Abstract
Pyruvate, orthophosphate dikinase (PPDK; E.C.2.7.9.1) is most well known as a photosynthetic enzyme in C4 plants. The enzyme is also ubiquitous in C3 plant tissues, although a precise non-photosynthetic C3 function(s) is yet to be validated, owing largely to its low abundance in most C3 organs. The single C3 organ type where PPDK is in high abundance, and, therefore, where its function is most amenable to elucidation, are the developing seeds of graminaceous cereals. In this report, we suggest a non-photosynthetic function for C3 PPDK by characterizing its abundance and posttranslational regulation in developing Oryza sativa (rice) seeds. Using primarily an immunoblot-based approach, we show that PPDK is a massively expressed protein during the early syncitial-endosperm/-cellularization stage of seed development. As seed development progresses from this early stage, the enzyme undergoes a rapid, posttranslational down-regulation in activity and amount via regulatory threonyl-phosphorylation (PPDK inactivation) and protein degradation. Immunoblot analysis of separated seed tissue fractions (pericarp, embryo + aleurone, seed embryo) revealed that regulatory phosphorylation of PPDK occurs in the non-green seed embryo and green outer pericarp layer, but not in the endosperm + aleurone layer. The modestly abundant pool of inactive PPDK (phosphorylated + dephosphorylated) that was found to persist in mature rice seeds was shown to remain largely unchanged (inactive) upon seed germination, suggesting that PPDK in rice seeds function in developmental rather than in post-developmental processes. These and related observations lead us to postulate a putative function for the enzyme that aligns its PEP to pyruvate-forming reaction with biosynthetic processes that are specific to early cereal seed development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In C4-plant leaves, the enzyme pyruvate, orthophosphate (Pi) dikinase (PPDK; E.C. 2.7.9.1) is expressed abundantly in mesophyll chloroplasts where it plays a central role in the C4-photosynthetic pathway (Hatch 1987; Chastain and Chollet 2003). In this role, PPDK catalyzes the ATP- and Pi-dependent formation of phosphoenol pyruvate (PEP), the primary CO2 acceptor molecule in the C4 pathway, from pyruvate (Pyr):
Though the enzyme operates in the Pyr to PEP direction during C4 photosynthesis, catalysis is freely reversible. This is well illustrated in certain microorganisms, such as amitochondriate protozoa and endobacteria, where PPDK is deployed in the Pyr-forming direction for synthesis of cellular ATP (Plaxton 1996; Saavedra et al. 2005). In chloroplasts of C4 plants, photosynthetic PPDK activity is regulated in a light-/dark-dependent manner by reversible phosphorylation of an active site threonine residue (Thr-456 in maize C4 PPDK) (Burnell and Hatch 1985; and for a recent review, see Chastain and Chollet 2003). In its Thr-phosphorylated form, the enzyme is inactive. A single, bifunctional protein kinase/phosphatase, named the PPDK regulatory protein (RP), catalyzes this unusual ADP-/Pi-dependent regulatory phosphorylation/dephosphorylation cycle in the chloroplast stroma:

In C3 plants, PPDK is not a photosynthetic enzyme. It is, however, a ubiquitous enzyme found in virtually all organs and tissues of the plant (Chastain and Chollet 2003). Except for its low abundance and non-photosynthetic function, PPDK in C3 plants is similar to C4 dikinase with respect to primary structure, biochemical properties, and posttranslational light-/dark-dependent regulation in C3 chloroplasts by an RP-like activity (Aoyagi and Bassham 1984; Rosche et al. 1994; Imaizumi et al. 1997; Moons et al. 1998; Chastain et al. 2002). In both C3 and C4 plants, PPDK is co-localized in chloroplasts and the cytoplasm. Interestingly, these two intracellular isoforms are expressed from the same gene copy by a tandemly configured chloroplast-targeting and cytoplasmic-targeting promoter 5′ to the ORF of the shared gene (Sheen 1991; Imaizumi et al. 1997). In certain C4 and C3 plants, such as maize and rice, a second cytoplasmic isoform is encoded by a separate gene (Sheen 1991; Moons et al. 1998). Although much is known about the distribution and regulation of PPDK in C3 plants, its precise metabolic or physiological role(s) in these plant cells is yet to be established (Hausler et al. 2002; Chastain and Chollet 2003). Elucidating a function for C3 PPDK is complicated by several factors. Among these are its low abundance in C3 plant tissues, its freely reversible catalysis, and the presence of other enzymes that can catalyze PEP/Pyr interconversions. In this metabolic context, using conventional radiotracer techniques for assessing a metabolic function in vivo, for example, is rendered technically unfeasible. The one known exception in C3 plants where PPDK is in high abundance and, therefore, where its function should be highly amenable to elucidation is in the endosperm of developing cereal seeds such as rice and wheat (Meyer et al. 1982; Aoyagi and Bassham 1984; Aoyagi et al. 1984; Hata and Matsuoka 1987; Gallusci et al. 1996; Nomura et al. 2000; Kang et al. 2005). In two early studies (Meyer et al. 1982; Aoyagi and Bassham 1984;), two putative metabolic functions for PPDK in this highly specialized organ were advanced. One is a role linked to providing carbon skeletons for amino acid synthesis (e.g., Ala, Glu) via the ability of the enzyme to reversibly interconvert Pyr and PEP. A second proposed role is the synthesis of PEP for PEP carboxylase (PEPC) refixation of respired CO2. A logical assumption would be that these two long-standing hypotheses concerning PPDK function in developing cereal seeds would be resolved by isolation and characterization of a C3 plant PPDK null mutant. However, this does not seem to be the case as the first report of a PPDK T-DNA insertional knockout mutant showed that plants with inactivated PPDK genes failed to produce a gross phenotype that could resolutely validate a specific function for the enzyme in developing rice seeds (Kang et al. 2005). Specifically, T-DNA insertion mutants of the rice OsPPDKB gene (flo4) were isolated that lacked the seed-expressed cytoplasmic (or chloroplastic) PPDK isoforms. Knockout plants, apparently without a severe phenotype affecting growth or development, were recovered by visualizing the phenotypic specific opaque seeds these plants produced. A corresponding histochemical analysis of a promoter-less GUS gene driven by the intact OsPPDKB promoter in developing flo4 seeds showed a high level of GUS expression in the endosperm, aleurone, and scutellum of developing seeds, with the peak expression occurring at 10 days post-anthesis. Based on additional biochemical analyses, it was proposed that the endosperm-expressed cytoplasmic PPDK functions primarily to regulate the flow of glycolytically derived carbon into either free fatty acid (FA) or starch biosynthesis during the grain-filling stage. The key to this recent model is the ability of PPDK to reversibly catalyze PEP/Pyr interconversions in the cytoplasm.
