. Representative kinetics of three measurements, each
The response to Glu is somewhat different in that a voltage-gated channel cannot be the primary AP-releasing factor, since even 50 mM Glu does not depolarize the cells sufficiently (Fig. 3). As Fig. 5b shows, the principal apoplastic responses of Ca2+ and Cl– are apparently the same as with the depolarizing agents, the onset of the ion movements cannot be discriminated, however. The Ca2+ channel inbititor La3+ (1 mM LaCl3), added to the cut leaf to be transported by the transpiration stream to the measuring site, abolished both AP and Ca2+ flux (Fig. 5b). 0.1 mM Ruthenium Red, added the same way, had no effect (data not shown).
The pH response
Apoplastic pH responds rapidly and strongly during an action potential that has been released in a neighbouring leaf by 50 mM KCl or 1 mM glutamic acid. As shown in Fig. 6a, b, apoplastic pH rapidly increases by over a pH unit in an oscillating manner to return slowly close to the value measured prior to stimulation. Simultaneously to the apoplastic alkalinization the cytoplasmic pH strongly drops by almost half-a-unit, but recovers within minutes to the control level. Clearly, APs triggered by these two different agents, yielded very similar pH responses.
Apoplastic pH (pH apo ) and cytoplasmic pH (pH cyt ) during an action potential E(apo). An AP was released by the addition of 50 mM KCl (a) or 1 mM glutamic acid (Glu, b) at the leaf tip of one leaf and apoplastic voltage and pH measured simultaneously on another leaf (condition Fig. 1b; propagating distance, 30–35 cm). Representative kinetics of three measurements. In another experiment, cytoplasmic pH was recorded during an AP, using a double-barreled microelectrode. See Materials and methods
Root-to-leaf signalling
Within the plant, information may be transferred systemically either through electrical and/or chemical signals. In Fig. 7a, both possibilities are demonstrated. Addition of 50 mM KCl to the roots triggers an AP which rapidly propagates to the leaf under investigation. At the same time K+ is transported from the root to the leaf, at a much slower speed, however. Whereas it takes the AP just about a minute or two to overcome the distance from the root to the measuring site on the leaf, it takes 8–10 min for K+ to reach the measuring site, which is 20–25 cm away. The latter transfer velocity obviously depends on the transpiration rate (Felle et al. 2005), the propagation of the AP does not. KCl concentrations lower than 50 mM seemingly do not trigger an AP at the roots; nevertheless, the information “K+ increase” or “salt increase” is transferred as such and is detected in the leaf apoplast thereafter (Fig. 7b).
Root-to-shoot signalling. a Action potential, released through the addition of 50 mM KCl to the root system, but measured on a leaf (condition Fig. 1c). The AP is accompanied by the transport of K+ from the root to a leaf (propagating distance approx. 30 cm). b Transport of K+ from the root to a leaf, initiated by different KCl concentrations, as indicated. APs are not triggered by these lower K+ concentrations. Representative kinetics of at least five equivalent experiments, each
Discussion
With respect to systemic signalling, monocots are an almost unploughed field yet. Whereas some evidence of systemic chemical signalling in monocots has been brought forward (e.g. Alborn et al. 1997; Oostendorp et al. 2001), with respect to electrical signalling the available information is apparently limited so far. Malone (1992) reports wound-induced variation potentials in wheat, which travel through the xylem at 10 cm s−1, i.e. much faster than the APs in barley. Using the aphid technique, Fromm and Bauer (1994) measured electric- and cold-shock triggered APs in maize sieve tubes which travel at 3–5 cm s−1. It was suggested that the loss of K+ and Cl– was a consequence of the cytoplasmic Ca2+ increase. A signalling role of electrical events was also discussed for maize by Fromm and Fei (1998) and for Sorghum by Mishra et al. (2001).
In this report it is demonstrated that in barley through relatively mild treatment APs are released, which propagate not only along the leaf they were triggered on, but also from leaf to leaf or from root to leaf. Characteristic properties identify this electrical response as a genuine action potential: it is an ‘all-or-none’ phenomenon. Thus, these APs are not to be compared with the so-called variation potentials (Roblin 1985) or slow waves (Julien et al. 1991), the characteristics of which were outlined by Stankovic et al. (1998).
