Abstract
Raman spectroscopy can be used for sensitive detection of carotenoids in living tissue and Raman mapping provides further information about their spatial distribution in the measured plant sample. In this work, the relative content and distribution of the main carrot (Daucus carota L.) root carotenoids, α-, β-carotene, lutein and lycopene were assessed using near-infrared Fourier transform Raman spectroscopy. The pigments were measured simultaneously in situ in root sections without any preliminary sample preparation. The Raman spectra obtained from carrots of different origin and root colour had intensive bands of carotenoids that could be assigned to β-carotene (1,520 cm−1), lycopene (1,510 cm−1) and α-carotene/lutein (1,527 cm−1). The Raman mapping technique revealed detailed information regarding the relative content and distribution of these carotenoids. The level of β-carotene was heterogeneous across root sections of orange, yellow, red and purple roots, and in the secondary phloem increased gradually from periderm towards the core, but declined fast in cells close to the vascular cambium. α-carotene/lutein were deposited in younger cells with a higher rate than β-carotene while lycopene in red carrots accumulated throughout the whole secondary phloem at the same level. The results indicate developmental regulation of carotenoid genes in carrot root and that Raman spectroscopy can supply essential information on carotenogenesis useful for molecular investigations on gene expression and regulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carotenoids are ubiquitous natural pigments that serve essential functions in photosystems of higher plants (Demmig-Adams et al. 1996). Moreover, they occur in non-photosynthetic tissues of some flowers and fruits enhancing attractiveness to pollinators and animals dispersing seeds. Carrot storage root is also a rich source of carotenoids, which are bioavailable to animals and human beings (Horvitz et al. 2004). It is well known that α- and β-carotene can be converted to vitamin A (Olsen 1989) and carrot is one of the main sources of these compounds in the US diet (Rubatzky et al. 1999). The ability of quenching singlet oxygen by carotenoids implies their antioxidant property that plays an important role in the prevention of several diseases. Lycopene and lutein present in some carrot types are valuable phytonutrients in the prevention of cancer, cardiovascular disease and macular degeneration (Fraser and Bramley 2004).
Carrot root has a very complex carotenogenesis and various carotenes and xanthophylls may be synthesized and deposited there. The carotenoid composition of the typical coloured forms is well agreed. Orange carrot contains predominantly β-carotene (45–80%) accompanied by α-carotene that together constitute up to 95% of total carotenoids (Simon and Wolff 1987). Although orange rooted carrots are the most familiar nowadays, yellow and purple types were domesticated first in Afghanistan about tenth century and were cultivated since then in Asia and Europe. Orange and white carrots have been known in Europe for the recent 400 years only and were transferred to North America and Japan. Red coloured carrots evolved in China and India around the eighteenth century where they are still available (Rubatzky et al. 1999). In the yellow carrot lutein and β-carotene are mainly found, but traces of α-carotene are also reported. Significant amounts of lycopene are present only in red roots that contain also β-carotene while α-carotene is usually below the detection limit. Purple roots can possess a similar carotene composition as orange roots, but the presence of dark anthocyanins masks the orange colour (Buishand and Gabelman 1980; Nicolle et al. 2004; Surles et al. 2004).
Carotenoids determine not only root colour but they also affect the perception of carrot taste and flavour that influence the consumer preference (Alasalvar et al. 2001; Habegger and Schnitzler 2005). Colour and aroma compounds are highly associated and thus the carotenoid degradation pathway is considered a key route for the formation of volatile compounds in many plants, also during a long-term storage of carrots (Ayers et al. 1964; Lewinsohn et al. 2005). Furthermore, it has been found that carotenoid cleavage products may also act in plant defence (Bouvier et al. 2005).
Carotenoid molecules consist of a long central chain with a conjugated double-bond system, which is a light-absorbing chromophore responsible for the characteristic colour of these compounds. The colour of main carrot carotenoids, lutein, α-carotene, β-carotene and lycopene varies from yellow to red (Simon and Wolff 1987). Several major genes determine the accumulation of orange, yellow or red pigments in carrot (Simon 1984; Poon and Goldman 2002), but their quantitative composition is additionally affected by quantitative trait loci (QTL, Santos and Simon 2002). Differential level of carotenoids between the secondary phloem and xylem is also under genetic control and is commonly used as a genotype descriptor. Moreover, the colour of both tissues is composite that apparently complicates further study on mechanisms regulating carotenogenesis. Variability in qualitative and quantitative composition of carotenoids in a whole carrot root is well documented; some authors analysed the secondary phloem and xylem separately as they are easily distinguishable (Buishand and Gabelman 1979). However, ambiguous colour delimitation within the tissue and the limits of standard analytical methods constrain more detailed investigations.