In the present study, we primarily utilized the technique of immunoblot analysis to provide evidence that places the endosperm-localized, cytosoplasmic PPDK isoform’s function in the early syncitial-/endosperm-cellularization stage of cereal seed development, rather than in the grain-filling (e.g., storage-product accumulation) stage (Kang et al. 2005). At this early phase in seed development, we show that PPDK is a massively expressed protein with measured enzyme activities on par with PPDK abundance and activity in C4 leaves. As seed development proceeds beyond this early phase, PPDK activity and protein level are rapidly down-regulated by the combined posttranslational mechanisms of protein phosphorylation and degradation. Because of the timing of expression in the amount and activity of PPDK in nascent rice seed tissues, we propose a function for the endosperm-localized cytoplasmic isoform based on the enzyme’s kinetically favored operation in the PEP to Pyr direction. In the PEP to Pyr-forming direction, the enzyme would be ideally integrated for supporting the predominant biosynthetic processes in the nascent endosperm. Furthermore, since developing cereal seed tissues are hypoxic and presumably unable to generate ATP aerobically (Rolletschek et al. 2004; van Dongen et al. 2004), we propose an additional role for this enzyme as providing an efficient mechanism for glycolytic ATP synthesis in oxygen depleted tissues.
Materials and methods
Plant materials
The japonica variety Kitake of rice (Oryza sativa L.) was selected for this research. Plants were grown in pots, with one plant per pot (28 cm diameter×25 cm height), and maintained in a greenhouse. The pots were filled with a mixture of clay soil and SUNSHINE medium (Sun Gro® Horticulture Canada Ltd., Seba Beach, Alberta, Canada) supplemented with slow-release fertilizer pellets (20–20–20, N–P–K) and watered daily with distilled water. The day/night temperatures were set at 29/21°C, and the day length set at a minimum of 14 h in the greenhouse. Supplementary light was applied daily before 10.00 and after 14.00 hours. The plants flowered after ~8 weeks of vegetative growth (mid-August). In order to synchronize the seed developmental environment, only the main panicles of individual plants were selected to harvest seeds for analysis. To collect seed material at discrete developmental stages, selected spikelets were checked for fertilization at 4 days after flowering. Seeds were harvested starting from 10 days post-pollination and ending at 40 days after flowering at 5-day intervals. At each harvest, the seeds were randomly collected from different plants, with each selected panicle contributing about five seeds to a seed sample. The harvested seeds were immediately immersed in liquid nitrogen and then stored at -70°C before analysis. Other seeds utilized in this study for preparation of soluble protein extracts were maize (Zea mays [hybrid field corn] cv unspecified Texas variety), cotton (Gossypium hirsutum L. cv Upland Short Staple), Sorghum (Sorghum bicolor (L) Moench cv Hegari), red winter wheat (Triticum aestivum L. cv ‘812’), garden cucumber (Cucumis sativa L. cv Bush), spinach (Spinacia oleracea cv Bloomsdale Longstanding), sunflower (Helianthus annuus cv Mammoth Russian), oat (Avena sativa L. cv Coronado), and Arabidopsis (Arabidopsis thaliana, Columbia ecotype).
Preparation of soluble protein seed extracts for immunoblot analysis
During all extraction procedures, seed material and extracts were kept on dry ice or wet ice. Soluble protein was prepared from developing rice seeds as follows. Eight to ten dehulled seeds were weighed and then homogenized via mortar and pestle in 1-ml ice-cold extraction buffer (50 mM Tris–HCl, pH 8.0, 2 mM EDTA, 2 μM orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 1% [v/v] Sigma protease inhibitor cocktail). The homogenate was clarified by a 5-min centrifugation at 75,000g and 4°C. Aliquots of this soluble extract were combined with SDS-PAGE sample buffer and stored at −80°C. Preparation of soluble protein extracts from separated seed tissues was accomplished by microdissection of individual caryopses as follows. Prior to microdissection and separation of seed tissues into pericarp, endosperm + aleurone, and seed embryos, mature seeds (40 days post-pollination) were soaked in ice-cold water for 3 min to soften the outer pericarp layers. Using forceps and a dissecting microscope, each respective seed tissue component was dissected away from the caryopsis and placed on dry ice until extraction of soluble protein as above. Prior to freezing on dry ice, the pericarp and seed embryo tissues were briefly rinsed in ice-cold H2O to remove starch granules. Each tissue-extract sample was derived from 15 to 20 seeds. Soluble protein seed extracts from other seeds included in this study were prepared in the same way as for rice seeds but with the following modifications. Whole seeds of maize, Sorghum, and all dicotyledons were used directly for homogenization. Seeds of wheat and oats were first dehulled prior to homogenization.
Immunoblot analyses
Immunoblots of 10% SDS-PAGE gels were prepared using standard techniques as previously described (Chastain et al. 2002). Two primary rabbit antibodies were used in this study. For detection of regulatory phosphorylated PPDK, affinity-purified polyclonal antibodies raised against a synthetic phosphopeptide conjugate corresponding to the highly conserved Thr-456 phosphorylation domain of maize C4 PPDK (encompassing residues 445–464 [AVGILTERGGMpTSHAAVVAR]; Chastain et al. 2000, 2002; Chastain and Chollet 2003) were used. The corresponding Thr-phosphorylation domain of rice PPDK is identical to the maize sequence in 19 out of 20 residues, with alanine in place of glutamate at the P-5 position as the sole difference. For detection of total PPDK (phospho and dephospho) affinity-purified polyclonal antibodies raised against the recombinant maize, C4 PPDK monomer was used (Chastain et al. 2002). The chemiluminescent detection of the respective antigen/antibody complexes on immunoblots was accomplished using the alkaline phosphatase substrate CDP-Star™ (Applied Biosystems). Quantitative estimates of band optical density from scanned immunoblot autoradiographs were accomplished using the Quantity-One™ software package (Bio-Rad Laboratories). The scanning methodology that was utilized for this study was shown previously to be linear for lane loads of 0.05 to 1 μg of total PPDK per band (Chastain et al. 2002). PPDK and ThrP-PPDK standards used to estimate PPDK protein on immunoblots were generated by in vitro phosphorylation (inactivation) of purified recombinant maize C4 PPDK as described previously (Chastain et al. 2002). Briefly, 70 μg of affinity-purified recombinant maize C4 PPDK (fully activated, dephospho form) was introduced into a 180-μl reaction mixture containing 50 mM Bicine-KOH, pH 8.3, 10 mM MgCl2, 5 mM DTT, 1 mg ml−1 BSA, 1 mM ADP, 0.2 mM ATP. Site-specific phosphorylation of PPDK was initiated by addition of 20 μl (~5 μg protein) of a rapidly prepared and desalted maize leaf extract which provided the source for RP. During incubation at 30°C, duplicate 10-μl aliquots were removed at 5-min intervals with one of the duplicate aliquots combined with 90 μl SDS-PAGE sample buffer to quench the RP-catalyzed phosphorylation reaction and the other aliquot assayed directly in a coupled spectrophotometric assay for PPDK enzyme activity. Aliquots of the quenched assays were loaded onto 10% SDS-polyacrylamide gels, electrophoresed, and electroblotted onto nitrocellulose membrane. The resulting blots were hybridized with anti-PPDK antibody or anti-ThrP-PPDK antibody for immunoblot analysis as described above. Time points corresponding to the linear phase of the PPDK inactivation reaction [time = 0 min, 100% enzyme activity (i.e. 100% non-phospho PPDK)] were used to assess percent ThrP-PPDK versus total PPDK at the various reaction time points. For example, the ThrP-PPDK protein standard shown in Fig. 2a is the 10-min quenched aliquot with a ThrP-PPDK content of 11 ng μl−1 that corresponds to a 31% inactivation of initial PPDK enzyme activity.