Ion fluxes and depolarization as AP-determinants
A suggested, sequence of events is given in Fig. 8. Apart from spontaneously released APs (Zawadski et al. 1995), several factors may contribute to the release of an AP: mechanical and heat injury, chilling, electrical stimulation, ligand binding, and depolarization. As demonstrated here, a number of substances strongly depolarize the plasma membrane and thus presumably activate (voltage-gated) ion channels. It has been shown for giant algal cells, that Cl– and K+ effluxes may be integral parts of an AP (Samejima and Sibaoka 1980), probably preceded by Ca2+ influx (Kikujama and Tazawa 1982; Williamson and Ashley 1982; Beilby 1984) which, after elevating cytosolic Ca2+, supposedly stimulates Cl– efflux as shown in Mimosa (Abe 1981) or Conocephalum (Trebacz et al. 1997b). This sequence has also been suggested by Davies (1987) for Helianthus and by Fromm and Bauer (1994) for maize. Although in principle it is possible that (anion-) channels are directly activated by depolarization, the temporal sequence of the ion flux kinetics (Fig. 5a) shows that Ca2+ is lost from the apoplast well before apoplastic anion concentration (measured as Cl–) starts to increase. As shown and discussed for giant algae (Shepherd et al. 2002), Conocephalum (Trebacz et al. 1997b) or dicots (Fisahn et al. 2004), but also for other signalling events like elicitor action (Nürnberger et al. 1994) or Nod factor action (Felle et al. 1998), elevated cytosolic free Ca2+ will activate anion channels and thus cause subsequent rapid anion efflux down its steep electrochemical gradient. The more the channels are activated the more rapid the depolarization will be, finally resulting in an accelerated depolarization and is measured as membrane potential ‘break-through’, typical of an AP. During this depolarization, the equilibrium potential of K+ (EK +) is passed, which inverts the usually inwardly directed driving force for K+ into an outwardly directed one. The resulting rapid K+ efflux neutralizes the anion charges, finally causing the depolarization to stop when positive and negative charges balance each other. This explains why apoplastic Cl– still increases although repolarization is already in action (Fig. 5a, b). The subsequent repolarization inverts the driving force for K+ and (after passing EK + again) induces a rapid re-uptake of K+ (Fig. 5a, b). In the following, apoplastic Cl– slowly recovers, probably through H+-cotransport fuelled by the pump. These data are supported by observations of Fromm and Bauer (1994) who found that the cytoplasmic concentrations of K+ and Cl– in sieve tubes of maize dropped when electrically stimulated while that of Ca2+ may have increased.
Proposed sequence of events leading to action potentials after treating a barley leaf with so-called AP-releasing factors. As in the text, the releasing factors are separated into depolarizing factors (e.g. K+, NH4), Ca2+, and non-depolarizing factors (Glu, GABA). The activations of the Ca2+ and anion channels are regarded as the central events. See also Wayne (1994)
The response to CaCl2 is a special case. Although 50 mM CaCl2 depolarize the mesophyll cells just as much as the same concentration of l-serine (Fig. 3), CaCl2 triggers an AP, l-serine just a propagating transient. Obviously, the depolarization was not sufficient to overcome the threshold. It is suggested that due to the increase in external Ca2+ from 0.1 to 50 mM, Ca2+ rapidly entered the cells, elevated the cytosolic free Ca2+ sufficiently to activate the depolarizing anion channels with the consequences lined out above.
The pH changes measured during an AP are interpreted to be a result from transmembrane ion fluxes. Anion channels do not only release the depolarizing inorganic anions but at the same time organic acid anions which, due to the acidic apoplastic pH and their pK S-values bind protons and thus cause a pH increase. Inversely, the same scenario may lead to the initial cytoplasmic acidification, which turns out smaller because of the buffer capacity being about ten times that of the apoplast. But even taking this into consideration, cytoplasmic and apoplastic pH changes cannot be explained through one mutual process.