New prospects of carotenoid analysis directly in an intact plant tissue have been recently demonstrated by the use of Fourier transform (FT) Raman spectroscopy (Baranski et al. 2005; Schulz et al. 2005). Although carotenoids occur in carrot as minor components at the ppm level, a very sensitive detection can be achieved by Raman measurements due to a signal enhancement caused by the pre-resonance effect of the analyte (Ozaki et al. 1992). Moreover, laser excitation in the near-infrared (NIR) region allows the obscuring fluorescence of biological material to be avoided. Raman spectroscopy is also considered to be non-destructive to plant tissue and thus spectral information can be obtained directly from the investigated sample without the need for carotenoid extraction (Schrader et al. 1999). That feature allows several measurements to be performed at the same point without any harm. Additionally, a spatial distribution of individual carotenoids can be assessed when spectra are collected point by point across a defined root section and then integrated at their characteristic wavelengths. Such data are further reconstructed into 2- or 3-dimensional maps and compared to a visual image of the sample.
The main carrot carotenoids synthesized according to the isoprenoid biosynthetic pathway differ in the length of their central polyene chain and side groups (Fig. 1). Stretching modes of conjugated C=C and C–C bonds in the central chain give in Raman spectra two intense bands near 1,525 and 1,155 cm−1, respectively. Generally, the wavenumber position of the first band is correlated with the length of the polyene chain and can be used for identification purposes. Thus carotenoids with 11, 9, 8 and 7 conjugated C=C bonds have their characteristic bands at about 1,510, 1,525, 1,530 and 1,536 cm−1, respectively. The band position can be additionally slightly influenced by carotenoid side groups and covalent bonds to other plant constituents. Thus the spectral position of –C=C– stretching vibrations provide information about the structure of the investigated carotenoids that have already enabled the main carotenoids occurring in orange and yellow carrot roots to be detected (Schulz et al. 2005).
In spite of the fact that carrot is an important dietary source of carotenoids and that inheritance of root colour has been studied for several years, little is known about carotenogenesis in root tissue. The demand for improved carrot cultivars of high nutritional value requires detailed information on the regulation of the carotenoid biosynthesis and sequestration. Raman spectroscopy can provide insight into carotenoid accumulation directly in living tissue that is not available using previously applied analytical techniques. In this paper, we assess a complex carotenoid composition directly in various intact carrot roots of different colour using FT−Raman spectroscopy; the main carotenoids are identified and their relative contents are compared. We also evaluate spatial distribution and define tissue specific accumulation of these carotenoids across individual root sections. The achieved results demonstrate the potential of Raman spectroscopy for non-destructive carotenoid analysis and are believed to serve as a background for the study on the regulation of carotenoid accumulation.
Materials and methods
Plant material
Cultivated carrots (Daucus carota L. ssp. sativus (Hoffm.) of different root colour and origin were grown in the experimental garden of the BAZ and used for analyses: typical orange ‘Bolero’ F1 (Vilmorin, FR) and ‘Regulus Imperial’ (Nordic Gene Bank, Alnarp, SE, Acc. No. 13955), dark orange ‘Beta III’ and ‘HCM’ (USDA, ARS, University of Wisconsin, Madison, USA), orange with purple outer phloem ‘Purple Haze’ F1 (Bejo Zaden, NL), completely purple line ‘bejo 01-0102’ (Bejo Zaden, NL), yellow line ‘Purple Stem Selection’ (Warwick HRI, Genetic Resources Unit, Wellesbourne, UK, Acc. No. HRIGRU 6519) and red ‘Panipat Special’ (Warwick HRI, Acc. No. HRIGRU 6754); additionally, white wild carrot from BAZ collection 32793/BAZ-GB. Root discs of about 3 mm thickness were transversely cut and used for analyses without any further preparation. Pure α-carotene, β-carotene, lutein and lycopene standards were purchased from Sigma-Aldrich.
Raman measurements
Raman spectra were recorded using a Bruker NIR-FT-Raman Spectrometer (model RFS 100) equipped with a Nd:YAG laser, emitting at 1,064 nm, and a germanium detector cooled with liquid nitrogen. The instrument was equipped with an xy stage, a mirror objective and a prism slide for redirection of the laser beam. Compared with the standard vertical sampling arrangement, the samples were mounted horizontally. Carrot root discs were mounted between two glass slides to avoid any movement and deformation during the measurement.
Single measurements from carrot roots were obtained with 128 scans and an unfocused laser beam of 200 mW. One hundred and twenty-eight scans and a laser power of 10 mW were used for spectral analysis of carotenoid standards in the solid state. Most spectra were obtained with a spectral resolution of 4 cm−1 (for yellow carrot resolution of 2 cm−1 was used) in the wavenumber range from 100 to 4,000 cm−1. Line and 2D Raman measurements were obtained point by point moving the xy stage; x and y directions of the accessory were automatically controlled by the spectrometer software. The samples were irradiated with a focused laser beam of 100–200 mW of a diameter about 0.1 mm; in most cases four scans were collected at each measured point. The spectra collected from the mapped areas were baseline corrected and processed by the Bruker Opus/map software package v. 4.3. The maps were obtained by integration of the spectra at a band characteristic for an analyte and coloured according to the Raman intensity.