PPDK enzyme assay
Soluble protein seed extracts for enzyme assays were prepared by homogenizing 8–10 dehulled seeds via mortar and pestle in 400 μl ice-cold extraction buffer containing 100 mM Tris–HCl, pH 8.0, 10 mM MgSO4, 1 mM EDTA, 2 mM Pi (K+ salt), 5 mM pyruvate, 14 mM 2-mercaptoethanol and 1% (v/v) Sigma protease inhibitor cocktail. The homogenate was clarified by a 3-min, 4°C centrifugation at 42,000g and then desalted via gel filtration prior to assay. Aliquots of the desalted seed extracts were assayed for PPDK activity using a coupled, PEPC/malate dehydrogenase-based spectrophotometric assay at 30°C as described previously (Chastain et al. 2002). Prior to assay, desalted extracts were preincubated at 30°C for 10 min to ensure full heat-reactivation of PPDK. Assays were initiated by addition of ATP.
Quantitation of proteins
Proteins were assayed quantitatively using a modified Coomassie dye-binding method (Coomassie Plus™, Pierce-Endogen) with BSA as standard.
Results
PPDK protein abundance, regulatory phosphorylation, and activity during rice seed development
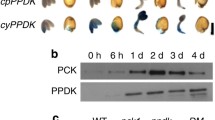
Rice is similar to most cereals in how the caryopsis undergoes development and maturation during a 40-day period after the carpel is fertilized. In general, a succession of developmental events is initiated at the syncitial stage at around 5 days post-pollination (Bewley et al. 2000; Kurata 2005). These post-pollination stages encompass differentiation and growth of the seed embryo (0–10 days), cellularization of endosperm and cell division in the starchy endosperm tissue (7–14 days), aleurone differentiation (15–20 days) and storage-product accumulation and enlargement of endosperm cells (20–40 days). Therefore, in order to accurately assess the role of PPDK in rice seed development, the expression of its protein abundance, activity, and posttranslational regulation must be framed within the context of the developmental state of the seed as described above. We addressed these potential concerns by carefully tagging clusters of rice florets within a single portion of a spikelet as they initiated synchronous flowering. This synchronized “cohort” of florets was then harvested with respect to a discrete post-pollination developmental stage. In this manner, we were able to reliably estimate PPDK enzyme expression at discrete stages of seed development by use of immunoblot analysis and enzyme assay of seed extracts prepared from pooling dehulled seeds from a similar developmental stage. Such immunoblot analyses, shown in Fig. 2a–c, demonstrate that at 10 days post-pollination, the earliest developmental stage examined, rice seeds contained the highest amount of PPDK protein per mass of seed tissue or per unit soluble protein in seed extracts. The absolute amount of PPDK per soluble seed protein, as approximated via densitometric analysis of scanned immunoblots (Fig. 2 b, c), indicated that PPDK is a massively expressed enzyme in the early seed tissues, being comparable in abundance to photosynthetic PPDK in C4 leaves. It should be noted, however, that our approach utilized a maize polyclonal antibody for quantitatively detecting rice PPDK. Thus, the estimates provided in Fig. 2 b and c should be considered only as semi-quantitative. However, the accompanying PPDK activity determinations (Fig. 2d) indicate these estimates are within the range of the PPDK protein amount that would be predicted based on published values of higher plant PPDK specific activity (Chastain et al. 1996, 2000). In this context, these determinations suggest that PPDK represented ~3% of total soluble seed protein at 10 days post-pollination (Fig. 2b). By comparison, PPDK in C4 leaves is typically≅10% of total soluble leaf protein (Sugiyama and Hirayama 1983; Usuda et al. 1985). Similarly, the high rate of PPDK activity measured in 10 days post-pollination stage seed extracts (Fig. 2d) is comparable to the high rates of photosynthetic PPDK activity reported for C4 leaf extracts (approximately 50% of the extractable PPDK activity of C4 leaves; Yamamoto et al. 1974; Usuda et al. 1985; Hocking and Anderson 1986; Fukayama et al. 2001). The 10-day post-pollination stage was the earliest sampled in this study because the small size of the caryopsis ensheathed in the floret glumes precluded its accurate extraction at time points prior to this. Therefore, it is not known if this time point represents the exact peak expression level of PPDK protein and activity, or if it occurs prior to the 10-day post-pollination stage. After 10-days post-pollination, PPDK protein level was shown to undergo a steep 70% decline as seed maturation proceeded to the fully mature, 40-day post-pollination stage (Fig. 2a–c). PPDK enzyme activity was also shown to decline during seed maturation, but more precipitously in time and magnitude. Between 10 and 20 days post-pollination, enzyme activity declined from ~70 to 90%, with only 6–10% of the original 10-day post-pollination activity remaining in mature seeds (Fig. 2d).