APs released by glutamic acid and GABA
Already 1 mM glutamic acid (Glu) or 5 mM GABA triggered APs which propagated from leaf to leaf. In contrast to the observations of Dennison and Spalding (2000) and Meyerhoff et al. (2005) who observed a strong depolarization in Arabidopsis mesophyll cells following the application 1 mM or 0.5 mM Glu, respectively, barley mesophyll cells hardly responded to such concentrations (Fig. 3). Immediately upon addition of Glu, apoplastic Ca2+ transiently decreased in a manner quite comparable to the increase in cytosolic free Ca2+ measured by Meyerhoff et al. (2005). Although Ca2+ and Cl– were measured simultaneously during the AP, the onset of the Cl− and Ca2+ movements were experimentally indistinguishable, making it impossible to give a definite temporal run of the events. From the minor depolarization response on mesophyll cells it becomes clear, however, that not depolarization, but Glu presumably through binding to a specific receptor (Lam et al. 1998) or possibly directly to a Ca2+ channel could initiate the signal cascade that leads to the release of an AP (Fig. 7). Since GABA at 5 mM also triggered APs without considerable depolarization of mesophyll cells (Fig. 3), the same arguments would apply. Alternatively, Glu may gate Cl− conducting channels, which has been shown in animal membranes (e.g. Cully et al. 1994; Schwartz-Bloom and Sah 2001). To find out whether Ca2+ or anion channels are directly activated by Glu, the application of inhibitors could be helpful. However, since anions apparently are the main depolarizing charge carriers in the APs shown here, the use of anion channel inhibitors would not be discriminative enough. On the other hand, feeding of the leaves with 1 mM La3+ into the xylem clearly prevented the apoplastic Ca2+ loss (Fig. 5b) and subsequently the release of an AP, which points to the activation of a Ca2+ channel that releases Ca2+ into the cytoplasm. Whether release of Ca2+ from intracellular stores also plays a role therein, as demonstrated by Plieth et al. (1998) in Chara or Fisahn et al. (2004) for heat-induced APs in Solanum has to remain open, because 0.1 mM Ruthenium Red had no effect. This, however could result from the mode of addition, from which it was not clear at which concentration Ruthenium Red actually reached the site of measurement or whether it arrived there at all.
The problem of the propagating transients
Following the addition of different substances, we observe two types of propagating voltage responses: the just described APs and voltage transients that do not fulfil the requirements of an AP. Some of these responses shown in Fig. 4 originate from agents that do not trigger APs at physiologically meaningful concentrations, others are caused by sub-threshold concentrations of agents that otherwise do trigger APs. It is stressed that their propagation is not electrotonic, because due to system resistance (cytoplasm, plasmodesmata) any superimposed voltage change will, according to cable properties (Cole 1968), suffer a strong decrement already inside a cell, but definitely from cell-to-cell: the voltage amplitude will decrease in a mathematically more or less complicated exponential manner with growing distance from the origin. For instance, not much of a voltage pulse given at the tip of a 5 mm long root hair would reach the cortex or a neighbouring root hair (Felle 1981), unless it got amplified somehow. How do non-AP voltage changes propagate then? There are two explanations: (1) a genuine AP was triggered, but was not picked up because the measuring electrode was too far away. If we reasonably assume that only phloem elements are excitable, then APs will, while propagating, cause more or less pronounced depolarizations in the cells nearby. Thus, in case the electrodes are placed close enough to the AP-conducting cells, an AP-shaped response will be picked up, as shown in this report. A few cell layers away, however, only reduced depolarizations of variable amplitude can be measured (according to the system resistance). In spite of the fact that these voltage transients would then not fulfil the requirements of an AP, they are still remnants of APs that cause these transients while passing by. Basically, such a scenario has been shown in tomato, where heat-induced voltage changes, intra- as well as extracellularly measured, where recorded in different tissues (Rhodes et al. 1996). Since we have never observed APs in the presence of these substances at the given concentrations, we would rather exclude this interpretation for the transients shown in Fig. 4. (2) No AP was triggered, but a sub-critical amount of ion channels was activated, enough to cause the depolarization of a certain membrane area, but not enough to cause the typical ‘break-through’. We assume that, like an AP such a voltage transient may propagate along the measuring leaf, however, unlike an AP may not easily jump electrical obstacles like nodi, albeit we were able to record such signal transfers (data not shown).