Results
Fourier transform Raman spectroscopy was applied for carotenoid analysis in intact white, yellow, orange, red and purple carrot storage roots. The obtained spectra differed in the position of the bands characteristic for the main carrot carotenoids as well as in their intensity. These features allowed visualization of the carotenoid spatial distribution present in the analysed roots.
Identification of the main carotenoids
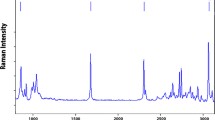
In the Raman spectra obtained from various carrots, the –C=C– stretching vibration of the carotenoid signal was observed within a wavenumber range between 1,500 and 1,540 cm−1, but the position of this band depended on the colour of the analysed root (Fig. 2). Only for white carrots no signal was detected in this range, which confirmed that the observed bands for coloured roots were related to carotenoids and not to the other plant constituents. For both orange and purple roots the band was symmetric and located at 1,520 cm−1 that enabled its identification as a characteristic band of β-carotene according to previous reports (Schulz et al. 2005). For yellow roots the bands were shifted and much broader in comparison to the band of β-carotene. The evident asymmetry suggested their complex composition resulting from the presence of various carotenoids in the tissue.
It is well documented that lutein and α-carotene accompany β-carotene in yellow roots (Alasalvar et al. 2001; Nicolle et al. 2004); thus, the observed band can be considered as consisting of two overlapped signals: the first dominating at 1,520 cm−1 (β-carotene) and the second of lower intensity at about 1,527 cm−1, which can be assigned to lutein or α-carotene (Schulz et al. 2005). The later both pigments have nine conjugated C=C bonds in the central chain and the only structural difference between them is restricted to the presence of hydroxyl groups in the terminal rings. In such case the wavenumber position of ν1 stretching vibration for both carotenoids should be similar and thus discrimination between lutein and α-carotene can be hardly observed. Confirmation of this fact comes from the spectra of pure lutein and α-carotene standards where the discussed difference in the position of the ν1 band is only 1 cm−1 (Schulz et al. 2005). Therefore, both carotenoids could contribute to the observed band at 1,527 cm−1, so we did not discriminate between them.
The spectrum obtained from red carrot was also asymmetrical. The dominant band at 1,520 cm−1 could be assigned to β-carotene similarly as for the other coloured carrots whereas the lower wavenumber vibration seen as a shoulder at about 1,510 cm−1 was due to red lycopene, which is another main carotenoid of red carrot (Buishand and Gabelman 1980). Lycopene is an acyclic 11-conjugated carotene occurring as the principal pigment in red tomato fruits, where it has been already identified by FT-Raman spectroscopy as responsible for ν1 stretching vibration occurring at 1,510 cm−1 (Schulz et al. 2005).
Distribution of β-carotene
The occurrence of strong β-carotene signals in the spectrum of orange and purple carrot roots gives a good precondition to apply Raman mapping technique for investigation of its distribution. For that purpose, measurements were taken point by point in two dimensions and the collected spectra were integrated at 1,520 cm−1. The obtained map was then coloured according to the band intensity that corresponded to β-carotene concentration in the analysed tissue. In all roots, β-carotene distribution was heterogeneous across the root section independently on the carrot genotype and resembled a pattern of concentric rings of high and low carotene levels, which could not be distinguished so clearly by visual examination (Fig. 3). Generally, the highest accumulation could be found in the secondary phloem, whereas periderm/pericyclic parenchyma and the tissue along the vascular cambium were relatively poor in this compound. Significant accumulation of β-carotene in the secondary xylem could be seen for genotypes known as possessing an elevated carotenoid content such as ‘Beta III’ (Fig. 3b) or ‘HCM’ (Fig. 3c), which had a dark orange core. Additionally, carotene accumulation in the secondary phloem showed often a star-shape pattern, which could be compared to the rays of different intensity of orange colour. Repetitive measurements of the same root performed with a resolution of 100 μm produced maps that clearly demonstrated the presence of characteristic carotenoid rays (Fig. 4). Further increase of the resolution to 25 μm revealed that the carotene-rich rays are undoubtedly separated by parenchyma of lower carotene content. The level of carotenoids in the oil ducts and surrounding cells was below detection limit, and appeared as blue spots on Raman maps. Slightly lower colour intensity in such tissue was hardly distinguishable by visual colour inspection and it was not enough pronounced to state that the oil duct cells could be free of carotenoids (Fig. 4c, d).