In our previous work, we showed that immature seeds of rice contain an extractable RP-like, ADP-dependent protein kinase activity (Chastain and Chollet 2003). To indirectly assess how this bifunctional protein kinase/phosphatase enzyme is manifested in the developmental program of the rice seed, we next examined regulatory phosphorylation (inactivation) of PPDK via immunoblot analysis at these same developmental stages by probing blots with a previously developed and presently verified phosphopeptide antibody specific for the maize and rice-seed PPDK active-site regulatory ThrP residue (Chastain et al. 2000; Fig. 1). The specificity of this antibody for phosphorylated PPDK in rice seed extracts is documented in Fig. 1, where immuno-signal corresponding to the ~95-kD rice PPDK phospho-monomer is eliminated by the inclusion of 15 μg ml−1 of the parent phosphopeptide in the hybridization buffer (cf., panel b versus a). Conversely, inclusion of 15 μg ml−1 of the parent dephosphopeptide did not eliminate the same respective immuno-signals in Panel a (data not shown). Such immunoblots, shown in Fig. 2a, revealed that at the most immature seed stage examined (10-day post-pollination), only traces of phosphorylated/inactivated PPDK were detectable. However, within the next 10 days of development, phosphorylated PPDK was markedly more detectable and constituted a significant portion of the total seed PPDK. At around the 20-day developmental stage, no further increase in the amount of ThrP-PPDK protein is evident, with the level of the threonyl-P enzyme remaining constant through the 40-day mature seed stage. Plots based on densitometric analysis of the immunoblots shown in panel a indicated that during the early stages of seed development (10–20 days) the pool of phosphorylated PPDK comprises the lesser fraction of the total seed PPDK (i.e., dephospho > phospho) (Fig. 2 b, c). A noteworthy difference between the pool of PPDK that undergoes regulatory phosphorylation and the initially much larger pool of non-phosphorylated PPDK is the inherent relative stability of the former during development. This pool of PPDK, after its inactivation by regulatory phosphorylation, remained essentially intact throughout the seed maturation process (Fig. 2a–c). This is in contrast to the initially larger pool of non-phosphorylated PPDK that steadily declined in abundance during seed maturation, presumably by degradation as suggested by the truncated (<95 kD) immuno-reactive bands most detectable at 10–15 days post-pollination on the general-PPDK antibody probed immunoblots (Fig. 2a).
Specificity analysis of maize C4 PPDK phosphopeptide antibody. Identical immunoblots were probed with anti-ThrP-PPDK (anti-PPDK phosphopeptide) antibody in the absence of (a) or presence of (b) 15 μg ml−1 of the parent phosphopeptide used as antigen. c Blot from b stripped and reprobed with anti-PPDK antibody. Samples in lanes are total soluble proteins extracted from the rice pericarp layer (5 μg per lane), seed embryos (10 μg per lane), and endosperm + aleurone layer (10 μg per lane). Each blot included negative and positive control lanes of 0.25 μg purified maize recombinant PPDK (rPPDK) and 2.5 μg maize soluble crude extract prepared from dark-adapted leaves as respective dephospho- and phospho-PPDK reference lanes (Chastain et al. 2000). Arrowheads indicate position of bands corresponding to the ≈95-kDa PPDK monomer as estimated by molecular mass standards on the same blot. The doublet bands in the far right lanes (c) may represent either a separation of the similar-size molecular mass of OsPPDKA and OsPPDKB polypeptide monomers, or minor proteolysis of total PPDK isoforms within the sample
PPDK protein accumulation, regulatory phosphorylation, and activity in developing rice seeds. a Representative immunoblots of total soluble proteins extracted from developing rice seeds at 5-day intervals beginning at 10 days post-pollination. Blots were probed with either anti-PPDK (upper panel) or anti-ThrP-PPDK (lower panel) antibody. Each lane contained 20 μg soluble protein. Arrowheads indicate position of bands corresponding to the ≈95-kDa PPDK monomer as estimated by molecular mass standards on the same blot. The lane designated as std (PPDK standard) was loaded with 0.35 μg of recombinant maize C4 PPDK (comprised of 0.13 μg dephospho PPDK plus 0.22 μg of phosphorylated PPDK). b, c Plots showing changes in the abundance of total PPDK protein (square) versus phosphorylated PPDK protein (triangle) in developing rice seeds; b ng per μg soluble seed protein, or c ng per mg fresh weight (FW) of seed mass. Data points depicted in the plots are derived from scanning densitometric analysis of triplicate blots representing three independent experiments (including blots shown in (a). Estimates of total PPDK and ThrP-PPDK protein are based on the inclusion of reference lanes in each blot with known amounts of ThrP-PPDK and total PPDK as described in (a). n=3, ±SEM. d PPDK activity in desalted soluble protein extracts of developing rice seeds. Enzyme activity is plotted as μmol h−1 mg−1 soluble seed protein (square) or μmol h−1 mg−1 seed FW (triangle). Each point represents the mean of two independent determinations (8–10 pooled seeds per determination)
Localization of PPDK and ThrP-PPDK in mature rice seed tissue fractions
We initially assumed that the pool of phosphorylated PPDK we observed in our seed extracts (Fig. 2a) originated from chloroplasts of the outer green pericarp layer in immature rice seed. The basis for this assumption is that RP is known to be exclusive to the stromal compartment of C4- and C3-leaf chloroplasts, thus rendering stromal-localized PPDK as the sole isoform amenable to regulatory phosphorylation (Burnell and Hatch 1985, 1986; Chastain and Chollet 2003, and references therein). This assumption was examined directly by separating the mature rice seed into three distinct tissue fractions, the pericarp layer, the aleurone + endosperm tissue, and the seed embryo, for subsequent immunoblot analysis of the respective soluble protein extracts. These immunoblots (Fig. 3) revealed that ThrP-PPDK was indeed present in the pericarp tissue, but, surprisingly, a substantial portion was also localized in the seed embryo. Conversely, the lane corresponding to endosperm + aleurone tissue was found to be essentially devoid of ThrP-PPDK, yet, displayed the strongest immuno-signal on the general PPDK antibody-probed blot (Fig. 3). These collective findings indicate that the bulk of the non-phosphorylated PPDK in mature seeds is endosperm-localized.
Immunoblot analysis of ThrP-PPDK and total PPDK in pericarp, endosperm + aleurone, and seed embryo soluble protein extracts dissected from mature rice seeds (40 days post-pollination). Displayed are immunoblots of total soluble protein extracted from the pericarp layer (5 μg per lane), endosperm + aleurone layer (10 μg per lane), and seed embryos (10 μg per lane) probed with either anti-ThrP-PPDK antibody (a) or anti-PPDK antibody (b). Each blot included negative and positive control lanes of 0.1 μg purified maize recombinant PPDK (rPPDK) and 10 μg maize soluble crude extract prepared from dark-adapted leaves as respective dephospho- and phospho-PPDK reference lanes. Arrowheads indicate position of bands corresponding to the ≈95-kDa PPDK monomer as estimated by molecular mass standards on the same blot. Shown are blots representative of at least four independent experiments
PPDK protein content and activity in germinating rice seeds
Given that a modestly abundant amount of apparently intact PPDK polypeptide persists in the mature rice seed (Fig. 2a–c), we conjectured that it may have a role in post-seed developmental processes such as in seed dormancy or germination. Our hypothesis was that if all or part of the sizable, intact pool of seed-localized PPDK was dephosphorylated/reactivated during germination, it could implicate a possible role for PPDK in the seed-germination process. We examined this possibility by comparing PPDK protein level and activity between germinating (as indicated by radicle protrusion) and dormant seeds. Immunoblots prepared from these seed extracts showed that as the seed undergoes germination, the total amount of PPDK protein in the seed tissue remains relatively constant (Fig. 4a). Likewise, corresponding immunoblots of ThrP-PPDK indicated that the pool of phosphorylated PPDK in dormant seeds is also essentially unchanged during seed germination (data not shown). Corroborating this latter observation was the finding that PPDK activity in extracts of germinating seeds was comparable to the low activity measured from dormant seeds (Fig. 4b), indicating that reactivation (dephosphorylation) of PPDK in dormant seed tissues is negligible, at least at this early stage of germination.