Concluding remarks
Action potentials may be triggered by a variety of stimuli; however, only a few of these would truly qualify to be of known biological relevance. Since it could be demonstrated here that already low concentrations of certain agents trigger APs in barley, the physiological relevance of such electrical phenomena with respect to defence related signalling is beyond doubt. What kind of information could be carried by APs? As all-or-nothing responses they probably contain very little defense-related information, but they could act as a fast forerunners to signal injury or threat. Whereas very likely the anions released do not contain much defence related information, the pH changes do, however. Alkalinization of the apoplast is a typical indicator of stress. Wilkinson (1999) discussed this for drought and recently Felle et al. (2005) could show that apoplastic alkalinization is in general a stress indicator. The accompanying cytoplasmic acidification may serve as a precondition for upregulating genes or could act as trigger for gene activation (He et al. 1998). To amplify or confirm the information, the stimulus has to be repeated and more specific (chemical) information will have to travel either volatile (Mithöfer et al. 2005) or somewhat slower in the phloem or, depending on the direction, also in the xylem to distant plant organs. For salt stress such a scenario is shown in Fig. 7, where the AP triggered by KCl is accompanied or followed by substantial transport of K+ into the leaf. It should be interesting to find out whether voltage transients (not APs) are able to carry more specific information than APs, work that is currently in progress in this laboratory.
Abbreviations
- AP:
-
Action potential
- GABA:
-
γ-Aminobutyric acid
- Glu:
-
Glutamic acid
References
Abe T (1981) Chloride ion efflux during action potential in the main pulvinus of Mimosa pudica. Bot Mag 94:379–383
Alborn HT, Turlings TCJ, Jones TH, Stenhagen G, Loughrin JH, Tumlinson JH (1997) An elicitor of plant volatiles from beet armyworm oral secretion. Science 276:945–949
Beilby MJ (1984) Calcium in plant action potentials. Plant Cell Environ 7:415–421
Brenner ED, Stahlberg R, Mancuso S, Vivanco J, Baluska F, van Volkenburgh E (2006) Plant neurobiology: an integrated view of plant signaling. Trends Plant Sci 11:413–419
Cole KS (1968) Membrane, ions and impulses. University of California Press, Berkeley, pp 152–168
Cully DF, Vassilatis DK, Liu KK, Paress PS, Van der Ploeg LHT, Schaffer JM, Arena JP (1994) Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature 371:707–711
Davies E (1987) Action potentials as multifunctional signals in plants: a unifying hypothesis to explain apparently disparate wound responses. Plant Cell Environ 10:623–631
Davies E (2004) New functions for electrical signals in plants. New Phytol 161:607–610
Dennison KL, Spalding EP (2000) Glutamate-gated calcium fluxes in Arabidopsis. Plant Physiol 124:1511–1514
Dziubinska H, Trebacz K, Zawadzki T (2001) Transmission route for action potentials and variation potentials in Helianthus annuus L. J Plant Physiol 158:1167–1172
Favre P, Zawadzki T, Dziubinska A, Trebacz K, Greppin H, Degli Agosti R (1999) Repetitive action potentials induced in the liverwort Conocephalum conicum. Arch Sci 52:187–198
Favre P, Greppin H, Agosti RD (2001) Repetitive action potentials induced inArabidopsis thaliana leaves by wounding and potassium chloride application. Plant Physiol Biochem 39:961–969
Felle HH (1981) A study of the current-voltage relationships of electrogenic active and passive membrane elements in Riccia fluitans. Biochim Biophys Acta 646:151–160
Felle HH, Kondorosi É, Kondorosi Á, Schultze M (1998) The role of ion fluxes in Nod factor signaling in Medicago sativa. Plant J 13:455–463