Visual images of a quarter of transversely cut carrot roots and their Raman maps coloured according to the band intensity in the range of 1,517–1,523 cm−1 related to β-carotene content; orange ‘Bolero’ F1 (a), dark orange ‘Beta III’ (b), dark orange ‘HCM’ (c), purple-orange ‘Purple Haze’ F1 (d), purple line ‘bejo 01–0102’ (e). Mapping increment of 250 μm (a, b), 400 μm (c), 500 μm (d) and 300 μm (e). Microscopic section of carrot root at early secondary growth stained with toluidine blue (f )
Raman maps obtained from a section of orange ‘Bolero’ F1 root with mapping increment of 500 μm (a), 100 μm (b) and 25 μm (c), respectively. The maps are coloured according to the band intensity in the range of 1,517–1,523 cm−1 related to the β-carotene content in the carrot root. d Microscopic image of the same fragment as in map (c) stained with toluidine blue; od oil duct, pr phloem rays
Relative distribution of β-carotene and α-carotene/lutein
A line mapping was performed by collecting a series of spectra from measurements taken in one dimension along the radius of a transversely cut root. Several line mappings executed on the same orange root as well as roots of various cultivars confirmed heterogeneous distribution of β-carotene, but enabled additionally better visualization of differences in carotene content between adjusted tissues. Raman intensity is proportional to the concentration of the analyte molecule so the curve presented in Fig. 5 demonstrates the relative level of β-carotene in the tissue along the root radius. A general tendency was observed that despite homogeneous orange colouration in the secondary phloem the β-carotene level increased from the periderm towards the core, but then it declined rapidly in tissue close to the vascular cambium. The distribution was not symmetrical and the maximum accumulation was located in younger phloem. In the secondary xylem, a small increase of the β-carotene level was observed in the vicinity of the vascular cambium, which was not perceived by visual examination. The innermost tissue usually of lower colour saturation was relatively poor in β-carotene, except for carotene-rich cultivars.
FT-Raman measurements of carotenoids in carrot root of orange ‘Regulus Imperial’ (a) and yellow line ‘Purple Stem Selection’ (b). The plots show the intensity of the C=C vibrational mode at 1,520 cm−1 for β-carotene (a, b) and at 1,527 cm−1 for α-carotene/lutein (b); measurements were taken every 100 μm along a root radius; pp periderm and pericycle, sp secondary phloem, vc vascular cambium, sx secondary xylem
Visual inspection on yellow roots allowed the secondary phloem and xylem to be distinguished, but within those tissues differences in colour intensity were difficult to assess. The same line mapping technique revealed that the distribution of the main carotenoids across the root is complex and allowed to compare their relative level in the same tissue. For that purpose, the intensity of the –C=C– stretching mode was integrated at 1,520 cm−1 for β-carotene and at 1,527 cm−1 for α-carotene/lutein and presented as curves along the root radius (Fig. 5b). In spite of no orange colouration, β-carotene was detected and its distribution followed an asymmetrical pattern similar to that found for orange root with the maximum at intense yellow tissue. At the same time, α-carotene/lutein occurred at lower level and their distribution was more uneven. Elevated amounts of α-carotene/lutein were observed in several measured points along the root radius and were particularly well identified in the secondary xylem. In comparison to β-carotene, the maximum amount of α-carotene/lutein in the secondary phloem was shifted towards the vascular cambium and some of their local minima corresponded to β-carotene peaks. Periderm/pericycle parenchyma contained elevated amounts of α-carotene/lutein.
To visualize the shift between the maxima of β-carotene and α-carotene/lutein in a section of yellow root, 2D Raman mapping was performed with the band integration done at the same wavelengths as for the line mapping presented above. The obtained maps confirmed that β-carotene accumulated predominantly in the secondary phloem (Fig. 6b) while maxima of α-carotene/lutein (Fig. 6c) were localized at regions where the β-carotene level declined. Thus, high amounts of α-carotene/lutein were evident in the xylem, in the phloem along vascular cambium and periderm. Additionally, they accumulated in the secondary xylem produced between the adjusted vascular cambium developing across the secondary phloem towards periderm that was almost free of β-carotene.
Image of a root section of yellow ‘Purple Stem Selection’ (a) and its Raman maps coloured according to the band intensity in the range of 1,517–1,523 cm−1 for β-carotene (b) and 1,524–1,530 cm−1 for α-carotene/lutein (c); mapping increment of 500 μm; pp periderm and pericycle, sp secondary phloem, vc vascular cambium, sx secondary xylem
Relative distribution of β-carotene and lycopene
The Raman maps obtained from a quarter of transversely cut red root and generated from bands at 1,520 and 1,510 cm−1 revealed the distribution of β-carotene and lycopene, respectively (Fig. 7). The distribution of β-carotene was similar to that observed before in the roots of the other colours; also a characteristic ray pattern could be noticed. However, this distribution was not consistent with a visual root appearance since red colour of outer phloem was pronounced and masked any orange colouration coming from β-carotene. Mapping of lycopene showed that this pigment was also accumulated more in the secondary phloem than in the xylem. However, in contrary to the other carotenoids it was distributed relatively homogeneously in the secondary phloem and thus no distinctive maximum concentration of this pigment could be found. That finding was in contrast to visual examination, which showed a gradient of red colour from the periderm towards the secondary cambium.