PPDK protein and activity in dormant versus germinating rice seeds. Soluble protein extracts were prepared from dormant or germinating seeds just at the onset of radicle protrusion. a Immunoblot of soluble protein extracts of germinating (g) and dormant (d) rice seeds probed with anti-PPDK antibody. Each lane (10 μg soluble protein) represents an independent preparation of soluble seed extract. As depicted, each band corresponds to the ≈95-kDa PPDK monomer as estimated by molecular mass standards on the same blot. b PPDK activity in dormant versus germinating seeds (n=3 ±SEM, P=0.43)
Comparative immunoblot analysis of PPDK content in mature seeds of dicot and monocot species
The well-documented abundance of PPDK in all graminaceous cereal seeds examined to date implicates a common function for the enzyme in seed tissues of these species. However, to our knowledge, a similar survey of PPDK content in mature seeds of species other than graminaceous cereal plants has not been reported. In this study, we included an immunoblot survey of PPDK content in seed extracts of several dicotyledonous species and, for comparative purposes, several additional graminaceous cereal species. These blots indicated that the PPDK content of the several other cereal seeds we examined [Z. mays (maize), S. bicolor (Sorghum), A. sativa (oat), T. aestivum (wheat)], although apparently lower, as based on immunoblot signal strength was approaching that of rice (Fig. 5a). In contrast, four of the five dicotyledonous species seeds examined [C. sativa (cucumber), S. oleracea (spinach), G. hirsutum (cotton), A. thaliana] had undetectable amounts of PPDK on immunoblots with similar lane loads of seed extract (Fig. 5b). The inability to detect PPDK in these respective dicotyledonous species seed extracts most likely reflects the low abundance of the protein in these seeds, rather than a reduced affinity of the heterologous antibody. In a previous study, these antibodies were utilized to successfully detect total and ThrP-PPDK on immunoblots of soluble leaf extracts from several dicot species including Vicia faba, S. oleracea, and Flaveria pringlei (Chastain et al. 2002). A notable exception was the finding that H. annuus (striped sunflower) seeds, a representative oil-storing seed-type, contained a relative abundance of PPDK (Fig. 5b). Moreover, as in mature rice seeds, mature seeds of sunflower were found to contain a sizable pool of ThrP-PPDK (Figs. 2a, 5b).
PPDK content in mature seeds of several graminaceous cereals and dicotyledonous species. a Immunoblot of soluble proteins extracted from mature (dormant) seeds of the monocotyledonous cereal species O. sativa (C3), S. bicolor (C4), Z. mays (C4), A. sativa (C3), and T. aestivum (C3) probed with anti-PPDK antibody. Each lane was loaded with 20 μg of soluble protein extract. b Immunoblots of soluble seed extracts of rice and several C3 dicots probed with anti-PPDK (upper blot) or anti-ThrP-PPDK antibody (lower blot). Each lane was loaded with 20 μg of soluble protein extract. All experiments were repeated independently at least three times. Arrowheads indicate position of bands corresponding to the ≈95-kDa PPDK monomer as estimated by molecular mass standards on the same blot
Discussion
To date, a specific function for PPDK in C3 plants is yet to be established. Although significant progress towards this goal has been advanced by the recent isolation of C3 PPDK knockout lines of rice (Kang et al. 2005), analyses of these mutants are yet to elucidate a distinct and unequivocal function for the C3 enzyme. For example, the mutant plants, with an inactivated OsPPDKB gene that encodes both cytoplasmic and chloroplastic isoforms, were able to carry out normal growth and development. Kang and coworkers (2005) postulated that the “presence of masking compensatory metabolic pathways” obscured a phenotype that would allow them to readily discern the metabolic function(s) for C3 PPDK. Such inherent metabolic flexibility, which typifies plants as sessile organisms (Plaxton 2005), underscores the limitations in utilizing gene knockouts for understanding highly networked processes such as plant metabolism. Our approach for ultimately elucidating the metabolic role of PPDK in C3 plants was to first target for analysis the singular C3 plant organ in which it is known to be a highly expressed enzyme, namely the tissues of developing cereal seeds. Because of its high abundance in rice seeds, we were able to exploit conventional biochemical tools for accurately characterizing the time-dependent expression of PPDK protein and enzyme activity in this model C3 species. In so doing, we have provided evidence linking PPDK enzyme expression, and therefore, its function, to the early, syncitial-/endosperm-cellularization phase of seed development (~5- to 15-day post-pollination). As seed development progresses from this early stage into the final storage product accumulation stage (~20-day post-pollination), both PPDK protein level and activity are rapidly down-regulated by the posttranslational mechanisms of protein degradation and RP-catalyzed, site-specific protein phosphorylation, respectively. The former process is presumably mediated by the action of endoproteases shown to be coordinately up-regulated at this specific stage in cereal seed development (Dominguez and Cejudo 1996).