Felle HH, Hanstein S, Steinmeyer R, Hedrich R (2000) Dynamics of ionic-activities in the apoplast of the sub-stomatal cavity of intact Vicia faba leaves during stomatal closure evoked by ABA and darkness. Plant J 24:297–304
Felle HH, Herrmann A, Hanstein S, Hückelhoven R, Kogel K-H (2004) Apoplastic pH-signalling in barley leaves attacked by the powdery mildew fungus (Blumeria graminis f.sp. hordei). Mol Plant Microbe Interact 17:118–123
Felle HH, Herrmann A, Hückelhoven R, Kogel (2005) Root-to-shoot signalling: apoplastic alkalinization, a general stress signal and defence factor in barley (Hordeum vulgare). Protoplasma 227:17–24
Fisahn J, Herde O, Willmitzer L, Peña-Cortez H (2004) Analysis of the transient increase in cytosolic Ca2+ during the action potential of higher plants with high temporal resolution: requirement of Ca2+ transients for induction of jasmonic acid biosynthesis and PINII gene expression. Plant Cell Physiol 45:456–459
Forterre Y, Skotheim JM, Dumais J, Mahadevau L (2005) How the venus flytrap snaps. Nature 433:421–425
Fromm J, Bauer T (1994) Action potentials in maize sieve tubes change phloem translocation. J Exp Bot 45:463–469
Fromm J, Fei HM (1998) Electrical signalling and gas exchange in maize plants of drying soil. Plant Sci 132:203–213
Gradmann D, Mummert H (1980) Plant action potentials. In: Spanswick RM, Lucas WJ, Dainty J (eds) Plant membrane transport: current conceptual issues. Elsevier North-Holland Biomedical Press, Amsterdam, pp 333–337
Graham JS, Hall G, Pearce G, Uyan CA (1986) Regulation of synthesis of proteinase inhibitor-I and inhibitor-II messenger RNAs in leaves of wounded tomato plants. Planta 169:399–405
He DY, Yazaki Y, Nishizawa Y, Takai R, Yamada K, Sakano K, Shibuya N, Minami E (1998) Gene activation by cytoplasmic acidification in suspension-cultured rice cells in response to the elicitor, N-acetylchitoheptaose. Mol Plant Microbe Interact 11:1167–1174
Herde O, Peña-Cortes H, Willmitzer L, Fisahn J (1998) Time-resolved analysis of signals involved in systemic induction of Pin2 gene expression. Bot Acta 111:383–389
Hope AB, Walker NA (1975) Action potentials in charophyte cells. In: The physiology of giant algal cells. Cambridge University Press, Cambridge, pp 111–119
Julien JL, Desbiez M-O, De Jaegher G, Frachisse JM (1991) Characteristics of the wave of depolarization induced by wounding in Bidens pilosa L. J Exp Bot 42:131–137
Kikujama M, Tazawa M (1982) Transient increase of intracellular Ca2+ during excitation of tonoplast-free Chara cells. Protoplasma 117:62–67
Lam HM, Chiu J, Hsie MH, Meisel L, Oliveira IC, Shin M, Coruzzi G (1998) Glutamate-receptor genes in plants. Nature 396:125–126
Maffei M, Bossi S, Spiteller D, Mithöfer A, Boland W (2004) Effects of feeding Spodoptera littoralis on Lima bean leaves. I. Membrane potentials, intracellular calcium variations, oral secretions, and regurgitate components. Plant Physiol 134:1752–1762
Malone M (1992) Kinetics of wound-induced hydraulic signals and variation potentials in wheat seedlings. Planta 187:505–510
Malone M (1994) Wound-induced hydraulic signals and stimulus transmission in Mimosa pudica L. New Phytol 128:49–56
Malone M (1996) Rapid, long-distance signal transmission in higher plants. Adv Bot Res 22:163–228
Mancuso S (1999) Hydrolic and electrical transmission of wound-induced signals in Vitis vinifera. Aust J Plant Physiol 26:55–61
Meyerhoff O, Müller K, Rolfsema MRG, Latz A, Lacombe B, Hedrich R, Dietrich P, Becker D (2005) AtGLR3.4, a glutamate receptor channel-like gene is sensitive to touch and cold. Planta 222:418–427
Mishra NS, Mallick BN, Sopory SK (2001) Electrical signal from root to shoot in Sorghum bicolor: induction of leaf opening and evidence for fast extracellular propagation. Plant Sci 160:237–245