Image of a root section of red ‘Penipat Special’ (a) and its Raman maps coloured according to the band intensity in the range of 1,517–1,523 cm−1 for β-carotene (b) and 1,505–1,513 cm−1 for lycopene (c); mapping increment of 300 μm; pp periderm and pericycle, sp secondary phloem, vc vascular cambium, sx secondary xylem
Discussion
We have recently demonstrated that FT-Raman spectroscopy is a powerful technique that enables detection of carotenoids in orange and yellow carrots (Schulz et al. 2005). The Raman mapping provided further information on the carotenoid spatial distribution and thus heterogeneous carotene content in the orange roots was postulated (Baranska and Schulz 2005). Here, we have analysed carrots of different origin to assess the distribution of the main carrot carotenoids occurring in roots of different colour. Moreover, we observed relationships in their distribution and related them to the root tissues.
The registered Raman signals of β-carotene were very strong and partially overlapped the signals of the other carotenoids. However, shoulder bands could be clearly assigned to lycopene and α-carotene/lutein, and their characteristic wavenumbers have already been described in the previous report (Schulz et al. 2005). The difference between Raman signals of pure standards of lutein and α-carotene was too small to distinguish between both pigments, so generally we considered these carotenoids together. However, in yellow roots, lutein dominates while α-carotene is often not detectable (Alasalvar et al. 2001; Nicolle et al. 2004), so in those roots the observed band can be assigned mainly to lutein. The assignment of the characteristic bands gave a good precondition to apply the mapping technique, which supplies information about spatial distribution of the investigated molecules and enables a semi-quantitative comparison between different sample regions. Moreover, the ratio between the content of different molecules can be estimated. Such comparison is possible due to the fact that the data on all sample components are collected from the measured point at the same time and then information on the chosen molecules can be retrieved (Baranska et al. 2005). Finally, the principal advantage of FT-Raman spectroscopy with incitation in the NIR range over standard analytical methods is the possibility for taking measurements from living tissue, so the identified carotenoids can be analysed directly in the tissue/cells where they are deposited (Schrader et al. 1999). We therefore used this technique for mapping carotenoids along the root radius or across the surface of the transversely cut root to visualize the level as well as proportions of individual carotenoids accumulated in various root tissues.
Several reports on the difference in carotenogenesis between the secondary phloem and xylem are available and that difference is usually taken into account in detailed carrot examination. Buishand and Gabelman (1979) visually distinguished non-tinge roots where the secondary phloem and xylem had a similar colour, and tinge carrot with very different colour between phloem and xylem. However, tinge roots showed often continuous variation of colour, particularly in phloem, and several classes of colour intensity had to be considered. The similar approach was used for red carrots and their segregating progenies (Buishand and Gabelman 1980). Further chemical analysis of root sections comprising the secondary phloem or xylem confirmed that the observed colour difference was related to the carotenoid content in the tissue, but it was also found that roots of less intense orange colour could possess higher level of carotenoids than those of more intense colouration, so visual classification was not always accurate. Our results are clearly congruent with those findings as we usually observed a difference in carotenoid level between the secondary phloem and xylem. Distribution in the secondary xylem differed also between various carrots. Carotene-rich cultivars such as ‘Beta III’ and ‘HCM’ showed in our experiments elevated amounts of β-carotene while the other carrots were poor in this pigment. In many roots we observed, however, a notable β-carotene increase in a near distance from the vascular cambium while the innermost part of the root was usually almost free of this pigment. Moreover, we found that in the secondary phloem, β-carotene accumulated gradually and increased from very low at periderm to the maximum at about two-third of the phloem radius and then declined fast in cells close to the vascular cambium. Such distribution was observed in all orange, yellow and red carrots as well as in purple roots where anthocyanins completely masked visual colour perception. Variable carotene content across the root could affect visual colour assessment; however, such uneven distribution was found even in roots where no colour differentiation was perceived. These results indicate that both tinge and non-tinge roots have heterogeneous carotene accumulation within the secondary phloem as well as xylem. Therefore, the use of the whole phloem or xylem tissue sections for chemical analyses does not provide complete data for detailed characterization of carotenoid content in various root tissues.