The finding that PPDK is notably abundant and active during early seed development (i.e., syncitium/-cellularization stage) rather than in subsequent stages (i.e., storage-product synthesis) (Gallusci et al. 1996; Kang et al. 2005), is of crucial importance in establishing its putative function in this organ. For example, in the case of the endosperm cytoplasm-localized enzyme, its function is likely to be aligned with the metabolic and biosynthetic processes known to be specific to the early (syncitial-/endosperm cellularization) stages of cereal seed development (i.e., free amino acid synthesis, denovo free FA synthesis and lipid biosynthesis) (Fig. 6). Moreover, as the cytoplasm-localized isoform, the enzyme may function preferentially in the PEP to Pyr-forming direction, rather than in the opposing Pyr to PEP direction (as in mesophyll chloroplasts of C4 leaves). This assumption stems from what is known of the in vitro properties of maize C4 PPDK, which shows markedly different pH optima for its forward and reverse reactions (Jenkins and Hatch 1985). In the Pyr to PEP direction the pH optimum is pH 8.3. However, at pH 7.0, the competency of the enzyme in catalyzing its PEP-forming reaction is dramatically reduced, having only 6% of the rate at pH 8.3. Alternatively, at pH 7.0, the PEP to Pyr reaction is highly favored, with a pH optimum of ~pH 6.8, which is also the pH typical to the cytoplasmic compartment of plant cells. Furthermore, the absolute rate of the PEP to Pyr reaction is twice that of the Pyr to PEP reaction at its optimum pH 8.3. That cytoplasmic PPDK in rice may also share the same catalytic properties as the C4 stromal enzyme is suggested by related biochemical studies of the bacterial enzyme (McGuire et al. 1996) and the fact that the primary structure of the plant cytosoplasmic- and plastid-targeted isoforms is nearly identical (Sheen 1991; Imaizumi et al. 1997). Further restricting PPDK catalysis in the PEP-forming direction in the cytoplasm is that cytosolic [PPi] in plant cells is relatively high (~0.3 mM) and constant, with the notable absence of pyrophosphatase in this compartment (Dennis and Blakeley 2000). As depicted in Fig. 6, PPDK functioning primarily in the in the PEP to Pyr-forming mode may be more ideally integrated for supporting early-stage seed development metabolism via the conversion of glycolytically derived PEP to Pyr. Cytoplasmic Pyr, the primary carbon source for free FA synthesis in lipid-synthesizing plastids, is required to sustain the high rate of FA and lipid biosynthesis as the endosperm syncitium expands and cellularizes. Lipid biosynthesis would be supported by the PPDK function we propose here by cycling lipid biosynthesis by-products, namely AMP and PPi, via the PEP to Pyr reaction. These metabolites are generated during de novo lipid biosynthesis by the action of the ER (and plastid)-localized enzyme, fatty acyl-CoA synthetase (Ichihara et al. 2003). Notably, acyl-CoA synthetase (ACS) activity has been shown to undergo an abrupt, high-level of expression in developing rice seeds that peaks at 9- to 12-days post-anthesis, rapidly plummeting thereafter (Ichihara et al. 2003). This transient increase in activity is accompanied by a similar, rapid rise in total lipid accumulation. The kinetics of the rise and fall of ACS activity closely parallels the developmental expression kinetics of the PPDK enzyme documented herein. Yet an additional PPDK function that would likely be facilitated by PPDK operating in the PEP to Pyr direction is in the cytoplasmic interconversion of Pyr to alanine by transamination (Fig. 6). Alanine, among the most abundant free amino acids in developing cereal seeds (Schaeffer and Sharpe 1997; Wang and Larkins 2001), is synthesized from glutamate and Pyr via the action of Ala aminotransferase (Ireland and Joy 1990). Supporting evidence for this putative PPDK function is provided by a recent proteomic analysis of expressed proteins in developing rice seeds that showed the alanine aminotransferase and PPDK proteins to be co-expressed in the same class of early and abundantly expressed polypeptides in this organ (Lin et al. 2005).
Proposed metabolic scheme illustrating how cytoplasmic PPDK, operating in the PEP to Pyr-forming direction in the nascent endosperm, may be aligned with biosynthetic processes known to be specific to early cereal seed development. As depicted, a major portion of the glycolytically derived PEP is preferentially converted to Pyr by PPDK (versus PK) due to its relatively high abundance and activity. Pyr, in turn, is required to sustain the high rates of de novo FA and alanine biosynthesis (via alanine aminotransferase, AAT). FAs are subsequently exported from the plastids and converted into acyl-CoA thioesters for use as substrates in de novo lipid synthesis by the action of acyl-CoA synthetase (ACS). The by-products of the ACS reaction, AMP and PPi, would also serve to support the PPDK-mediated, PEP to Pyr substrate cycle in the cytoplasm. PPDK would provide an inherently more efficient mechanism (versus the PEP to Pyr PK reaction) for the anaerobic (glycolytic) synthesis of ATP because the PPi utilizing reaction mechanism can catalyze the net formation of two “ATP equivalents” from AMP in the glycolytic PEP to Pyr direction, compared to the single ATP generated by PK operating in this same direction (Plaxton 2005). To maintain hexose-P to PEP carbon flow through the NAD+-coupled glyceraldehyde-3-Pi-dehydrogenase (GaP-DH) glycolytic step, a portion of glycolytically derived PEP (depicted above) is shown shunted into a PEPC/malate dehydrogenase (MDH) mediated by-pass, thus enabling oxidation of NADH in this hypoxic organ
Paramount to the above early seed development biosynthetic processes is the requirement for ATP. Recent studies have shown that the central tissues of developing barley and wheat seeds are highly hypoxic, with measured O2 concentrations as low as ≤0.3% (Rolletschek et al. 2004; van Dongen et al. 2004). At such low O2 concentrations, it can be inferred that ATP synthesis is largely anaerobic in nature. PPDK, operating in the PEP to Pyr-forming direction, may provide these hypoxic tissues with an inherently more efficient means of generating ATP anaerobically than by the classical action of cytoplasmic pyruvate kinase (PK)(Fig. 6). This is because PPDK’s PPi utilizing reaction mechanism can catalyze the net formation of two “ATP equivalents” from AMP in the glycolytic PEP to Pyr direction, compared to the single ATP generated by PK operating in this same direction (Plaxton, 2005). The proposition that cytoplasmic PPDK serves as the major means for producing cellular ATP seems plausible in light of its extreme concentration in the nascent caryopsis, e.g., being on the order of ~50% of PPDK in a C4 leaf. Perhaps the means by which the competing ADP-dependent cytoplasmic PK reaction is bypassed is that PPDK is in such abundance in the cytoplasm that the default route of PEP to Pyr via PK is minimized.