Mithöfer A, Wanner G, Boland W (2005) Effects of feeding Spodoptera littoralis on Lima bean leaves. II. Continuous mechanical wounding resembling insect feeding is sufficient to elicit herbivory-related volatile emission. Plant Physiol 137:1160–1168
Nürnberger T, Nennstiel D, Jabs T, Sacks WR, Hahlbrock K, Scheel D (1994) High affinity binding of a fungal oligopeptide elicitor to parsley plasma membranes triggers multiple defence responses. Cell 78:449–460
Oostendorp M, Kunz W, Dietrich B, Staub T (2001) Induced resistance in plants by chemicals. Eur J Plant Pathol 107:19–28
Pickard BG (1973) Action potentials in higher plants. Bot Rev 39:172–201
Plieth C, Sattelmacher B, Hansen UP, Thiel G (1998) The action potential in Chara: Ca2+ release from internal stores visualized by Mn2+-induced quenching of fura-dextran. Plant J 13:167–175
Rhodes JD, Thain JF, Wildon DC (1996) The pathway for systemic electrical signal conduction in the wounded tomato plant. Planta 200:50–57
Rhodes JD, Thain JF, Wildon DC (1997) Evidence for physically distinct systemic signalling pathways in the wounded tomato plant. Ann Bot 84:109–116
Roblin G (1985) Analysis of the variation potential induced by wounding in plants. Plant Cell Physiol 26:255–261
Samejima M, Sibaoka T (1980) Changes in the extracellular ion concentration in the main pulvinus of Mimosa pudica during rapid movement and recovery. Plant Cell Physiol 21:467–479
Schwartz-Bloom RD, Sah R (2001) γ-Aminobutyric acid neurotransmission and cerebral ischemia. J Neurochem 77:353–371
Shepard VA, Beilby MJ, Shimmen T (2002) Mechanosensory ion channels in charophyte cells: the response to touch and salinity stress. Eur Biophys J 31:341–355
Stankovic B, Davies E (1997) Intercellular communication in plants: electrical stimulation of proteinase inhibitor gene expression in tomato. Planta 202:402–406
Stankovic B, Witters DL, Zawadzki T, Davies E (1998) Action potentials and variation potentials in sunflower: an analysis of their relationships and distinguishing characteristics. Physiol Plant 103:51–58
Steigner W, Köhler K, Simonis W, Urbach W (1987) Transient cytoplasmic pH changes in correlation with opening of potassium channels in Eremosphaera. J Exp Bot 39:23–36
Trebacz K, Stolarz M, Dziubinska H, Zawadzki T (1997a) Electrical control of plant development. In: Greppin H, Pennel C, Simon P (eds) Travelling shot on plant development. University of Geneva, Geneva pp 163–179
Trebacz K, Simonis W, Schönknecht G (1997b) Effects of anion channel inhibitors on light-induced potential changes in the liverwort Conocephalum conicum. Plant Cell Physiol 38:550–557
Walling LL (2000) The myriad plant responses to herbivores. J Plant Growth Regul 19:195–216
Wayne R (1994) The excitability of plant cells: with special emphasis on characean internodial cells. Bot Rev 60:265–367
Wildon DC, Thain JF, Minchin PEH, Gubb IR, Reilly AJ, Skipper YD, Doherty HM, O’Donnell PJ, Bowles DJ (1992) Electrical signalling and systemic proteinase inhibitor induction in the wounded plant. Nature 360:62–65
Wilkinson S (1999) pH as a stress signal. Plant Growth Regul 29:87–99
Williamson RE, Ashley CC (1982) Free Ca2+ and cytoplasmic streaming in the alga Chara. Nature 296:647–651
Zawadzki T, Dziubinska H, Davies E (1995) Characteristics of action potentials generated spontaneously in Helianthus annuus. Physiol Plant 93:291–297
Acknowledgments
The financial support by the Deutsche Forschungsgemeinschaft (Fe 213/15-1) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Felle, H.H., Zimmermann, M.R. Systemic signalling in barley through action potentials. Planta 226, 203–214 (2007). https://doi.org/10.1007/s00425-006-0458-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-006-0458-y