Microscopic observations revealed that carotenes deposited in root chromoplasts show crystalline appearance (Frey-Wyssling and Schwegler 1965). However, the density of crystalline β-carotene (1.00 g ml−1) indicates that chromoplasts do not contain pure carotene crystals. Reiter et al. (2003) have proposed that a hydrophobic core of crystalloid β-carotene is stabilized by amphophilic constituents such as protein or residual membranes. The application of a non-ionic detergent for carotene extraction from carrot root allowed carotenoproteins to be isolated (Milicua et al. 1991; Dietz Bryant et al. 1992). Later, Zhou et al. (1994) have found that the 18 kDa protein-containing carotenoid complex was the major one associated with the pigments within the carrot chromoplasts although associated only with a small percentage of total carotenoids. They also postulated that this complex might function enzymatically in carotenoid synthesis. However, it has not been shown precisely which carotenoids occur in complexes, so far. In Raman spectroscopy, the signal is obtained from both crystalloid and amorphic forms of a molecule, but also if a molecule binds to other plant constituents, for example to proteins. Thus the spectra obtained from the carrot root may comprise information from various carotenoid forms. The applied Raman mapping confirmed that β-carotene was accumulated predominantly in phloem rays (Esau 1940), but additionally revealed that parenchymatic cells also contain this pigment although at a lower level. The characteristic star-shape pattern of the rays can be particularly well recognized when a higher mapping resolution is applied. At the same resolution, no carotenoid signals were recorded in some areas. Comparison of the Raman map and visual picture of the same fragment of intact root tissue showed that the region free of carotenoids is occupied by oil ducts and accompanying cells (Fig. 4c, d). These cells accumulate mono- and sesquiterpenoids responsible for carrot flavour. The observed lack of carotenoids indicates that after cell differentiation during root development the mechanism for sequestration of carotenoids, which are tetraterpenoids, is no longer supported.
α-Carotene or lutein, accompany β-carotene in orange or yellow carrots and a big difference in their content was observed between the secondary phloem and xylem in tinge roots (Buishand and Gabelman 1979). The presented results demonstrate that the level of α-carotene/lutein is also heterogeneous within each of those tissues. A general trend in the distribution of α-carotene/lutein was similar to that of β-carotene, but interestingly several significant deviations were observed: (1) the maxima of these pigments were usually shifted towards the vascular cambium, (2) another local maximum close to the periderm was often recognized, (3) an elevated amount was observed in the secondary xylem produced between adjusted cambium across the secondary phloem where β-carotene was almost not detectable, (4) in the secondary xylem their ratio to β-carotene was usually elevated, and (5) in the secondary xylem local maxima corresponded to local minima of β-carotene. Carrot undergoes a secondary root growth initiated at the vascular cambium. In both outer and inner root parts, new cells are produced continuously and thus the cells along the vascular cambium are the youngest. Due to that expanding growth, the primary meristematic tissue, pericycle, is moved outwards and localized close to periderm, but is still active and produce a thin layer of new cells (Esau 1940). Therefore, the first three findings may indicate that α-carotene/lutein are synthesized in younger cells with a higher rate than β-carotene, but later, in older cells, β-carotene dominates. The observed decline of β-carotene and α-carotene/lutein in more outer phloem may be related to the root developmental stage. Young carrot roots are free of carotenoids and start to accumulate them after the first month of growth. Carotenogenesis intensifies during vegetation and the maximum of deposited carotenoids occurs usually short before the secondary growth is completed (Phan and Hsu 1973). Therefore, the difference in the carotenoid level within the secondary phloem observed in Raman maps reflects changes occurring during root growth with the oldest root parts possessing the lowest amounts of carotenoids. The similar tendency can be observed also in the secondary xylem although it is less pronounced as this root part is usually much smaller and thus the decreasing carotenoid level inwards is evident in some roots only.
Unlike previously discussed pigments, lycopene in red carrots is accumulated throughout the whole secondary phloem at the same level. Also no pattern was found in the secondary xylem, which contained low amount of lycopene. Visually, red carrots exhibited more dark red colour in the outer phloem while the younger part had lower saturation. The uniform distribution of lycopene and heterogeneous accumulation of β-carotene in the same root indicates that the perception of red colour is due to decreased amount of orange β-carotene in the outer phloem rather than to elevated amounts of red lycopene.