We also showed that appreciable amounts of ThrP-PPDK begin to appear in the developing rice seed at approximately 15 to 20-days post-pollination. Our presumption was that this pool of phosphorylated PPDK originated entirely from chloroplasts of the green pericarp layer, the only portion of the immature seed that contains chloroplasts (van Dongen et al. 2004; Kurata 2005). The inference that ThrP-PPDK could only originate from within this photosynthetic organelle stems from previous work that has established the stroma as the only known intracellular location for PPDK’s dedicated RP kinase/phosphatase, RP. In this regard, a surprising discovery in this investigation was that a comparable amount of ThrP-PPDK was found to be co-localized to the seed embryo as well. Since the embryos of developing cereal seeds are devoid of chlorophyll (van Dongen et al. 2004), the likely origin of ThrP-PPDK, and hence RP, are proplastids in the embryonic cotyledon and leaf primordia. Most intriguing is the manner in which PPDK is down-regulated by “reversible” phosphorylation in these tissues. In previous work we established that PPDK in the chloroplasts of C3 leaves is strictly a light-/dark-regulated enzyme (Chastain et al. 2002). We also documented that a C3-isoform of RP catalyzes this posttranslational regulation by rapid, reversible site-specific phosphorylation/dephosphorylation (Chastain et al. 2002; Chastain and Chollet 2003). Chloroplast RP, in turn, is believed to be regulated by photosynthesis-mediated, light-/dark-induced fluctuations in stromal [ADP], with the high dark-induced levels of stromal ADP favoring the phosphorylation/inactivation of PPDK (Burnell and Hatch 1985; Nakamoto and Young 1990). However, in the dimly illuminated chloroplasts of the pericarp layer (or proplastids of the seed embryo), regulatory phosphorylation of PPDK is apparently mediated not by light and dark per se, but by seed maturation. Ascribing a function to the pool of dikinase represented by the ThrP-PPDK present in the pericarp and seed embryo is more problematic than for the larger pool of endosperm-localized cytoplasmic PPDK since the metabolic processes occurring in cereal seed chloroplasts (or proplastids) are poorly understood. Nevertheless, we provide new insight into a putative function(s) by revealing that the developing seed, for unknown reasons, requires that PPDK-mediated processes in the chloroplasts of the pericarp, and proplastids of the endosperm, undergo down-regulation as the seed advances beyond the syncitial-endosperm, cellularization stage (>10 days post-pollination).
We could not find supporting evidence that the large amount of PPDK synthesized in rice seeds provides any function for the seed (e.g., seed germination) other than in early seed development. Based on our pre- and post-germination analysis of rice seed PPDK protein level and activity, we showed that the sizable amount of apparently intact dephospho PPDK and ThrP-PPDK in mature rice seeds does not become activated upon imbibition and germination, at least in the single, early stage of the germination process that was examined.
A number of previous reports have consistently documented PPDK abundance in developing and/or mature seeds of maize, wheat, barley, and oats (Meyer et al. 1982; Aoyagi and Bassham 1984; Aoyagi et al. 1984; Gallusci et al. 1996). The metabolic function we propose herein (Fig. 6) for the endosperm-localized cytoplasmic PPDK isoform in developing rice seeds is therefore likely to be operating in these related graminaceous cereal species as well. We provide supporting evidence by demonstrating that mature seeds of Sorghum, maize, oats, and wheat are similar to rice seeds in terms of PPDK content. In contrast, with one exception, we could not detect PPDK in mature seeds of the several dicotyledonous species we included in this study. The single but striking exception was the finding that mature seeds of striped sunflower contain appreciable amounts of both PPDK and ThrP-PPDK. This is potentially significant in that sunflower seeds differ markedly from cereal seeds in at least two important respects. The first is that the primary seed storage tissues are the cotyledons rather than the endosperm as in cereal seeds. Second, seeds of sunflowers have oil as a major storage product in addition to carbohydrate, versus primarily carbohydrate for rice (cereal) seeds (Bewley et al. 2000). The readily detectable amounts of PPDK and ThrP-PPDK in sunflower seeds implies that a highly expressed C3 PPDK enzyme, and its regulation via reversible phosphorylation, may not be a unique feature of developing graminaceous seeds.
Abbreviations
- ACS:
-
Acyl-CoA synthetase
- FA:
-
Fatty acid
- GaP-DH:
-
Glyceraldehyde-3-Pi dehydrogenase
- GUS:
-
β-Glucuronidase
- MDH:
-
Malate dehydrogenase
- OAA:
-
Oxaloacetic acid
- PEP:
-
Phosphoenol pyruvate
- PEPC:
-
PEP carboxylase
- Pi:
-
Orthophosphate
- PK:
-
Pyrvuate kinase
- PPDK:
-
Pyruvate, orthophosphate dikinase
- PPi:
-
Pyrophosphate
- Pyr:
-
Pyrvuate
- RP:
-
PPDK regulatory protein
- ThrP:
-
Threonyl phosphorylated
References
Aoyagi K, Bassham JA (1984) Pyruvate orthophosphate dikinase of C3 seeds and leaves as compared to the enzyme from maize. Plant Physiol 75:387–392
Aoyagi K, Bassham JA, Greene FC (1984) Pyruvate orthophosphate dikinase gene expression in developing wheat seeds. Plant Physiol 75:393–396
Bewley JD, Hempel FD, McCormick S, Zambryski P (2000) Reproductive development. In: Buchanan BB, Gruissem W, Jones RL (eds) Biochemistry and molecular biology of plants. American Society of Plant Physiologists, Rockville pp 988–1043
Burnell JN, Hatch MD (1985) Regulation of C4 photosynthesis: Purification and properties of the protein catalyzing ADP-mediated inactivation and Pi-mediated activation of pyruvate, Pi dikinase. Arch Biochem Biophys 237:490–503
Burnell JN, Hatch MD (1986) Activation and inactivation of an enzyme catalyzed by a single, bifunctional protein: a new example and why. Arch Biochem Biophys 245:297–304
Chastain CJ, Chollet R (2003) Regulation of pyruvate, orthophosphate dikinase by ADP/Pi-dependent reversible phosphorylation in C3 and C4 plants. Plant Physiol Biochem 41:523–532
Chastain CJ, Botschner M, Harrington GS, Thompson BJ, Mills SE, Sarath G, Chollet R (2000) Further analysis of maize C4-pyruvate, orthophosphate dikinase phosphorylation by its bifunctional regulatory protein using selective substitutions of the regulatory Thr-456 and catalytic His-458 residues. Arch Biochem Biophys 375:165–170
Chastain CJ, Fries JP, Vogel JA, Randklev CL, Vossen AP, Dittmer SK, Watkins EE, Fiedler LJ, Wacker SA, Meinhover KC, Sarath G, Chollet R (2002) Pyruvate, orthophosphate dikinase in leaves and chloroplasts of C3 plants undergoes light/dark-induced reversible phosphorylation. Plant Physiol 128:1368–1378