In the recent years much work has been devoted to genetics and molecular biology of carotenoids. These approaches have resulted in the identification and cloning of genes critical in the carotenoid biosynthesis (Cunningham and Gantt 1998). According to the biosynthetic pathway of carotenoids in plants, phytoene is the first molecule with a long polyene chain, which is typical for this chemical class. A series of sequential desaturations catalysed first by pytoene desaturase (PSY) and then by ζ-carotene desaturase (ZDS) converts phytoene to phytofluene, ζ-carotene and neurosporene and finally lycopene; formation of lycopene requires additionally carotene isomerase. Cyclic carotenoids are formed from lycopene by cyclization of its end groups. Lycopene β-cyclase (LCY-b) catalyses the formation of two β-rings of β-carotene and one of α-carotene while lycopene ε-cyclase (LCY-e) is additionally required for the creation of the ɛ-ring of α-carotene. β- and ε-ring hydroxylases (CRTR-b and CRTR-e, respectively) convert α-carotene to lutein (Taylor and Ramsay 2005). Several work on gene expression and their regulation clarified the mechanism undergoing in fruit chromoplasts in parallel to that in chloroplasts of photosynthetic tissue. Tomato fruit has become a model for the investigation of regulation in chromoplasts, but relatively little is known about these mechanisms in underground storage organs in spite of intensifying works in potato. The inheritance of the genes conferring carrot root colour has been studied for the last few decades, but carrot has become the target of molecular investigations just recently. So far, only Santos et al. (2005) commenced work in this field and suggested that phytoene is an essential substrate for lycopene and β-carotene production in carrot. Previous works on tomato fruits indicate that carotene isomerase is required for carotenoid biosynthesis that occurs in chromoplasts in the dark and thus may be also critical in carrot root (Isaacson et al. 2002). Different rate of α-carotene/lutein and β-carotene accumulation in root tissues presented in our work may result from the fact that β,ε-ring carotenoids are synthesized via a separate pathway branch than β,β-carotene. The former requires the activation of two independent genes Lcy-e and Lcy-b while for the latter Lcy-b expression seems to be sufficient. In tomato chromoplasts additional cyclase (CYC-b) has been identified that also participates in the β-carotene synthesis (Ronen et al. 2000), but there is no report whether this enzyme is active in carrot root chromoplasts too. Our observations of different distribution of the discussed pigments may indicate that they are developmentally regulated and the expression of the lycopene cyclase genes in carrot is at least partially controlled independently. It is also probable that the regulation occurs directly at the level of accumulation. Carotenoids are sequestered as both crystals and lipoprotein complexes depending on the chromoplast type and some homologous genes involved in that process have been found in tomato (Vishnevetsky et al. 1999). Carrot root also deposits carotenoids in various forms so an analogous mechanism may function there.
The importance of carotenoids in plant ontogenesis as well as for human nutrition has burst the molecular study of carotenoid biosynthesis. However, the knowledge about the regulation mechanisms of their synthesis, accumulation and decomposition is still very limited. The information available on the genetics of carrot carotenoids is based on either visual colour perception or/and chemical analyses. So far, only root sections comprising the secondary phloem or xylem have been used for analyses due to the method limitations. However, the presented results achieved by applying Raman spectroscopy indicate that carotenoid composition is tissue specific and the whole phloem or xylem sections are too big to follow the pattern of pigment accumulation. The recorded distributions of individual carotenoids demonstrate that visual observations of root colour also do not provide enough evidence for the accurate assessment of carotenoid accumulation. Raman spectroscopy offers direct insight into carotenogenesis in living tissue and allows to study carotenoid sequestration even at microscopic dimensions. This new approach can supply valuable information on spatial differentiation of carotenogenesis and serve as a basis for further molecular investigations on gene expression and regulation.
Abbreviations
- NIR:
-
Near-infrared
- FT:
-
Fourier transform
References
Alasalvar C, Grigor JM, Zhang D, Quantick PC, Shahidi F (2001) Comparison of volatiles, phenolics, sugars, antioxidant vitamins, and sensory quality of different colored carrot varieties. J Agric Food Chem 49:1410–1416
Ayers JE, Fishwick MJ, Land DG, Swain T (1964) Off-flavor of dehydrated carrot stored in oxygen. Science 203:81–82
Baranska M, Schulz H (2005) Spatial distribution of polyacetylenes in carrot root. Analyst 130:855–859
Baranska M, Schulz H, Baranski R, Nothnagel T, Christensen L (2005) In situ simultaneous analysis of polyacetylenes, carotenoids and polysaccharides in carrot roots. J Agric Food Chem 53:6565–6571
Baranski R, Baranska M, Schulz H (2005) Changes in carotenoid content and their distribution in fresh plant tissue can be observed and mapped in situ using NIR-FT-Raman spectroscopy. Planta 222:448–457