Chastain CJ, Thompson BJ, Chollet R (1996) Maize recombinant C4-pyruvate, orthophosphate dikinase: expression in Escherichia coli, partial purification, and characterization of the phosphorylatable protein. Photosynth Res 49:83–89
Dennis DT, Blakeley SD (2000) Carbohydrate metabolism. In: Buchanan BB, Gruissem W, Jones RL (eds) Biochemistry and molecular biology of plants. American Society of Plant Physiologists, Rockville, pp 630–675
Fukayama H, Tsuchida H, Agarie S, Nomura M, Onodera H, Ono K, Lee B-H, Hirose S, Toki S, Ku MSB, Makino A, Matsuoka M, Miyao M (2001) Significant accumulation of C4-specific pyruvate,orthophosphate dikinase in a C3 plant, rice. Plant Physiol 127:1136–1146
Dominguez F, Cejudo FJ (1996) Characterization of the endoproteases appearing during wheat grain development. Plant Physiol 112:1211–1217
Gallusci P, Varotto S, Matsuoka M, Maddaloni M, Thompson MD (1996) Regulation of cytosolic pyruvate, orthophosphate dikinase expression in developing maize endosperm. Plant Mol Biol 31:45–55
Hata S, Matsuoka M (1987) Immunological studies on pyruvate orthophosphate dikinase in C3 plants. Plant Cell Physiol 28:635–641
Hatch MD (1987) C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochim Biophys Acta 895:81–106
Hausler RE, Hirsch HJ, Kreuzaler F, Peterhansel C (2002) Overexpression of C4-cycle enzymes in transgenic C3 plants: a biotechnological approach to improve C3-photosynthesis. J Exp Bot 53:591–607
Hocking CG, Anderson JW (1986) Survey of pyruvate, phosphate dikinase activity of plants in relation to C3, C4 and CAM mechanisms of CO2 assimilation. Phytochemistry 25:1537–1543
Ichihara K, Kobayashi N, Saito K (2003) Lipid synthesis and acyl-CoA synthetase in developing rice seeds. Lipids 38:881–884
Imaizumi N, Ku MSB, Ishihara K, Samejima M, Kaneko S, Matsuoka M (1997) Characterization of the gene for pyruvate, orthophosphate dikinase from rice, a C3 plant, and comparison of structure and expression between C3 and C4 genes for this protein. Plant Mol Biol 34:701–716
Ireland RJ, Joy KW (1990) Aminotransferases. In: Lea PJ (eds) Methods in Plant Biochemistry. Academic, San Diego, Vol 3, pp 39–72
Jenkins CLD, Hatch MD (1985) Properties and reaction mechanism of C4 leaf pyruvate,Pi dikinase. Arch Biochem Biophys 239:53–62
Kang HG, Park S, Matsuoka M, An G (2005) White-core endosperm floury endosperm-4 in rice is generated by knockout mutations in the C4-type pyruvate orthophosphate dikinase gene (OsPPDKB). Plant J 42:901–911
Kurata N (2005) Oryzabase. National Institute of Genetics, Japan, http://www.shigen.nig.ac.jp/rice/oryzabase/development/
Lin SK, Chang MC, Tsai YG, Lur HS (2005) Proteomic analysis of the expression of proteins related to rice quality during caryopsis development and the effect of high temperature on expression. Proteomics 5:2140–2156
McGuire M, Carroll LJ, Yankie L, Thrall S, Dunaway-Mariano D, Herzberg O, Jayaram B, Haley BH (1996) Determination of the nucleotide binding site within Clostridium symbiosum pyruvate phosphate dikinase by photoaffinity labeling, site-directed mutagenesis, and structural analysis. Biochem 35:8544–8552
Meyer AO, Kelly GJ, Latzko E (1982) Pyruvate orthophosphate dikinase from immature grains of cereal grasses. Plant Physiol 69:7–10
Moons A, Valcke R, Van Montagu M (1998) Low-oxygen stress and water deficit induce cytosolic pyruvate orthophosphate dikinase (PPDK) expression in roots of rice, a C3 plant. Plant J 15:89–98
Nakamoto H, Young PS (1990) Light activation of pyruvate, orthophosphate dikinase in maize mesophyll chloroplasts: a role of adenylate energy charge. Plant Cell Physiol 31:1–6
Nomura M, Sentoku N, Tajima S, Matsuoka M (2000) Expression patterns of cytoplasmic pyruvate,orthophosphate dikinase of rice (C3) and maize (C4) in a C3 plant, rice. Aust J Plant Physiol 27:343–347
Plaxton WC (1996) The organization and regulation of plant glycolysis. Annu Rev Plant Physiol Plant Mol Biol 47:185–214
Plaxton WC (2005) Metabolic flexibility helps plants to survive stress. Web-essay for chapter 11. In: Taiz L, Zeigler E (eds) Plant physiology, 4th edn. Sinauer, Sunderland. URL: http://www.plantphys.net/article.php?ch=e&id=124
Rolletschek H, Weschke W, Weber H, Wobus U, Borisjuk L (2004) Energy state and its control on seed development: starch accumulation is associated with high ATP and steep oxygen gradients within barley grains. J Exp Bot 55:1351–1359
Rosche E, Streubel M, Westhoff P (1994) Primary structure of the pyruvate orthophosphate dikinase of the C3 plant Flaveria pringlei and expression analysis of pyruvate orthophosphate dikinase sequences in C3, C3-C4 and C4 Flaveria species. Plant Mol Biol 26:763–769
Saavedra E, Encalada R, Pineda E, Jasso-Chavez R, Moreno-Sanchez R (2005) Glycolysis in Entamoeba histolytica. Biochemical characterization of recombinant glycolytic enzymes and flux control analysis. FEBS J 272:1767–1783
Schaeffer GW, Sharpe FT (1997) Free and bound amino acids and proteins in developing grains of rice with enhanced lysine/proteins. Theor Appl Genet 94:878–881
Sheen J (1991) Molecular mechanisms underlying the differential expression of maize pyruvate, orthophosphate dikinase genes. Plant Cell 3:225–245
Sugiyama T, Hirayama Y (1983) Correlation of the activities of phosphoenolpyruvate carboxylase and pyruvate,orthophosphate dikinase with biomass in maize seedlings. Plant Cell Physiol 24:783–787
Usuda H, Ku MSB, Edwards GE (1985) Influence of light intensity during growth on photosynthesis and activity of key photosynthetic enzymes in a C4 plant (Zea mays). Physiol Plant 63:65–70
van Dongen JT, Roeb GW, Dautzenberg M, Froehlich A, Vigeolas H, Minchin PEH, Geigenberger P (2004) Phloem import and storage metabolism are highly coordinated by the low oxygen concentrations within developing wheat seeds. Plant Physiol 135:1809–1821
Wang X, Larkins BA (2001) Genetic analysis of amino acid accumulation in opaque-2 maize endosperm. Plant Physiol 125:1766–1777
Yamamoto E, Sugiyama T, Miyachi S (1974) Action spectrum for light activation of pyruvate, phosphate dikinase in maize leaves. Plant Cell Physiol 15:987–992
Acknowledgements
This work was supported by U.S. National Science Foundation Grant No. RUI-0318568 to C.J.C. The authors wish to acknowledge the generous assistance of Dr. Raymond Chollet in providing helpful insights during the preparation of this manuscript and in its review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chastain, C.J., Heck, J.W., Colquhoun, T.A. et al. Posttranslational regulation of pyruvate, orthophosphate dikinase in developing rice (Oryza sativa) seeds. Planta 224, 924–934 (2006). https://doi.org/10.1007/s00425-006-0259-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-006-0259-3