Bouvier F, Isner J-C, Dogbo O, Camara B (2005) Oxidative tailoring of carotenoids: a prospect towards novel functions in plants. Trends Plant Sci 10:187–194
Buishand JG, Gabelman WH (1979) Investigations on the inheritance of color and carotenoid content in phloem and xylem of carrot root (Daucus carota L.). Euphytica 28:611–632
Buishand JG, Gabelman WH (1980) Studies on the inheritance of root color and carotenoids content in red × yellow and red × white crosses of carrot, Daucus carota L. Euphytica 29:241–260
Cunningham FX, Gantt E (1998) Genes and enzymes of carotenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 49:557–583
Demmig-Adams B, Gilmore AM, Adams WW (1996) Carotenoiods 3: In vivo function of carotenoids in higher plants. FASEB J 10:403–412
Dietz Bryant J, McCord JD, Knight-Unlu L, Erdman JW Jr (1992) Isolation and partial characterization of α- and β-carotene-containing carotenoprotein from carrot (Daucus carota L.) root chromoplasts. J Agric Food Chem 40:545–549
Esau K (1940) Developmental anatomy of the fleshy storage organ of Daucus carota. Hilgardia 13:175–209
Fraser PD, Bramley PM (2004) The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res 43:228–265
Frey-Wyssling A, Schwegler F (1965) Ultrastructure of chromoplasts in the carrot root. J Ultrastruct Res 13:543–559
Habegger R, Schnitzler WH (2005) Aroma compounds of colored carrots (Daucus carota L. ssp. sativus Hoffm.). J Appl Bot Food Qual Angew Bot 79:130–136
Horvitz MA, Simon PW, Tanumihardjo S (2004) Lycopene and β-carotene are bioavailable form lycopene red carrots in humans. Eur J Clin Nutr 58:803–811
Isaacson T, Ronen G, Zamir D, Hirschberg J (2002) Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of β-carotene and xanthophylls in plants. Plant Cell 14:333–342
Lewinsohn E, Sitrit Y, Bar E, Azulay Y, Ibdah M, Meir A, Yosef E, Zamir D, Tadmor Y (2005) Not just colors-degradation as a link between pigmentation and aroma in tomato and watermelon fruit. Trends Food Sci Tech 16:407–415
Milicua JCG, Juarros JL, De Las Rivas J, Ibarrondo J, Gomez R (1991) Isolation of a yellow carotenoprotein from carrot. Phytochemistry 30:1535–1537
Nicolle C, Simon G, Rock E, Amouroux P, Rémésy C (2004) Genetic variability influences carotenoid, vitamin, phenolic, and mineral content in white, yellow, purple, orange, and dark-orange carrot cultivars. J Am Soc Hortic Sci 129:523–529
Olsen JA (1989) Provitamin A function of carotenoids. J Nat 119:105–108
Ozaki Y, Cho R, Ikegawa K, Muraishi S, Kawauchi K (1992) Potential of near-infrared Fourier transform Raman spectroscopy in food analysis. Appl Spectrosc 46:1503–1507
Phan CT, Hsu H (1973) Physical and chemical changes occurring in the carrot root during growth. Can J Plant Sci 53:629–634
Poon WYL, Goldman IL (2002) Comparative carotenoid accumulation and retention in near-isogenic rprp and RPRP inbred carrot lines. J Am Soc Hortic Sci 127:284–289
Reiter M, Neidhart S, Carle R (2003) Sedimentation behaviour and turbidity of carrot juices in relation to the characteristics of their cloud particles. J Sci Food Agric 83:745–751
Ronen G, Carmel-Goren L, Zamir D, Hirschberg J (2000) An alternative pathway to β-carotene formation in plant chromoplasts discovered by map-based cloning of Beta and old-gold color mutations in tomato. Proc Natl Acad Sci USA 97:11102–11107
Rubatzky VE, Quiros CF, Simon PW (1999) Carrots and related vegetable Umbelliferae. CABI Publishing, New York
Santos CAF, Senalik D, Simon PW (2005) Path analysis suggests phytoene accumulation is the key step limiting the carotenoid pathway in white carrot roots. Gen Mol Biol 28:287–293
Santos CAF, Simon PW (2002) QTL analyses reveal clustered loci for accumulation of major provitamin A carotenes and lycopene in carrot roots. Mol Genet Genomics 268:122–129
Schrader B, Klump HH, Schenzel K, Schulz H (1999) Non-destructive NIR FT Raman analysis of plants. J Mol Struct 509:201–212
Schulz H, Baranska M, Baranski R (2005) Potential of NIR-FT-Raman spectroscopy in natural carotenoid analysis. Biopolymers 77:212–221
Simon PW (1984) Carrot genetics. Plant Mol Biol Rep 2:54–63
Simon PW, Wolff XY (1987) Carotenes in typical and dark orange carrots. J Agric Food Chem 35:1017–1022
Surles RL, Weng N, Simon PW, Tanumihardjo SA (2004) Carotenoid profiles and consumer evaluation of specialty carrots (Daucus carota L.) of various colors. J Agric Food Chem 52:3417–3421
Taylor M, Ramsay G (2005) Carotenoid biosynthesis in plant storage organs: recent advances and prospects for improving plant food quality. Physiol Plant 124:143–151
Vishnevetsky M, Ovadis M, Vainstein A (1999) Carotenoid sequestration in plants: the role of carotenoid-associated proteins. Trends Plant Sci 4:232–235
Zhou JR, Gugger ET, Erdman JW Jr (1994) Isolation and partial characterisation of an 18 kDa carotenoid-protein complex from carrot roots. J Agric Food Chem 42:2386–2390
Acknowledgments
The financial support of the “Deutsche For-schungsgemeinschaft (DFG)” in Bonn, Germany (grant number: Schu 566/7-2) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Baranska, M., Baranski, R., Schulz, H. et al. Tissue-specific accumulation of carotenoids in carrot roots. Planta 224, 1028–1037 (2006). https://doi.org/10.1007/s00425-006-0289-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-006-0289-x